Merkel Cell Polyomavirus Co-Infection in HIV/AIDS Individuals: Clinical Diagnosis, Consequences and Treatments
Abstract
:1. Introduction
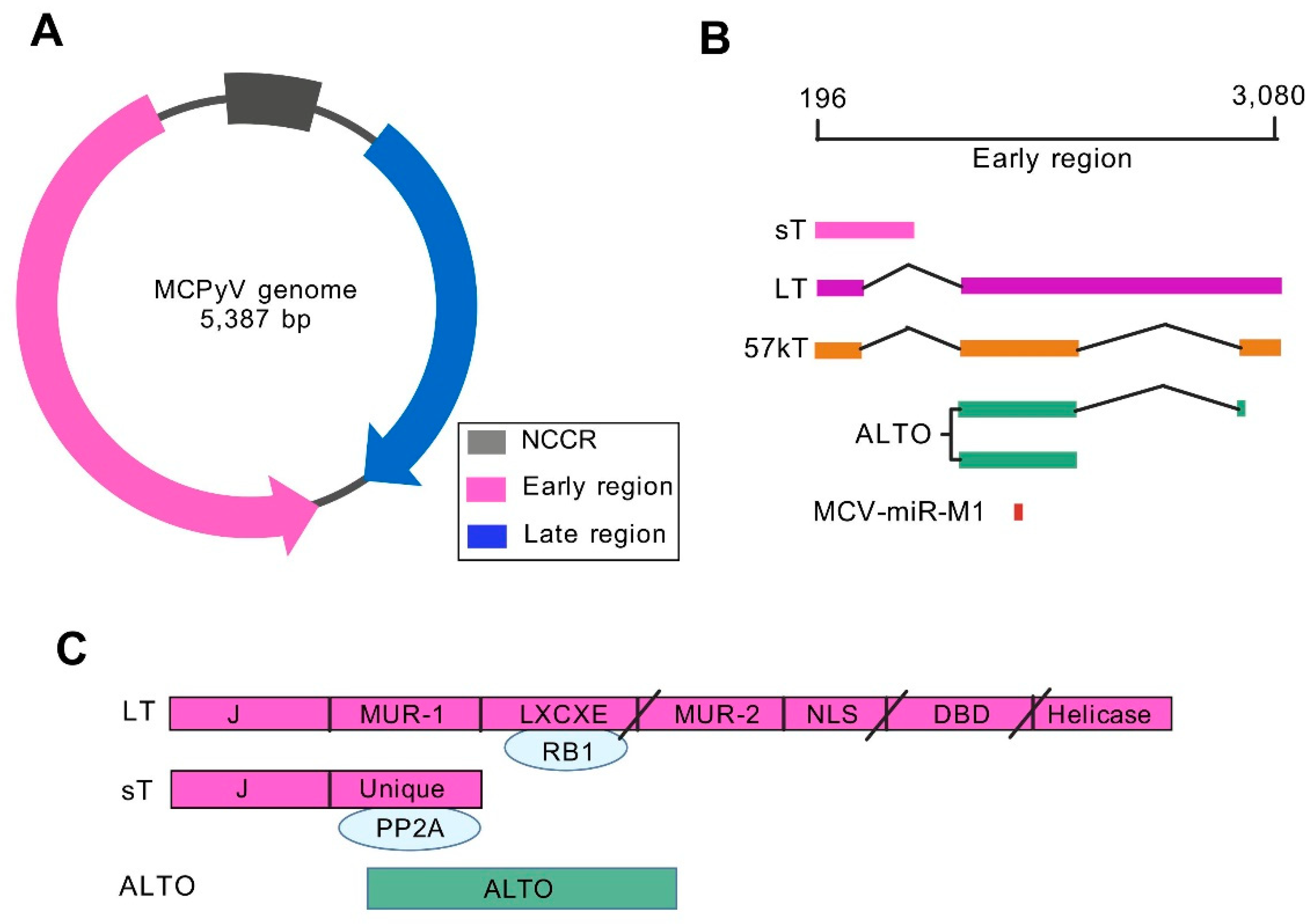
2. Genomic Characteristics and Regulatory Mechanisms of MCV
3. Epidemiological Characteristics of MCV
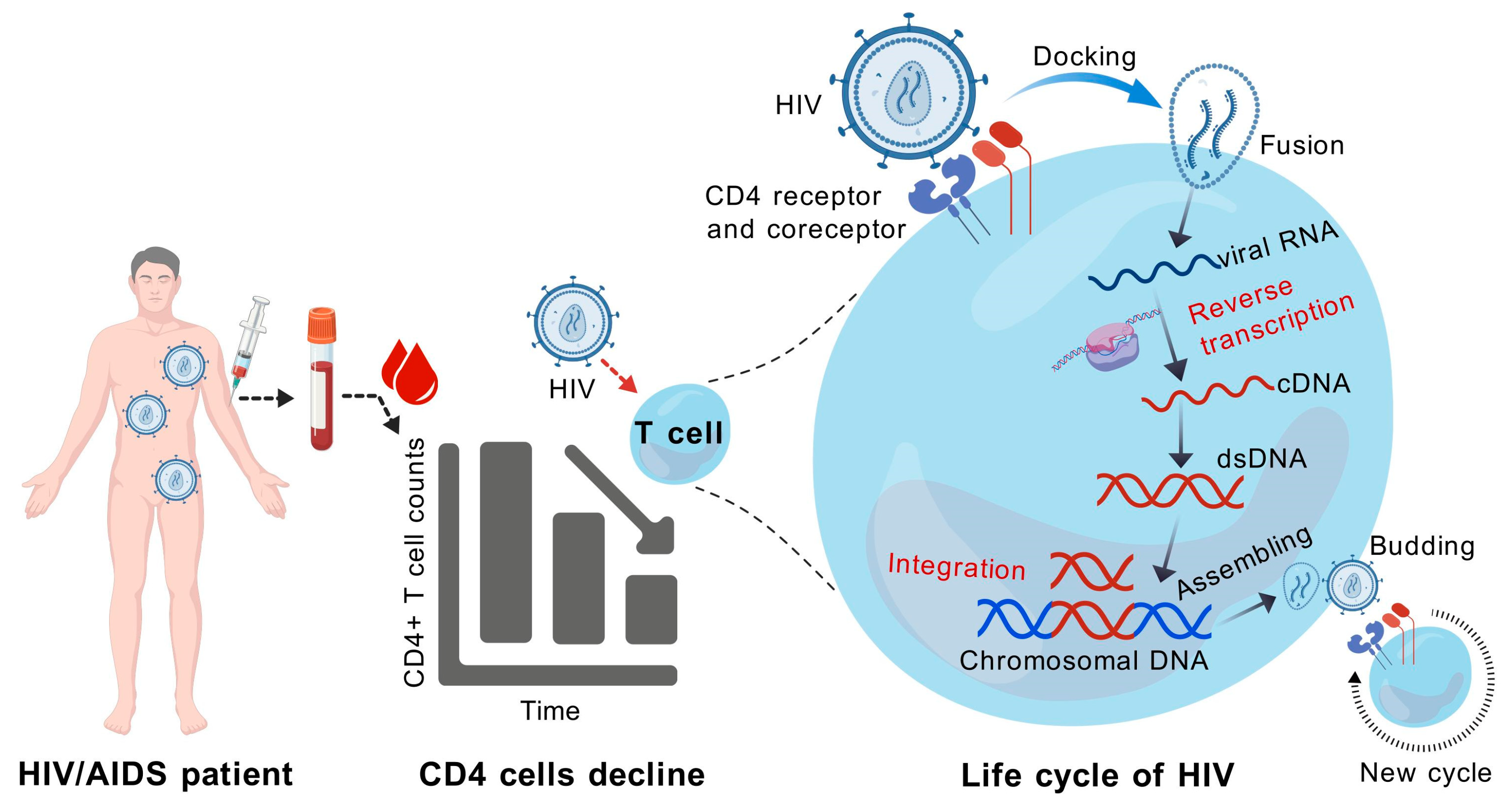
4. HIV Infection Increases the Risk of MCV Activation and MCC Occurrence
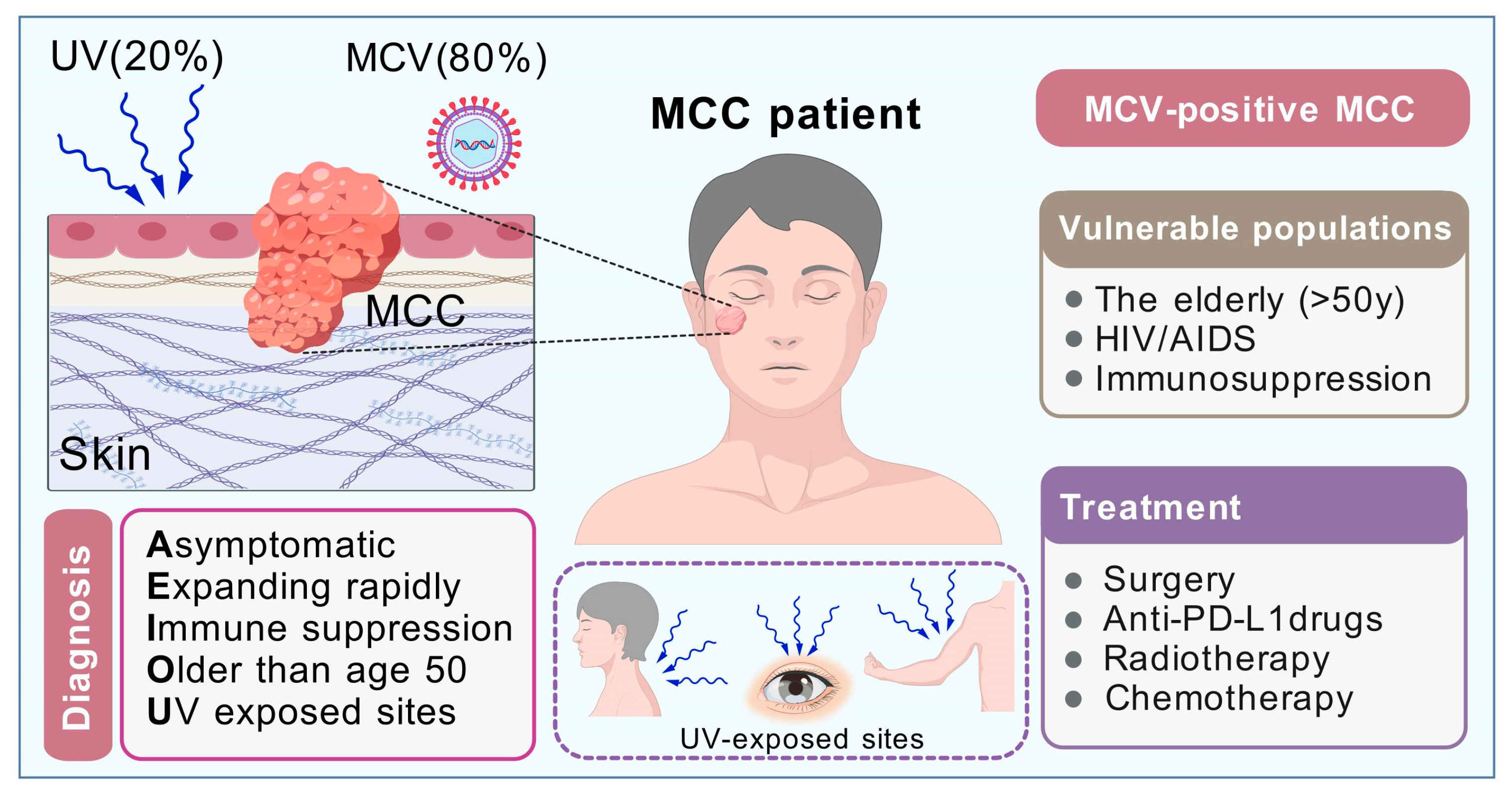
5. MCC Occurrence in HIV/AIDS Individuals
6. Clinical Characteristics of MCC
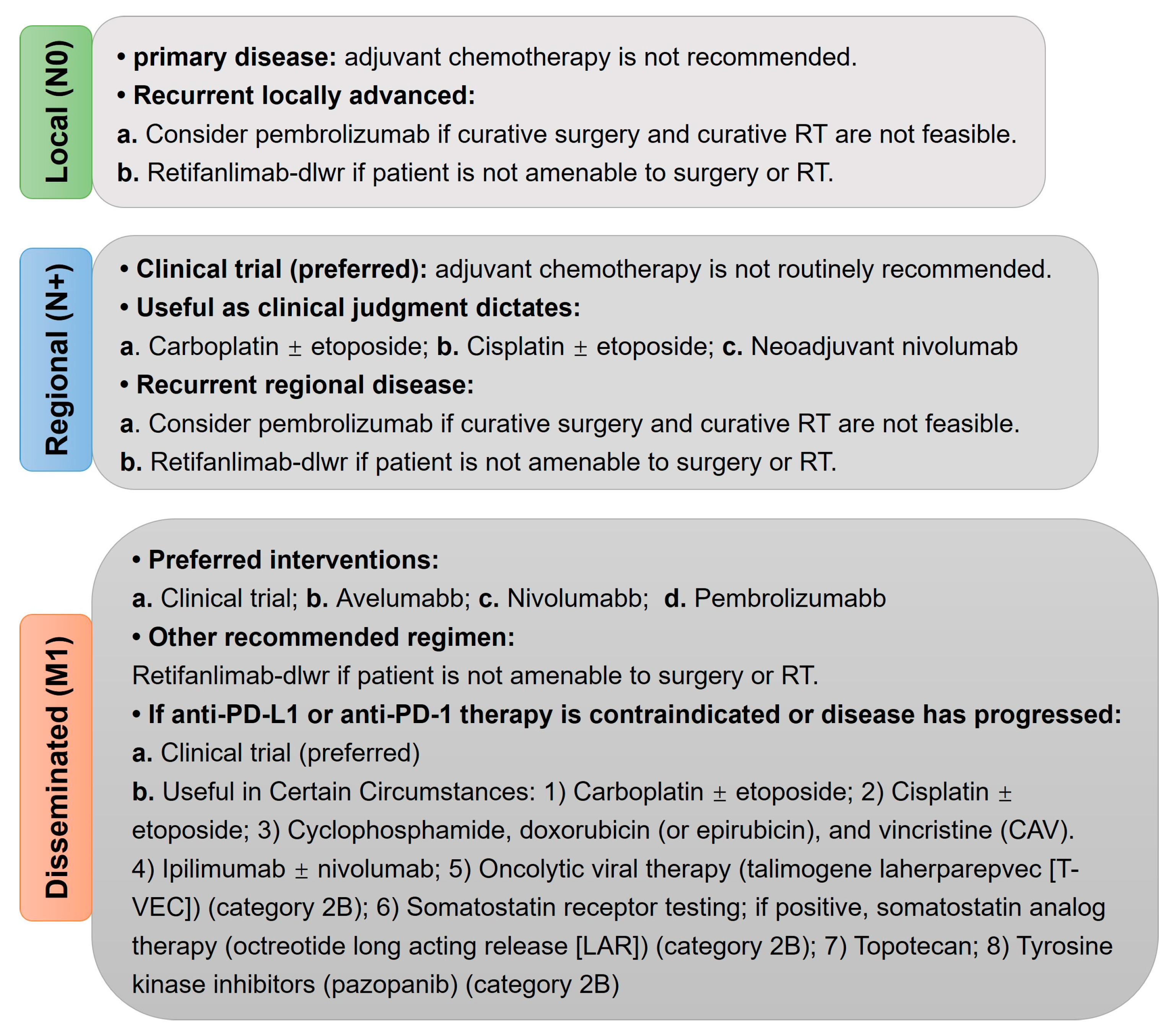
7. Molecular Characteristics and Clinical Treatments of MCC
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, S.O.M.; Campos-do-Carmo, G.; Dos Santos, A.L.S.; Martins, C.; de Melo, A.C. Merkel cell carcinoma: Epidemiology, clinical features, diagnosis and treatment of a rare disease. An. Bras. Dermatol. 2023, 98, 277–286. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, C.; Li, H. BK polyomavirus: Latency, reactivation, diseases and tumorigenesis. Front. Cell. Infect. Microbiol. 2023, 13, 1263983. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cliften, P.F.; Duncavage, E.J.; Lewis, J.S., Jr.; Chernock, R.D. UV Signature Mutations Reclassify Salivary High-grade Neuroendocrine Carcinomas as Occult Metastatic Cutaneous Merkel Cell Carcinomas. Am. J. Surg. Pathol. 2019, 43, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.H.; DeCaprio, J.A. Merkel Cell Carcinoma in the HIV-1/AIDS Patient. In HIV/AIDS-Associated Viral Oncogenesis; Cancer Treatment and Research; Springer: Cham, Switzerland, 2019; Volume 177, pp. 211–229. [Google Scholar] [CrossRef]
- Kassem, A.; Schopflin, A.; Diaz, C.; Weyers, W.; Stickeler, E.; Werner, M.; Zur Hausen, A. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008, 68, 5009–5013. [Google Scholar] [CrossRef]
- Houben, R.; Shuda, M.; Weinkam, R.; Schrama, D.; Feng, H.; Chang, Y.; Moore, P.S.; Becker, J.C. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J. Virol. 2010, 84, 7064–7072. [Google Scholar] [CrossRef]
- Starrett, G.J.; Marcelus, C.; Cantalupo, P.G.; Katz, J.P.; Cheng, J.; Akagi, K.; Thakuria, M.; Rabinowits, G.; Wang, L.C.; Symer, D.E.; et al. Merkel Cell Polyomavirus Exhibits Dominant Control of the Tumor Genome and Transcriptome in Virus-Associated Merkel Cell Carcinoma. mBio 2017, 8, e02079-16. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.M.; Ortiz, L.E.; Lukacher, A.E.; Kwun, H.J. Merkel Cell Polyomavirus Large T Antigen Induces Cellular Senescence for Host Growth Arrest and Viral Genome Persistence through Its Unique Domain. Cells 2023, 12, 380. [Google Scholar] [CrossRef] [PubMed]
- Passerini, S.; Prezioso, C.; Prota, A.; Babini, G.; Coppola, L.; Lodi, A.; Epifani, A.C.; Sarmati, L.; Andreoni, M.; Moens, U.; et al. Detection Analysis and Study of Genomic Region Variability of JCPyV, BKPyV, MCPyV, HPyV6, HPyV7 and QPyV in the Urine and Plasma of HIV-1-Infected Patients. Viruses 2022, 14, 2544. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.Y.; Lurain, K.; Yarchoan, R. How immunodeficiency can lead to malignancy. Hematol. Am. Soc. Hematol. Educ. Program. 2021, 2021, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, C.; Obregon, F.; Ambroselli, D.; Petrolo, S.; Checconi, P.; Rodio, D.M.; Coppola, L.; Nardi, A.; de Vito, C.; Sarmati, L.; et al. Merkel Cell Polyomavirus (MCPyV) in the Context of Immunosuppression: Genetic Analysis of Noncoding Control Region (NCCR) Variability among a HIV-1-Positive Population. Viruses 2020, 12, 507. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.; Celikdemir, B.; Kervarrec, T.; Schrama, D. Merkel Cell Polyomavirus: Infection, Genome, Transcripts and Its Role in Development of Merkel Cell Carcinoma. Cancers 2023, 15, 444. [Google Scholar] [CrossRef] [PubMed]
- Kamminga, S.; van der Meijden, E.; Feltkamp, M.C.W.; Zaaijer, H.L. Seroprevalence of fourteen human polyomaviruses determined in blood donors. PLoS ONE 2018, 13, e0206273. [Google Scholar] [CrossRef]
- Kamminga, S.; van der Meijden, E.; de Brouwer, C.; Feltkamp, M.; Zaaijer, H. Prevalence of DNA of fourteen human polyomaviruses determined in blood donors. Transfusion 2019, 59, 3689–3697. [Google Scholar] [CrossRef] [PubMed]
- DeCaprio, J.A. Merkel cell polyomavirus and Merkel cell carcinoma. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160276. [Google Scholar] [CrossRef] [PubMed]
- Rapchak, K.; Yagobian, S.D.; Moore, J.; Khattri, M.; Shuda, M. Merkel cell polyomavirus small T antigen is a viral transcription activator that is essential for viral genome maintenance. PLoS Pathog. 2022, 18, e1011039. [Google Scholar] [CrossRef] [PubMed]
- Akhbari, P.; Tobin, D.; Poterlowicz, K.; Roberts, W.; Boyne, J.R. MCV-miR-M1 Targets the Host-Cell Immune Response Resulting in the Attenuation of Neutrophil Chemotaxis. J. Investig. Dermatol. 2018, 138, 2343–2354. [Google Scholar] [CrossRef]
- Moore, P.S.; Chang, Y. Are There More Human Cancer Viruses Left to Be Found? Annu. Rev. Virol. 2024, 11, 239–259. [Google Scholar] [CrossRef]
- Hashida, Y.; Higuchi, T.; Matsui, K.; Shibata, Y.; Nakajima, K.; Sano, S.; Daibata, M. Genetic Variability of the Noncoding Control Region of Cutaneous Merkel Cell Polyomavirus: Identification of Geographically Related Genotypes. J. Infect. Dis. 2018, 217, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Hesbacher, S.; Pfitzer, L.; Wiedorfer, K.; Angermeyer, S.; Borst, A.; Haferkamp, S.; Scholz, C.J.; Wobser, M.; Schrama, D.; Houben, R. RB1 is the crucial target of the Merkel cell polyomavirus Large T antigen in Merkel cell carcinoma cells. Oncotarget 2016, 7, 32956–32968. [Google Scholar] [CrossRef]
- Kwun, H.; Wendzicki, J.; Shuda, Y.; Moore, P.S.; Chang, Y. Merkel cell polyomavirus small T antigen induces genome instability by E3 ubiquitin ligase targeting. Oncogene 2017, 36, 6784–6792. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Park, D.E.; Berrios, C.; White, E.A.; Arora, R.; Yoon, R.; Branigan, T.; Xiao, T.; Westerling, T.; Federation, A.; et al. Merkel cell polyomavirus recruits MYCL to the EP400 complex to promote oncogenesis. PLoS Pathog. 2017, 13, e1006668. [Google Scholar] [CrossRef]
- White, M.K.; Safak, M.; Khalili, K. Regulation of gene expression in primate polyomaviruses. J. Virol. 2009, 83, 10846–10856. [Google Scholar] [CrossRef]
- Pietropaolo, V.; Prezioso, C.; Bagnato, F.; Antonelli, G. John Cunningham virus: An overview on biology and disease of the etiological agent of the progressive multifocal leukoencephalopathy. New Microbiol. 2018, 41, 179–186. [Google Scholar]
- Olsen, G.H.; Hirsch, H.H.; Rinaldo, C.H. Functional analysis of polyomavirus BK non-coding control region quasispecies from kidney transplant recipients. J. Med. Virol. 2009, 81, 1959–1967. [Google Scholar] [CrossRef]
- Gosert, R.; Kardas, P.; Major, E.O.; Hirsch, H.H. Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy patient samples increase virus early gene expression and replication rate. J. Virol. 2010, 84, 10448–10456. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, C.; Scribano, D.; Rodio, D.M.; Ambrosi, C.; Trancassini, M.; Palamara, A.T.; Pietropaolo, V. COS-7-based model: Methodological approach to study John Cunningham virus replication cycle. Virol. J. 2018, 15, 29. [Google Scholar] [CrossRef]
- Blackard, J.T.; Davies, S.M.; Laskin, B.L. BK polyomavirus diversity—Why viral variation matters. Rev. Med. Virol. 2020, 30, e2102. [Google Scholar] [CrossRef] [PubMed]
- Bethge, T.; Hachemi, H.A.; Manzetti, J.; Gosert, R.; Schaffner, W.; Hirsch, H.H. Sp1 sites in the noncoding control region of BK polyomavirus are key regulators of bidirectional viral early and late gene expression. J. Virol. 2015, 89, 3396–3411. [Google Scholar] [CrossRef]
- Borchert, S.; Czech-Sioli, M.; Neumann, F.; Schmidt, C.; Wimmer, P.; Dobner, T.; Grundhoff, A.; Fischer, N. High-affinity Rb binding, p53 inhibition, subcellular localization, and transformation by wild-type or tumor-derived shortened Merkel cell polyomavirus large T antigens. J. Virol. 2014, 88, 3144–3160. [Google Scholar] [CrossRef]
- Nicol, J.T.; Robinot, R.; Carpentier, A.; Carandina, G.; Mazzoni, E.; Tognon, M.; Touze, A.; Coursaget, P. Age-specific seroprevalences of merkel cell polyomavirus, human polyomaviruses 6, 7, and 9, and trichodysplasia spinulosa-associated polyomavirus. Clin. Vaccine Immunol. 2013, 20, 363–368. [Google Scholar] [CrossRef]
- van der Meijden, E.; Bialasiewicz, S.; Rockett, R.J.; Tozer, S.J.; Sloots, T.P.; Feltkamp, M.C. Different serologic behavior of MCPyV, TSPyV, HPyV6, HPyV7 and HPyV9 polyomaviruses found on the skin. PLoS ONE 2013, 8, e81078. [Google Scholar] [CrossRef] [PubMed]
- Prado, J.C.M.; Monezi, T.A.; Amorim, A.T.; Lino, V.; Paladino, A.; Boccardo, E. Human polyomaviruses and cancer: An overview. Clinics 2018, 73, e558s. [Google Scholar] [CrossRef]
- Lasithiotaki, I.; Tsitoura, E.; Koutsopoulos, A.; Lagoudaki, E.; Koutoulaki, C.; Pitsidianakis, G.; Spandidos, D.A.; Siafakas, N.M.; Sourvinos, G.; Antoniou, K.M. Aberrant expression of miR-21, miR-376c and miR-145 and their target host genes in Merkel cell polyomavirus-positive non-small cell lung cancer. Oncotarget 2017, 8, 112371–112383. [Google Scholar] [CrossRef]
- Zhou, X.; Nakashima, K.; Ito, M.; Zhang, X.; Sakai, S.; Feng, C.; Sun, H.; Chen, H.; Li, T.C.; Suzuki, T. Prevalence and viral loads of polyomaviruses BKPyV, JCPyV, MCPyV, TSPyV and NJPyV and hepatitis viruses HBV, HCV and HEV in HIV-infected patients in China. Sci. Rep. 2020, 10, 17066. [Google Scholar] [CrossRef] [PubMed]
- Paulson, K.G.; Lewis, C.W.; Redman, M.W.; Simonson, W.T.; Lisberg, A.; Ritter, D.; Morishima, C.; Hutchinson, K.; Mudgistratova, L.; Blom, A.; et al. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: A prospective validation study. Cancer 2017, 123, 1464–1474. [Google Scholar] [CrossRef]
- Pastrana, D.V.; Tolstov, Y.L.; Becker, J.C.; Moore, P.S.; Chang, Y.; Buck, C.B. Quantitation of human seroresponsiveness to Merkel cell polyomavirus. PLoS Pathog. 2009, 5, e1000578. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Amini, A.; Bibee, K.; Bolotin, D.; Bordeaux, J.; Chen, P.L.; Contreras, C.M.; et al. NCCN Guidelines® Insights: Merkel Cell Carcinoma, Version 1.2024. J. Natl. Compr. Cancer Netw. 2024, 22, e240002. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.H.; Wang, J. Merkel cell carcinoma. Arch. Pathol. Lab. Med. 2010, 134, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Diaz, J.; Tsang, S.H.; Buck, C.B.; You, J. Merkel cell polyomavirus large T antigen disrupts host genomic integrity and inhibits cellular proliferation. J. Virol. 2013, 87, 9173–9188. [Google Scholar] [CrossRef] [PubMed]
- Haymerle, G.; Janik, S.; Fochtmann, A.; Pammer, J.; Schachner, H.; Nemec, L.; Mildner, M.; Houben, R.; Grasl, M.C.; Erovic, B.M. Expression of Merkelcell polyomavirus (MCPyV) large T-antigen in Merkel cell carcinoma lymph node metastases predicts poor outcome. PLoS ONE 2017, 12, e0180426. [Google Scholar] [CrossRef]
- Shuda, M.; Feng, H.; Kwun, H.J.; Rosen, S.T.; Gjoerup, O.; Moore, P.S.; Chang, Y. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc. Natl. Acad. Sci. USA 2008, 105, 16272–16277. [Google Scholar] [CrossRef] [PubMed]
- Panou, E.; Nikolaou, C.; Payagala, S.; Bakkour, W.; Shaw, H.; Perrett, C.; French, P.; Ratynska, M.; Brock, C.; Rayment, M.; et al. HIV-related Merkel cell carcinoma: A report of three cases from the UK. Int. J. STD AIDS 2022, 33, 1084–1086. [Google Scholar] [CrossRef]
- Ma, J.E.; Brewer, J.D. Merkel cell carcinoma in immunosuppressed patients. Cancers 2014, 6, 1328–1350. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Lebbe, C.; Zur Hausen, A.; Avril, M.F.; Hariharan, S.; Bharmal, M.; Becker, J.C. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur. J. Cancer 2017, 71, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.; Jaimes, N.; Lemos, B.; Mostaghimi, A.; Wang, L.C.; Penas, P.F.; Nghiem, P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol. 2008, 58, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Paulson, K.G.; Iyer, J.G.; Blom, A.; Warton, E.M.; Sokil, M.; Yelistratova, L.; Schuman, L.; Nagase, K.; Bhatia, S.; Asgari, M.M.; et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J. Investig. Dermatol. 2013, 133, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Douek, D.C.; Brenchley, J.M.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Okamoto, Y.; Casazza, J.P.; Kuruppu, J.; Kunstman, K.; Wolinsky, S.; et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 2002, 417, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Primers 2015, 1, 15035. [Google Scholar] [CrossRef]
- Wieland, U.; Silling, S.; Scola, N.; Potthoff, A.; Gambichler, T.; Brockmeyer, N.H.; Pfister, H.; Kreuter, A. Merkel cell polyomavirus infection in HIV-positive men. Arch. Dermatol. 2011, 147, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Wieland, U.; Kreuter, A. Merkel cell polyomavirus infection and Merkel cell carcinoma in HIV-positive individuals. Curr. Opin. Oncol. 2011, 23, 488–493. [Google Scholar] [CrossRef]
- Fukumoto, H.; Sato, Y.; Hasegawa, H.; Katano, H. Frequent detection of Merkel cell polyomavirus DNA in sera of HIV-1-positive patients. Virol. J. 2013, 10, 84. [Google Scholar] [CrossRef]
- Passerini, S.; Fracella, M.; Benvenuto, D.; Bugani, G.; D’Auria, A.; Coratti, E.; Babini, G.; Moens, U.; Cavallari, E.N.; Torti, C.; et al. High rates of anal Merkel Cell Polyomavirus and HPV co-infection among people living with HIV. J. Med. Virol. 2024, 96, e29883. [Google Scholar] [CrossRef] [PubMed]
- Gossai, A.; Waterboer, T.; Nelson, H.H.; Michel, A.; Willhauck-Fleckenstein, M.; Farzan, S.F.; Hoen, A.G.; Christensen, B.C.; Kelsey, K.T.; Marsit, C.J.; et al. Seroepidemiology of Human Polyomaviruses in a US Population. Am. J. Epidemiol. 2016, 183, 61–69. [Google Scholar] [CrossRef]
- Ramachandran, P.; Erdinc, B.; Gotlieb, V. An Unusual Presentation of Merkel Cell Carcinoma in a HIV Patient: A Case Report and Literature Review. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619836695. [Google Scholar] [CrossRef]
- Brugnaro, P.; Morelli, E.; Fiscon, M.; Ebo, F.; Rosini, G.; Belussi, F.; Eseme, F.; Mione, C.A.; Donisi, P.M.; Raise, E. Sustained remission of a primary nodal Merkel cell carcinoma in an HIV-positive patient. Onkologie 2011, 34, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Atallah-Yunes, S.A.; Murphy, D.J.; Noy, A. HIV-associated Burkitt lymphoma. Lancet Haematol. 2020, 7, e594–e600. [Google Scholar] [CrossRef]
- Cesarman, E.; Damania, B.; Krown, S.E.; Martin, J.; Bower, M.; Whitby, D. Kaposi sarcoma. Nat. Rev. Dis. Prim. 2019, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Gauci, M.L.; Aristei, C.; Becker, J.C.; Blom, A.; Bataille, V.; Dreno, B.; Del Marmol, V.; Forsea, A.M.; Fargnoli, M.C.; Grob, J.J.; et al. Diagnosis and treatment of Merkel cell carcinoma: European consensus-based interdisciplinary guideline—Update 2022. Eur. J. Cancer 2022, 171, 203–231. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, N.; Hunt, D.; Yan, J.; Jabbour, A.J.; Nghiem, P.; Choi, J.; Zhang, Y.; Freeman, A.F.; Bergerson, J.R.E.; Dell’Orso, S.; et al. Genetic Risk Factors for Early-Onset Merkel Cell Carcinoma. JAMA Dermatol. 2024, 160, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.Y.; Su, Q.J.; Zhu, Y.J.; Ji, P.Z.; Ma, J.W.; Liu, B.; Yang, Y.L. Merkel cell carcinoma: A clinicopathological study of 10 cases. Zhonghua Bing Li Xue Za Zhi 2021, 50, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Valiga, A.; Tababa, E.J.; Chung, H.J.; Cha, J. Merkel Cell Hyperplasia Versus Intraepidermal Merkel Cell Carcinoma: A Comparative Study of 2 Cases. Am. J. Dermatopathol. 2023, 45, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Blitzblau, R.; Bowen, G.M.; Contreras, C.M.; Daniels, G.A.; Decker, R.; et al. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 742–774. [Google Scholar] [CrossRef]
- Starrett, G.J.; Thakuria, M.; Chen, T.; Marcelus, C.; Cheng, J.; Nomburg, J.; Thorner, A.R.; Slevin, M.K.; Powers, W.; Burns, R.T.; et al. Clinical and molecular characterization of virus-positive and virus-negative Merkel cell carcinoma. Genome Med. 2020, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.W.; Collie, A.M.; Hovelson, D.H.; Cani, A.K.; Verhaegen, M.E.; Patel, R.M.; Fullen, D.R.; Omata, K.; Dlugosz, A.A.; Tomlins, S.A.; et al. Next generation sequencing of Cytokeratin 20-negative Merkel cell carcinoma reveals ultraviolet-signature mutations and recurrent TP53 and RB1 inactivation. Mod. Pathol. 2016, 29, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbe, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.L.; Sakai, S.; Kudchadkar, R.R.; Fling, S.P.; Day, T.A.; Vergara, J.A.; Ashkin, D.; Cheng, J.H.; Lundgren, L.M.; Raabe, V.N.; et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci. Transl. Med. 2019, 11, eaat2702. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Church, C.; Alexander, N.A.; Shinohara, M.M.; Paulson, K.G.; Lewis, K.D.; Lee, N.S.; Nghiem, P. Immune checkpoint inhibitor therapy in HIV-associated Merkel cell carcinoma: A case series of 3 patients. JAAD Case Rep. 2021, 8, 28–33. [Google Scholar] [CrossRef]
- Tello, T.L.; Coggshall, K.; Yom, S.S.; Yu, S.S. Merkel cell carcinoma: An update and review: Current and future therapy. J. Am. Acad. Dermatol. 2018, 78, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Montoto, S.; Shaw, K.; Okosun, J.; Gandhi, S.; Fields, P.; Wilson, A.; Shanyinde, M.; Cwynarski, K.; Marcus, R.; de Vos, J.; et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J. Clin. Oncol. 2012, 30, 4111–4116. [Google Scholar] [CrossRef] [PubMed]
- Little, R.F.; Dunleavy, K. Update on the treatment of HIV-associated hematologic malignancies. Hematol. Am. Soc. Hematol. Educ. Program. 2013, 2013, 382–388. [Google Scholar] [CrossRef]
- Lewis, C.W.; Qazi, J.; Hippe, D.S.; Lachance, K.; Thomas, H.; Cook, M.M.; Juhlin, I.; Singh, N.; Thuesmunn, Z.; Takagishi, S.R.; et al. Patterns of distant metastases in 215 Merkel cell carcinoma patients: Implications for prognosis and surveillance. Cancer Med. 2020, 9, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Ungari, M.; Manotti, L.; Tanzi, G.; Varotti, E.; Ferrero, G.; Gusolfino, M.D.; Trombatore, M.; Cavazzuti, L.; Tolomini, M. NeuN, a DNA-binding neuron-specific protein expressed by Merkel cell carcinoma: Analysis of 15 cases. Pathologica 2021, 113, 421–426. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Yin, C.; Lin, Z.; Yan, Z.; Wang, J. Merkel Cell Polyomavirus Co-Infection in HIV/AIDS Individuals: Clinical Diagnosis, Consequences and Treatments. Pathogens 2025, 14, 134. https://doi.org/10.3390/pathogens14020134
Zhou X, Yin C, Lin Z, Yan Z, Wang J. Merkel Cell Polyomavirus Co-Infection in HIV/AIDS Individuals: Clinical Diagnosis, Consequences and Treatments. Pathogens. 2025; 14(2):134. https://doi.org/10.3390/pathogens14020134
Chicago/Turabian StyleZhou, Xianfeng, Chenxue Yin, Ziqi Lin, Zhangren Yan, and Jiangang Wang. 2025. "Merkel Cell Polyomavirus Co-Infection in HIV/AIDS Individuals: Clinical Diagnosis, Consequences and Treatments" Pathogens 14, no. 2: 134. https://doi.org/10.3390/pathogens14020134
APA StyleZhou, X., Yin, C., Lin, Z., Yan, Z., & Wang, J. (2025). Merkel Cell Polyomavirus Co-Infection in HIV/AIDS Individuals: Clinical Diagnosis, Consequences and Treatments. Pathogens, 14(2), 134. https://doi.org/10.3390/pathogens14020134






