Exploiting Wolbachia as a Tool for Mosquito-Borne Disease Control: Pursuing Efficacy, Safety, and Sustainability
Abstract
:1. Introduction
1.1. The Constant Challenge of Vector Control in the Fight Against Arboviral Diseases
1.2. Mosquito-Borne Viral Diseases
1.3. Control Methods Against Mosquito Vectors: Evaluating Effectiveness and Sustainability
1.4. Wolbachia and Its Manipulation for Vector and Disease Control
| Mosquito Host | Wolbachia Strain | Transinfection Method b | Desirable Traits for Vector or Disease Control c | Stability of the Infection and Fitness Effects on Host If Any d,e | Blocked Pathogens |
|---|---|---|---|---|---|
| Ae. aegypti | wMel | Wolbachia microinjection in wild-type embryos [79,85,98]; Wolbachia introgression [99,100,101] | Uni-CI, PI | Stable infection; costs to fecundity, fertility, and quiescent egg viability [85,102]; high temperatures during preimaginal stages increase these negative effects but can also lead to a decrease in Wolbachia density [102,103,104,105,106,107]; CI leakage when males are obtained from eggs stored for a long time [107] | DENV [85,87,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126], ZIKV [87,127,128,129,130], CHIKV [130,131,132], YFV [131,133], MAYV [134,135], SFV [87], KUNV [109] |
| wAlbB | Wolbachia microinjection in wild-type embryos [68,86,135,136]; Wolbachia introgression [137,138] | Uni-CI, PI | Stable infection [136,139]; costs to fertility, longevity, and quiescent egg viability [107]; high temperatures associated with long-term egg storage can further reduce egg viability, female fecundity, and Wolbachia density [87,107] | DENV [87,117,140,141,142,143], ZIKV [87], SFV [87] | |
| wAlbA | Wolbachia microinjection in wild-type embryos [79,87] | Uni-CI, PI | Stable infection; costs to longevity and quiescent egg viability [87] | ZIKV [144] | |
| wAu | Wolbachia microinjection in wild-type embryos [87] | PI | Stable infection; costs to longevity and quiescent egg viability | DENV [87], ZIKV [87], SFV [87] | |
| wMelPop | Wolbachia microinjection in wild-type embryos [84] | Uni-CI, PI | Stable infection; substantial costs to longevity, egg fertility, and other traits [84,145,146,147,148]; CI, Wolbachia density, and vertical inheritance of the infection affected by heat stress during preimaginal stages [149] | DENV [85,113,116,124,150,151], CHIKV [150], YFV [131] | |
| wMelCS | Wolbachia microinjection in wild-type embryos [152] | Uni-CI, PI | Stable infection; costs to fertility and quiescent egg viability | DENV [117,152] | |
| wMelM | Wolbachia microinjection in wild-type embryos [98] | Uni-CI, PI | Stable infection; costs to fertility and quiescent egg viability | DENV [98] | |
| wPip | Wolbachia microinjection in wild-type embryos [152] | Uni-CI | Stable infection; costs to fertility, longevity, and quiescent egg viability | No effects against DENV and KUNV [109] | |
| wRi | Wolbachia microinjection in wild-type embryos [152] | Uni-CI, PI | Stable infection | DENV [152] | |
| wAlbA + wAlbB | Wolbachia microinjection in adult females [153]; Wolbachia microinjection in wild-type embryos [79] | Uni-CI | Stable infection [79]; imperfect vertical inheritance [153] | No data | |
| wAu + wAlbB | Wolbachia microinjection in wild-type embryos [87] | Uni-CI | No data | No data | |
| wMel + wAlbA | Wolbachia microinjection in wild-type embryos [79] | No data | No data | No data | |
| wMel + wAlbB | Wolbachia microinjection in wild-type embryos [79,116] | Uni-CI, PI | Reduced longevity and egg hatching compared to uninfected and wMel- wAlbB- single-infected lines [116] | DENV [116] | |
| wMel + wAlbA + wAlbB | Wolbachia microinjection in wild-type embryos [79] | Uni-CI | Unstable infection; self-CI, displacement of wAlbA Wolbachia from the ovaries | No data | |
| Ae. albopictus | wPip | Wolbachia microinjection in Wolbachia-cured wild-type embryos [89,154] | Bi-CI, PI | Stable infection | ZIKV [155] |
| wMel | Wolbachia microinjection in Wolbachia-cured wild-type embryos [90] | Bi-CI, PI | Stable infection; sensitive to high temperatures during preimaginal stages | DENV [90], CHIKV [156] | |
| wMelPop | Wolbachia microinjection in Wolbachia-cured wild-type embryos [91] | Bi-CI, PI | Stable infection; costs to longevity | No data | |
| wRi | Wolbachia microinjection in Wolbachia-cured wild-type embryos [157] | Bi-CI (incomplete) | Imperfect maternal transmission, partial self CI | No data | |
| wRiversi | Wolbachia microinjection in Wolbachia-cured wild-type embryos [158] | Uni-CI | No data | No data | |
| wPip + wMel | Wolbachia microinjection in Wolbachia-cured wild-type embryos [155] | Bi-CI; PI | Stable infection | DENV [155], ZIKV [155], CHIKV [155] | |
| wAlbA + wAlbB + wAu | Wolbachia microinjection in wild-type embryos [159] | PI | Moderate fitness effects | DENV [159], ZIKV [159] | |
| wAlbA + wAlbB + wRi | Wolbachia microinjection in wild-type embryos [160] | Uni-CI | Stable infection | No data | |
| wAlbA + wAlbB + wPip | Wolbachia microinjection in wild-type embryos [161] | Uni-CI; PI | Stable infection | DENV [161], ZIKV [161] | |
| wAlbA + wAlbB + wMel | Wolbachia microinjection in wild-type embryos [79] | Uni-CI | Stable infection; self-CI, displacement of wAlbA Wolbachia from the ovaries | No data | |
| wAlbA + wAlbB + wMelPop | Wolbachia microinjection in wild-type embryos [162] | Uni-CI (incomplete) | Maternal inheritance affected by blood type; costs to fecundity, fertility, and longevity | No data | |
| Ae. polynesiensis | wRiversi | Wolbachia introgression [163] | Uni-CI | Stable infection | No data |
| wAlbB | Wolbachia microinjection in Wolbachia-cured wild-type embryos [164,165] | Uni-CI; PI | Stable infection | DENV [164], Brugia pahangi [166] | |
| Ae. vexans | wAlbB | Wolbachia microinjection in wild-type adults [86] | No data | Unstable infection | No data |
| An. stephensi | Wolbachia microinjection in wild-type embryos [88] | Uni-CI | Stable infection | Plasmodium falciparum [88,97] | |
| Cx. quinquefasciatus | wAlbB | Wolbachia microinjection in Wolbachia-cured wild-type embryos [92,167] | Bi-CI | Stable infection | No effects on Plasmodium relictum [167] |
| wPip + wAlbA | Wolbachia microinjection in wild-type embryos [92] | Uni-CI | Stable infection | No data |
2. The Exploitation of Wolbachia for Disease Control: A Practical Guide to Open Field Deployment
2.1. Genetic Control Strategies: Potential and Practical Issues with a Specific Focus on Wolbachia-Based Strategies
2.1.1. Vector Population Suppression: Pros and Cons
2.1.2. Vector Population Modification: Pros and Cons
2.2. Safety of Wolbachia-Based Control Strategies
2.3. Legal Framework Related to the Use of Wolbachia as a Vector Control Tool
2.4. Public Acceptance and Initiatives to Favor Community Engagement
2.5. Main Wolbachia-Based Programs of Disease Control Worldwide and Their Results
2.5.1. Incompatible Insect Technique
2.5.2. Population Replacement Strategy
| Program Name (If Any)/Region | Open Field Activities a | Target Species | Involved Wolbachia Infection (Name of the Transinfected Line, If Any) | Control Strategy | Level of the Intervention b | Target Area c | Measured Effect d | Partners and Supporters e |
|---|---|---|---|---|---|---|---|---|
| - | 2012 | Ae. polynesiensis | wRiversi (CP [163]) | IIT (at about 0.6:1 release ratio) | Pilot trial [291] | French Polynesia | Significant reduction of adult females | Public Bodies Institut Louis Malardé (French Polynesia); Government of French Polynesia; University of Kentucky (Lexington, KY, USA); National Institutes of Health (USA) Private Bodies Bill and Melinda Gates Foundation (Seattle, WA, USA) |
| - | 2014 | Ae. albopictus | wPip (ARwPUS; [295]) | IIT (release ratio not available) | Pilot trial [290] | Lexington (KY, USA) | Significant reduction of egg hatching rate; Significant reduction of adult females | Public Bodies University of Kentucky (KY, USA); Kentucky Cabinet for Economic Development; National Institutes of Health (KY, USA) Private Bodies MosquitoMate, Inc. (Lexington, KY, USA) |
| - | 2015–2018 | Ae. albopictus | wAlbA + wAlbB + wPip (HC line [161]) | IIT-SIT combined (at 10–50:1 release ratio) | Large-scale trial [161] | Guangzhou (China) | >94% reduction of the wild population | Public Bodies China: Sun Yat-sen University in Guangzhou; Hunan Normal University; Guangzhou Center for Disease Control and Prevention; Center for Applied Mathematics, College of Mathematics and Information Sciences, Guangzhou University; School of Medicine, Hunan Normal University, Changsha; Nanjing Agricultural University; Guangdong Provincial Center for Disease Control and Prevention; National Natural Science Foundation of China; Chinese Center for Disease Control and Prevention, Beijing; Natural Science Foundation of Hunan Province, Hunan CDC, Hunan Educational Committee, Hunan Province Other countries: Michigan State University (MI, USA); IAEA (Joint FAO/IAEA, Programme of Nuclear Techniques in Food and Agriculture, Vienna International Centre, Austria); University of Melbourne (Australia) Private Bodies Guangzhou Wolbaki Biotech Co. (Guangzhou, China) |
| - | 2016 | Ae. aegypti | wAlbA + wAlbB (ThAB line [153]) | IIT-SIT combined (release ratio not available) | Pilot trial [302] | Plaeng Yao District (Thailand) | 85% reduction of egg hatch rate; 97% reduction of adult females | Public Bodies Thailand: Mahidol University Hua Sam Rong Administrative Authority, Plaeng Yao District Health Office, Plaeng Yao Hospital, Nong Satit School Other countries: International Development Research Centre (IDRC, Canada); International Atomic Energy Agency (IAEA, Austria) |
| - | 2019 | Ae. aegypti | wAlbB (introgression from WB2 line [68]) | IIT-SIT combined (at 10:1 estimated release ratio) within an IVM plan | Large-scale trial | Merida (Mexico) | 76–0–88% reduction of egg hatch rate (depending on the phase of the experiment); 55–61–75% reduction of biting females (depending on the phase of the experiment) | Public Bodies Mexico: Ministry of Health (MoH); Collaborative Unit for Entomological Bioassays (UCBE) and Laboratory of Biological Control (LCB) of Autonomous University of Yucatan (UADY); Fondo Mixto Consejo Nacional de Ciencia y Tecnología; Gobierno del Estado de Yucatán Other countries: University of Michigan (MI, USA); U.S. Agency for International Development (USAID) |
| Wolbachia Singapore | 2016–present | Ae. aegypti | wAlbB | IIT and IIT/SIT combination (release ratio not available) | Operational program [264,293,297] | Singapore | >90% reduction of the wild population after 12 months of sustained intervention; 56–88% reduction of dengue incidence [264,293]; 61% reduction of dengue incidence after 12 months of sustained intervention [297] | Public Bodies Singapore: National Environment Agency (NEA); Singapore Ministry of Finance, Ministry of Sustainability, and the National Environment Agency; Singapore National Robotics Program Private Bodies Verily Life Sciences LLC (South San Francisco, CA, USA); Orinno Technology Pte. Ltd. (Singapore) |
| Debug/Debug Fresno | 2017–2018 | Ae. aegypti | wAlbB (WB1 [68]) | IIT (release ratio not available) | Large-scale trials [220] | Fresno (CA, USA) | 95% reduction of the wild population | Public Bodies University of Kentucky (KY, USA); Consolidated Mosquito Abatement District (CMAD) (CA, USA) Private Bodies Verily Life Sciences LLC (South San Francisco, CA, USA); MosquitoMate (Lexington, KY, USA) |
| 2018 | Ae. aegypti | wAlbB (WB1 [68]) | IIT (release ratio not available) | Large-scale trial [292] | Miami (FL, USA) | Significant reduction of egg hatching rate; 78% reduction of adult females | Public Bodies Florida Department of Health (FL, USA); Mosquito Control Division, Department of Solid Waste Management, Miami-Dade County (FL, USA) Private Bodies MosquitoMate (Lexington, KY, USA), Clarke Mosquito Control Services (St. Charles, IL, USA) | |
| Innisfail Mozzie Program/Debug Innisfail | 2018 | Ae. aegypti | wAlbB (wAlbB2-F4 line) | IIT (5–10:1 release ratio) | Large-scale trial [300,318] | Innisfail (Queensland, Australia) | Significant reduction of larval productivity; >80% reduction of adult females | Public Bodies Australia: University of Queensland; CSIRO; James Cook University; QIMR Berghofer Medical Research Institute; Australian National Health and Medical Research Council Other countries: Michigan State University (MI, USA) Private Bodies Verily Life Sciences LLC (South San Francisco, CA, USA) |
| ARwP | 2018–2019 | Ae. albopictus | wPip (ARwP line [89]) | IIT (0.7–1.1:1 ratio releases) | Pilot trials [288,289] | Rome (Italy) | 15–40% reduction of the egg hatch rate (depending on the year) | Public Bodies National Italian Agency for New Technologies, Energy, and Sustainable Economic Development (ENEA, Italy); Università degli Studi di Roma “La Sapienza” (Italy) Private Bodies BiovecBlok s.r.l. (Camerino, Italy, 2019–2024) |
| 2019 | Ae. aegypti | wAlbB (WB1 line) | IIT (release ratio not available) | Large-scale trial [299] | Houston (TX, USA) | 94% reduction of Ae. aegypti females; Significant increase of Ae. albopictus adults | Public Bodies University of Texas Medical Branch (TX, USA); Mosquito and Vector Control Division of Harris County Public Health (TX, USA); Texas Department of State Health Services (TX, USA) Private Bodies MosquitoMate, Inc. (Lexington, KY, USA) | |
| 2020–2021 | Ae. albopictus | wAlbA + wAlbB + wPip (HC line) | IIT (at 1–7:1 release ratio) | Large-scale trial [80] | Changsha (China) | 97–85% reduction of egg hatch rate (respectively, after once-, or twice-per-week releases); 94% reduction of mosquito biting | Public Bodies China: Hunan Normal University, Central South University; Sun Yat-sen University; Hunan Provincial Center for Disease Control and Prevention; Guangzhou Center for Disease Control and Prevention; Hunan Academy of Agricultural Sciences; National Natural Science Foundation of China; Natural Science Foundation of Hunan Province; Hunan CDC; Hunan Educational Committee Other countries: Michigan State University, (MI, USA) Private Bodies Guangzhou Wolbaki Biotech Co. (Guangzhou, China) | |
| Communities Organized to Prevent Arboviruses (COPA) Wolbachia Project | 2020–present | Ae. aegypti | wAlbB | IIT (release ratio not available) | Large-scale trial [298,301] | Ponce (Puerto Rico) | 49% reduction of wild population [298] | Public Bodies Puerto Rico: Ponce Health Sciences University (Puerto Rico); Puerto Rico Vector Control Unit (Puerto Rico) Other countries: US Centers for Disease Control (GA, USA) Private Bodies Verily Life Sciences LLC (South San Francisco, CA, USA) |
| BugOut Wolbachia | 2022–present | Ae. aegypti | wAlbB | IIT (release ratio not available) | Large-scale trial [319,320] | Virgin Gorda (British Virgin Islands) | Open field releases since 2022, data still not available | Public Bodies Government of Virgin Islands; Ministry of Health and Social Development Private Bodies Verily Life Sciences LLC (South San Francisco, CA, USA); GreenVI (Tortola, BVI) |
| World Mosquito Program—Australia/Dengue Safe Project Ingham/Dengue Out Program | 2011–present | Ae. aegypti | wMel | PRS | Operational program [321] | Cairns, Cassowary Coast, Douglas Shire, Charters Towers, Townsville (Queensland, Australia) | With a few local and often only momentary exceptions, mean Wolbachia (wMel) frequency stably above 80–90% in treated areas; 96% reduction in dengue incidence in Wolbachia-treated populations [85,87,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126]; Locally acquired dengue cases decreased to zero [322] | Public Bodies Australia: Monash University; Queensland Health Government; Queensland Government; Townsville Hospital and Health Service (Townsville HHS); Hinchinbrook Shire Council (HSC); Tropical Public Health Service; Northern Peninsula Area Regional Council; National Health and Medical Research Council of Australia; College of Public Health, Medical and Veterinary Sciences, James Cook University; Bio21 Institute, University of Melbourne Other countries: School of Public Health, University of California, (CA, USA); London School of Hygiene and Tropical Medicine (London, UK) Private Bodies WMP (Melbourne, Victoria, Australia); Bill & Melinda Gates Foundation (Seattle, WA, USA); Wellcome Trust (London, UK); Gillespie Family Foundation (New York, NY, USA); Foundation for the National Institutes of Health (Bethesda, MD, USA) |
| World Mosquito Program—Oceania | 2018–present | Ae. aegypti | wMel/Fij-wMel, Van-wMel, and Kir-wMel | PRS | Operational programs [99,323] | Pacific islands of Oceania (Fiji, Kiribati, Vanuatu, New Caledonia) | Fiji: >80% wMel prevalence in trapped Ae. aegypti in five of six reporting areas [99,324] Kiribati: intermediate wMel prevalence (Eastern Site: 14.3–31.8%, Western Site: 50–100%) [99,320] Vanuatu: wMel established in ten of the twelve reporting areas, with five reporting areas having >95% of Ae. aegypti infected with wMel [99,325] New Caledonia: in Nouméa, Aedes aegypti individuals carrying Wolbachia reached 89%; in Mont-Dore (extended districts), Aedes aegypti individuals carrying Wolbachia reached 70%; in Dumbéa, Aedes aegypti individuals carrying Wolbachia reached 85% [323] | Fiji [324] Public Bodies Ministry for Health and Medical Services (Government of Fiji); Australian Government, Department of Foreign Affairs and Trade (Australia); USAID (USA); New Zealand Foreign Affairs and Trade, Aid Program (New Zealand) Private Bodies WMP (Melbourne, Victoria, Australia); Live and Learn Environmental Education (Melbourne, Victoria, Australia); Rotary Foundation (Evanston, IL, USA) Kiribati [326] Public Bodies Ministry for Health and Medical Services (Government of Kiribati); Australian Government, Department of Foreign Affairs and Trade (Australia) Private Bodies WMP (Melbourne, Victoria, Australia) Vanuatu [325] Public Bodies Ministry of Health (Government of Vanuatu); Australian Government, Department of Foreign Affairs and Trade (Australia) Private Bodies WMP (Melbourne, Victoria, Australia); Vanuatu Red Cross (Port Vila, Vanuatu) New Caledonia [323] Public Bodies Insitute Pasteur de Nouvelle Calédonie; Government de la Novelle-Calédonie; Ville de Nouméa; Ville du Mont-Dore; Ville de Dumbéa; Province Sud; Haut-Commissariat de la République en Nouvelle-Calédonie (France); Fonds Pacifique-Republique Française (France); Health Security Initiative 2017–2022 Private Bodies WMP (Melbourne, Victoria, Australia) |
| World Mosquito Program—Brazil | 2015–present | Ae. aegypti | wMel | PRS | Operational program [196,316,327,328] | Rio de Janeiro, Niterói, Belo Horizonte, Campo Grande, Petrolina (Brazil) | Rio de Janeiro: 25–32% introgression of Wolbachia wMel in the wild population, 38% reduction in dengue incidence, 10% reduction in chikungunya incidence [101]; Niterói: 40–80% introgression of Wolbachia wMel in the wild population, 69.4% reduction in dengue incidence, 56.3% reduction in chikungunya incidence, 37% reduction in Zika incidence [196]; Belo Horizonte, Campo Grande, and Petrolina: ongoing studies, data still unavailable | Public Bodies Brazil: Oswaldo Cruz Foundation (Fiocruz, Brazil), Ministry of Health of Brazil; various Community Reference Groups (see [328]). Other countries: Monash University (Melbourne, Australia); European Research Council Private Bodies WMP (Melbourne, Victoria, Australia); Bill & Melinda Gates Foundation (Seattle, WA, USA) |
| World Mosquito Program—Colombia | 2017–present | Ae. aegypti | wMel (wMel-COL/wMel-COL2) [100]) | PRS | Operational program [100,329] | Bello, Medellin, Itagui | Bello: 81.1–96.6% introgression of Wolbachia wMel in the wild population [100]; About 95% reduction of dengue incidence [312]; Medellín: extremely variable results regarding the percentage of Wolbachia introgression, ranging from 18.4–98.1%, depending on the area and on the period [100]; 9.5–33.2% introgression of Wolbachia wMel in the wild population [330]; About 95% reduction of dengue incidence [312]; Itagui: 63.6–92.3% introgression of Wolbachia wMel in the wild population [100]; About 97% reduction of dengue incidence [312] | Public Bodies Colombia: Universidad de Antioquia; Secretaría de Salud, Medellín Other countries: U.S. Agency for International Development (USAID, USA); UK Department for International Development (UK) Private Bodies WMP (Melbourne, Victoria, Australia); Bill & Melinda Gates Foundation (Seattle, WA, USA); Wellcome Trust (London, UK) |
| World Mosquito Program—Central America | 2019–present | Ae. aegypti | PRS | Large-scale trials [331,332,333] | Central America (Mexico, Honduras, El Salvador) | Mexico: open field releases since 2019, data still not available Honduras: open field releases since 2023, data still not available El Salvador: open field releases since 2024, data still not available | Mexico Public Bodies Secretaria de Salud Gobierno de Baja California Sur (Mexico) Private Bodies WMP (Melbourne, Victoria, Australia), International Community Foundation (ICF, National City, CA, USA); Wellcome Trust (London, UK); Alumbra Innovations Foundation (Bentonville, AR, USA) Honduras Public Bodies Universidad National Autonoma de Honduras; Secretaria de Salud, Gobierno de Honduras Private Bodies WMP (Melbourne, Victoria, Australia); Medecins sans Frontieres (Geneva, Swiss ) El Salvador [333] Public Bodies Gobierno de El Salvador, Ministerio del Salud; PRVCU International (Unidad de Control De Vectores de Puerto Rico, Puerto Rico) Private Bodies WMP (Melbourne, Victoria, Australia) | |
| World Mosquito Program—Vietnam | 2013–present | Ae. aegypti | wMelPop wMel | PRS | Operational program [283] | Vietnam | Failure of wMelPop Wolbachia infection establishment [244]; heterogeneity in wMel Wolbachia infection prevalence [242] | Public Bodies Institute Pasteur Vietnam; Ministry of Health of Vietnam, Action on Poverty; National Institute of Hygiene and Epidemiology of Vietnam Private Bodies WMP (Melbourne, Victoria, Australia) |
| Applying Wolbachia to Eliminate Dengue (AWED)/World Mosquito Program—Indonesia | 2017–2020 | Ae. aegypti | wMel | PRS | Large-scale trials [294] | Yogyakarta, Indonesia | 95.8% Wolbachia introgression in intervention clusters; 77.1% reduction of dengue cases; 86.2% reduction of hospitalizations [294] | Public Bodies Universitas Gadjah Mada, Indonesia Private Bodies WMP (Melbourne, Victoria, Australia), Tahija Foundation (Jakarta, Java, Indonesia) |
| World Mosquito Program—Laos and Sri Lanka | 2021–present | Ae. aegypti | wMel | PRS | Operational program [334,335] | Laos, Sri Lanka | Laos: open field releases since 2022, data still not available Sri Lanka: open field releases since 2021, data still not available | Laos [335] Public Bodies Ministry of Health of Laos Private Bodies WMP (Melbourne, Victoria, Australia), Save the Children (London, UK) Sri Lanka [334] Public Bodies National Dengue Control Unit of Sri Lanka Private Bodies WMP (Melbourne, Victoria, Australia), Australian Aid (Camberra, ACT, Australia) |
| Wolbachia Malaysia | 2017–present | Ae. aegypti | wAlbB (wAlbB.MC line) | PRS | Operational program [141,142,311,336,337] | Malaysia | wAlbB frequency in the wild population at 98% in one year in release sites and reduction in dengue incidence higher than 40.3% [141]; wAlbB frequency in the wild population higher than 80% in release sites [336]; average reduction in dengue fever of 62.4% [311]; 37.69% reduction of dengue incidence in adjacent non-intervention areas [313] | Public Bodies Malaysia: Ministry of Health Malaysia; Institute for Medical Research; Health Department of Federal Territory of Kuala Lumpur & Putrajaya Other countries: 3MRC-University of Glasgow Centre for Virus Research (UK); University of Melbourne (Australia); Telethon Kids Institute, Perth Children’s Hospital, (Australia); Curtin University (Australia); Australian National Health and Medical Research Council Private Bodies Wellcome Trust (London, UK) |
2.6. Cost-Effectiveness of Mosquito Control with a Specific Focus on Wolbachia-Based Disease Control Strategies
2.7. Effects of Climate Change on Mosquito-Borne Diseases and on Wolbachia-Based Control Strategies
3. Discussion
3.1. Perspectives of Implementation of Wolbachia-Based Control Strategies and Possible Issues
3.2. The Importance of Involving Public and Private Partners and Communities
3.3. Development of Models to Enhance Cost-Effectiveness of Wolbachia-Based Control Strategies
3.4. Biotechnological Methods to Support Sustainability of Wolbachia-Based Vector Control
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Control Strategy a | Main Characteristics | Major Pros | Major Cons | Acceptance and Potential Risks | Main Specific Costs b | Stage of Deployment c |
|---|---|---|---|---|---|---|
| SIT [177] | Aimed at population suppression; self-limiting control strategy d | Lack of requirement for a special mosquito strain; in the case of species easily suitable for laboratory rearing, it is possible to directly rear and release the population from the target area | Not all species are suitable for SIT because full male sterility is generally associated with a reduction in their fitness e; sexing is required to avoid the release of biting females [206] | Generally accepted and regulated; no evidence of risks related to mutations induced by irradiation | Costs for irradiation f or for directly managing the radiation source | Open field trials [412,413] |
| IIT [287] | Aimed at population suppression; self-limiting control strategy | Lack of requirement for any prerelease treatment or genetic modification, so no added fitness costs, beyond effects of Wolbachia, and mosquitoes can be reared as normal aside from sexing | Not all species are suitable for Wolbachia infection; sexing is required to avoid the release of biting females and the spread of the new Wolbachia infection; very accurate sexing is required in Uni-CI-based IIT | Not accepted and regulated globally; risks of unintended population replacement which could reduce efficacy of further interventions | Initial costs to produce a Wolbachia-transinfected line; costs for monitoring Wolbachia frequencies in the case of non-perfect sexing methods | Open field trials (see Table 2) |
| SIT/IIT combination [414] | Aimed at population suppression; self-limiting control strategy | Lower risks of unintended population replacement compared to IIT, where contaminant females should be sterile; lower level of irradiation required compared to SIT | See cons related to both SIT and IIT | See both SIT and IIT regarding acceptance and potential risks | See main specific costs for both SIT and IIT | Open field trials (see Table 2) |
| Wolbachia-based PRS [415] | Aimed at population modification; self-sustaining control strategy if a threshold frequency is maintained | Lack of requirement for any prerelease treatment or genetic modification, so no added fitness costs, beyond effects of Wolbachia, and mosquitoes can be reared as normal; sexing is not required | Possibility of Wolbachia depletion or reduction of its effectiveness due to strain-specific constraints associated with climate; initial temporary increase in the number of biting females | Not accepted and regulated globally; risks of irreversible changes to native population and unintended impacts on arbovirus transmission | Initial costs to produce a Wolbachia-transinfected line; costs for monitoring Wolbachia frequencies | Open field trials, area-wide operational programs (see Table 2) |
| Transformation of mosquito vectors with CI-inducing genes from Wolbachia [171] | Aimed at population suppression; self-limiting control strategy | Lack of requirement for any prerelease treatment | Sexing is required to avoid the release of biting females and the spread of CI-inducing genes from Wolbachia; risks of inbreeding depression [192] | Not accepted and regulated globally due to the involvement of GMOs | Initial costs to produce the transgenic line; costs for monitoring persistence of transgenes | Laboratory assays [171,172] |
| Dominant lethal-based suppression (RIDL) [416] | Aimed at population suppression; self-limiting control strategy | Lack of requirement for any sterilization treatment; late-acting mortality | Sexing is required; use of antibiotics is required to switch off lethal genes for rearing; fitness costs; risks of inbreeding depression | Not accepted and regulated globally due to the involvement of GMOs | Initial costs to produce the transgenic line; costs for tetracycline treatments | Open field trials [417,418] |
| Doublesex suppression drive [185] | Aimed at population suppression; self-limiting control strategy | Lack of requirement for any prerelease treatment; strain is self-sexing so sexing is not required; population suppression acts across multiple generations | Use of antibiotics is required to switch off lethal genes for rearing; fitness costs; risks of inbreeding depression | Not accepted and regulated globally due to the involvement of GMOs | Initial costs to produce the transgenic line; costs for tetracycline treatments | Open field trials [185] |
| Transgenic sex ratio distortion [419] | Aimed at population suppression; self-sustaining control strategy | Lack of requirement for any sterilization treatment; the transgene is passed to the progeny that amplifies the effect across generations | Risks of inbreeding depression | Not accepted and regulated globally due to the involvement of GMOs | Initial costs to produce the transgenic line; | Semi-field trials [420,421] |
| CRISPR-based precision-guided SIT (pgSIT) [182] | Aimed at population suppression; self-limiting control strategy | Lack of requirement for any sterilization treatment; RNA-guided dominant knockout of specific genes determining male sterility and female inviability | Males to be released must be obtained by crossing two distinct strains; fitness costs; risks of inbreeding depression | Not accepted and regulated globally due to the involvement of GMOs | Initial costs to produce two transgenic lines; costs for maintaining two distinct transgenic strains | Laboratory assays [183] |
| CRISPR-based gene drive system targeting female reproduction [186] | Aimed at population suppression; self-sustaining control strategy | Lack of requirement for any sterilization treatment; the mutation is passed to the progeny that amplifies the effect across generations | Risks of inbreeding depression | Not accepted and regulated globally due to the involvement of GMOs | Initial costs to produce the transgenic line | Laboratory assays [186] |
| CRISPR-based gene drive system targeting female vector competence [235] | Aimed at population modification; self-sustaining control strategy | Lack of requirement for any sterilization treatment; the mutation is passed to the progeny that amplifies the effect across generations | Risks of inbreeding depression | Not accepted and regulated globally due to the involvement of GMOs | Initial costs to produce the transgenic line | Laboratory assays [235] |
References
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic Arboviral Diseases: Priorities for Research and Public Health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Pinotti, F.; Zanluca, C.; Fonseca, V.; Nakase, T.; Koishi, A.C.; Tscha, M.; Soares, G.; Dorl, G.G.; Marques, A.E.M.L.; et al. Genomic Epidemiology Unveils the Dynamics and Spatial Corridor behind the Yellow Fever Virus Outbreak in Southern Brazil. Sci. Adv. 2023, 9, eadg9204. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Pereira, L.A.; Santiago, G.A.; Fonseca, V.; Mendoza, M.P.G.; De Oliveira, C.; De Moraes, L.; Xavier, J.; Tosta, S.; Fristch, H.; et al. Emergence of Dengue Virus Serotype 2 Cosmopolitan Genotype, Brazil. Emerg. Infect. Dis. 2022, 28, 1725–1727. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.; Alcantara, L.C.J.; Fonseca, V.; Lima, M.; Castro, E.; Fritsch, H.; Oliveira, C.; Guimarães, N.; Adelino, T.; Evaristo, M.; et al. Increased Interregional Virus Exchange and Nucleotide Diversity Outline the Expansion of Chikungunya Virus in Brazil. Nat. Commun. 2023, 14, 4413. [Google Scholar] [CrossRef]
- Iani, F.C.D.M.; Mota Pereira, F.; De Oliveira, E.C.; Nascimento Rodrigues, J.T.; Hoffmann Machado, M.; Fonseca, V.; Ribeiro Adelino, T.E.; Rocha Guimarães, N.; Ribeiro Tomé, L.M.; Astete Gómez, M.K.; et al. Rapid Viral Expansion Beyond the Amazon Basin: Increased Epidemic Activity of Oropouche Virus Across the Americas. MedRxiv 2024. [Google Scholar] [CrossRef]
- Branda, F.; Nakase, T.; Maruotti, A.; Scarpa, F.; Ciccozzi, A.; Romano, C.; Peletto, S.; de Filippis, A.M.B.; Alcantara, L.C.J.; Marcello, A.; et al. Dengue Virus Transmission in Italy: Historical Trends up to 2023 and a Data Repository into the Future. Sci. Data 2024, 11, 1325. [Google Scholar] [CrossRef]
- Huang, Y.-J.S.; Higgs, S.; Vanlandingham, D.L. Emergence and Re-Emergence of Mosquito-Borne Arboviruses. Curr. Opin. Virol. 2019, 34, 104–109. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- World Health Organization. Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 18 October 2024).
- Camill, P. Global Change: An Overview|Learn Science at Scitable. Nat. Educ. Knowl. 2010, 3, 49. [Google Scholar]
- Sutherst, R.W. Global Change and Human Vulnerability to Vector-Borne Diseases. Clin. Microbiol. Rev. 2004, 17, 136–173. [Google Scholar] [CrossRef]
- Semenza, J.C.; Suk, J.E. Vector-Borne Diseases and Climate Change: A European Perspective. FEMS Microbiol. Lett. 2018, 365, fnx244. [Google Scholar] [CrossRef] [PubMed]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of Recent and Future Climate Change on Vector-borne Diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Romanello, M.; McGushin, A.; Di Napoli, C.; Drummond, P.; Hughes, N.; Jamart, L.; Kennard, H.; Lampard, P.; Solano Rodriguez, B.; Arnell, N.; et al. The 2021 Report of the Lancet Countdown on Health and Climate Change: Code Red for a Healthy Future. Lancet 2021, 398, 1619–1662. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.C.; Stanberry, L.R. Climate Change and Vectorborne Diseases. N. Engl. J. Med. 2022, 387, 1969–1978. [Google Scholar] [CrossRef]
- Harvey, J.A.; Tougeron, K.; Gols, R.; Heinen, R.; Abarca, M.; Abram, P.K.; Basset, Y.; Berg, M.; Boggs, C.; Brodeur, J.; et al. Scientists’ Warning on Climate Change and Insects. Ecol. Monogr. 2023, 93, e1553. [Google Scholar] [CrossRef]
- De Souza, W.M.; Weaver, S.C. Effects of Climate Change and Human Activities on Vector-Borne Diseases. Nat. Rev. Microbiol. 2024, 22, 476–491. [Google Scholar] [CrossRef]
- Eritja, R.; Palmer, J.R.B.; Roiz, D.; Sanpera-Calbet, I.; Bartumeus, F. Direct Evidence of Adult Aedes albopictus Dispersal by Car. Sci. Rep. 2017, 7, 14399. [Google Scholar] [CrossRef]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; De Lamballerie, X. Emerging Arboviruses: Why Today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The Emergence of Arthropod-Borne Viral Diseases: A Global Prospective on Dengue, Chikungunya and Zika Fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Cianchi, V.; Torelli, A.; Montomoli, E. Yellow Fever: Origin, Epidemiology, Preventive Strategies and Future Prospects. Vaccines 2022, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Mingione, M.; Branda, F.; Maruotti, A.; Ciccozzi, M.; Mazzoli, S. Monitoring the West Nile Virus Outbreaks in Italy Using Open Access Data. Sci. Data 2023, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Bron, G.M.; Strimbu, K.; Cecilia, H.; Lerch, A.; Moore, S.M.; Tran, Q.; Perkins, T.A.; Ten Bosch, Q.A. Over 100 Years of Rift Valley Fever: A Patchwork of Data on Pathogen Spread and Spillover. Pathogens 2021, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Ketkar, H.; Herman, D.; Wang, P. Genetic Determinants of the Re-Emergence of Arboviral Diseases. Viruses 2019, 11, 150. [Google Scholar] [CrossRef]
- Phillips, M.A.; Burrows, J.N.; Manyando, C.; Van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria. Nat. Rev. Dis. Primers 2017, 3, 17050. [Google Scholar] [CrossRef]
- Famakinde, D. Mosquitoes and the Lymphatic Filarial Parasites: Research Trends and Budding Roadmaps to Future Disease Eradication. TropicalMed 2018, 3, 4. [Google Scholar] [CrossRef]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The Global Economic Burden of Dengue: A Systematic Analysis. Lancet Infect. Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef]
- Koerich, L.B.; Sant’Anna, M.R.V.; Huits, R. Recent Technological Advances and Strategies for Arbovirus Vector Control. Trop. Med. Infect. Dis. 2022, 7, 204. [Google Scholar] [CrossRef]
- Kolimenakis, A.; Heinz, S.; Wilson, M.L.; Winkler, V.; Yakob, L.; Michaelakis, A.; Papachristos, D.; Richardson, C.; Horstick, O. The Role of Urbanisation in the Spread of Aedes Mosquitoes and the Diseases They Transmit—A Systematic Review. PLoS Neglected Trop. Dis. 2021, 15, e0009631. [Google Scholar] [CrossRef]
- Ainsworth, C. Tropical Diseases Move North. Nature 2023. [Google Scholar] [CrossRef]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.-P.; et al. Contemporary Status of Insecticide Resistance in the Major Aedes Vectors of Arboviruses Infecting Humans. PLoS Neglected Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef] [PubMed]
- Branda, F.; Giovanetti, M.; Ceccarelli, G.; Ciccozzi, M.; Scarpa, F. ArboItaly: Leveraging Open Data for Enhanced Arbovirus Surveillance in Italy. Front. Pharmacol. 2024, 15, 1459408. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Dengue. Available online: https://www.ecdc.europa.eu/en/dengue (accessed on 18 October 2024).
- CDC. Areas with Risk of Dengue. Available online: https://www.cdc.gov/dengue/areas-with-risk/index.html (accessed on 18 October 2024).
- Padane, A.; Tegally, H.; Ramphal, Y.; Seyni, N.; Sarr, M.; Diop, M.M.; Diedhiou, C.K.; Mboup, A.; Diouf, N.D.; Souaré, A.; et al. An Emerging Clade of Chikungunya West African Genotype Discovered in Real-Time during 2023 Outbreak in Senegal. MedRxiv 2023. [Google Scholar] [CrossRef]
- Mekonnen, F.; Khan, B.A.; Nibret, E.; Munshea, A.; Tsega, D.; Endalamaw, D.; Tadesse, S.; Yismaw, G.; Lankir, D.; Ali, J.; et al. Introduction of Dengue Virus Serotype 3 in the Afar Region, Ethiopia. Emerg. Microbes Infect. 2024, 13, 2429653. [Google Scholar] [CrossRef]
- Affara, M.; Lagu, H.I.; Achol, E.; Karamagi, R.; Omari, N.; Ochido, G.; Kezakarayagwa, E.; Kabatesi, F.; Nkeshimana, A.; Roba, A.; et al. The East African Community (EAC) Mobile Laboratory Networks in Kenya, Burundi, Tanzania, Rwanda, Uganda, and South Sudan—From Project Implementation to Outbreak Response against Dengue, Ebola, COVID-19, and Epidemic-Prone Diseases. BMC Med. 2021, 19, 160. [Google Scholar] [CrossRef]
- WHO. Dengue—Global Situation. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON518 (accessed on 18 October 2024).
- Giovanetti, M.; Faria, N.R.; Lourenço, J.; Goes De Jesus, J.; Xavier, J.; Claro, I.M.; Kraemer, M.U.G.; Fonseca, V.; Dellicour, S.; Thézé, J.; et al. Genomic and Epidemiological Surveillance of Zika Virus in the Amazon Region. Cell Rep. 2020, 30, 2275–2283.e7. [Google Scholar] [CrossRef]
- OECD. Cost-Benefit Analysis and the Environment: Recent Developments; OECD: Paris, France, 2006; ISBN 978-92-64-01004-8. [Google Scholar]
- Marcos-Marcos, J.; Olry De Labry-Lima, A.; Toro-Cardenas, S.; Lacasaña, M.; Degroote, S.; Ridde, V.; Bermudez-Tamayo, C. Impact, Economic Evaluation, and Sustainability of Integrated Vector Management in Urban Settings to Prevent Vector-Borne Diseases: A Scoping Review. Infect. Dis. Poverty 2018, 7, 83. [Google Scholar] [CrossRef]
- Jones, R.T.; Ant, T.H.; Cameron, M.M.; Logan, J.G. Novel Control Strategies for Mosquito-Borne Diseases. Phil. Trans. R. Soc. B 2021, 376, 20190802. [Google Scholar] [CrossRef]
- Aryaprema, V.S.; Steck, M.R.; Peper, S.T.; Xue, R.; Qualls, W.A. A Systematic Review of Published Literature on Mosquito Control Action Thresholds across the World. PLoS Neglected Trop. Dis. 2023, 17, e0011173. [Google Scholar] [CrossRef]
- Deng, S.-Q.; Cai, Y.; Wang, D.-Q. Editorial: Novel Strategies for Controlling Mosquito-Borne Diseases. Front. Public Health 2023, 11, 1171634. [Google Scholar] [CrossRef]
- Baldacchino, F.; Caputo, B.; Chandre, F.; Drago, A.; Della Torre, A.; Montarsi, F.; Rizzoli, A. Control Methods against Invasive Aedes Mosquitoes in Europe: A Review. Pest Manag. Sci. 2015, 71, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.M. Challenges and Opportunities in Controlling Mosquito-Borne Infections. Nature 2018, 559, 490–497. [Google Scholar] [CrossRef]
- Yakob, L.; Funk, S.; Camacho, A.; Brady, O.; Edmunds, W.J. Aedes aegypti Control Through Modernized, Integrated Vector Management. PLoS Curr. 2017, 9, ecurrents.outbreaks.45deb8e03a438c4d088afb4fafae8747. [Google Scholar] [CrossRef]
- Fouet, C.; Kamdem, C. Integrated Mosquito Management: Is Precision Control a Luxury or Necessity? Trends Parasitol. 2019, 35, 85–95. [Google Scholar] [CrossRef]
- AMCA. Best Management Practices; American Mosquito Control Association: Mount Laurel, NJ, USA, 2021; Available online: https://www.mosquito.org/bmp/ (accessed on 31 January 2025).
- WHO. Data Requirements and Protocol for Determining Comparative Efficacy of Vector Control Products. Available online: https://iris.who.int/handle/10665/376778 (accessed on 18 October 2024).
- World Health Organization. How to Design Vector Control Efficacy Trials: Guidance on Phase III Vector Control Field Trial Design Provided by the Vector Control Advisory Group; World Health Organization: Geneva, Switzerland, 2017; Available online: https://iris.who.int/bitstream/handle/10665/259688/WHO-HTM-NTD-VEM-2017.03-eng.pdf (accessed on 31 January 2025).
- Hosseini, M.-S.; Jahanshahlou, F.; Akbarzadeh, M.A.; Zarei, M.; Vaez-Gharamaleki, Y. Formulating Research Questions for Evidence-Based Studies. J. Med. Surg. Public Health 2024, 2, 100046. [Google Scholar] [CrossRef]
- Ortega, B. Cost-Benefit Analysis. In Encyclopedia of Quality of Life and Well-Being Research; Maggino, F., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–5. ISBN 978-3-319-69909-7. [Google Scholar]
- O’Mahony, T. Cost-Benefit Analysis and the Environment: The Time Horizon Is of the Essence. Environ. Impact Assess. Rev. 2021, 89, 106587. [Google Scholar] [CrossRef]
- Kazmi, A. Sustainability (World Commission on Environment and Development Definition). In Encyclopedia of Sustainable Management; Idowu, S.O., Schmidpeter, R., Capaldi, N., Zu, L., Del Baldo, M., Abreu, R., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 3222–3225. ISBN 978-3-031-25983-8. [Google Scholar]
- Rosen, M.A. Sustainability: Concepts, Definitions, and Applications. In Building Sustainable Cities; Alvarez-Risco, A., Rosen, M.A., Del-Aguila-Arcentales, S., Marinova, D., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 15–26. ISBN 978-3-030-45532-3. [Google Scholar]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of Pesticide Pollution at the Global Scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Ansari, M.S.; Moraiet, M.A.; Ahmad, S. Insecticides: Impact on the Environment and Human Health. In Environmental Deterioration and Human Health; Malik, A., Grohmann, E., Akhtar, R., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2014; pp. 99–123. ISBN 978-94-007-7889-4. [Google Scholar]
- Isbell, F.; Balvanera, P.; Mori, A.S.; He, J.; Bullock, J.M.; Regmi, G.R.; Seabloom, E.W.; Ferrier, S.; Sala, O.E.; Guerrero-Ramírez, N.R.; et al. Expert Perspectives on Global Biodiversity Loss and Its Drivers and Impacts on People. Front. Ecol. Environ. 2023, 21, 94–103. [Google Scholar] [CrossRef]
- Douglas, M.R.; Baisley, P.; Soba, S.; Kammerer, M.; Lonsdorf, E.V.; Grozinger, C.M. Putting Pesticides on the Map for Pollinator Research and Conservation. Sci. Data 2022, 9, 571. [Google Scholar] [CrossRef]
- Kim, D.; Burkett-Cadena, N.D.; Reeves, L.E. Pollinator Biological Traits and Ecological Interactions Mediate the Impacts of Mosquito-Targeting Malathion Application. Sci. Rep. 2022, 12, 17039. [Google Scholar] [CrossRef]
- Lee, N.S.M.; Clements, G.R.; Ting, A.S.Y.; Wong, Z.H.; Yek, S.H. Persistent Mosquito Fogging Can Be Detrimental to Non-Target Invertebrates in an Urban Tropical Forest. PeerJ 2020, 8, e10033. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Shropshire, J.D.; Cross, K.L.; Leigh, B.; Mansueto, A.J.; Stewart, V.; Bordenstein, S.R.; Bordenstein, S.R. Living in the Endosymbiotic World of Wolbachia: A Centennial Review. Cell Host Microbe 2021, 29, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Bourtzis, K.; Dobson, S.L.; Xi, Z.; Rasgon, J.L.; Calvitti, M.; Moreira, L.A.; Bossin, H.C.; Moretti, R.; Baton, L.A.; Hughes, G.L.; et al. Harnessing Mosquito-Wolbachia Symbiosis for Vector and Disease Control. Acta Trop. 2014, 132, S150–S163. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master Manipulators of Invertebrate Biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Porter, J.; Sullivan, W. The Cellular Lives of Wolbachia. Nat. Rev. Microbiol. 2023, 21, 750–766. [Google Scholar] [CrossRef]
- Xi, Z.; Khoo, C.C.H.; Dobson, S.L. Wolbachia Establishment and Invasion in an Aedes aegypti Laboratory Population. Science 2005, 310, 326–328. [Google Scholar] [CrossRef]
- Sinkins, S.P. Wolbachia and Cytoplasmic Incompatibility in Mosquitoes. Insect Biochem. Mol. Biol. 2004, 34, 723–729. [Google Scholar] [CrossRef]
- Hochstrasser, M. Cytoplasmic Incompatibility: A Wolbachia Toxin-Antidote Mechanism Comes into View. Curr. Biol. 2022, 32, R287–R289. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, H.; Wang, H.; Zhang, M.; Chen, X.; Berk, J.M.; Zhang, L.; Wei, Y.; Li, W.; Cui, W.; et al. Structural and Mechanistic Insights into the Complexes Formed by Wolbachia Cytoplasmic Incompatibility Factors. Proc. Natl. Acad. Sci. USA 2021, 118, e2107699118. [Google Scholar] [CrossRef]
- Kaur, R.; McGarry, A.; Shropshire, J.D.; Leigh, B.A.; Bordenstein, S.R. Prophage Proteins Alter Long Noncoding RNA and DNA of Developing Sperm to Induce a Paternal-Effect Lethality. Science 2024, 383, 1111–1117. [Google Scholar] [CrossRef]
- Kaur, R.; Meier, C.J.; McGraw, E.A.; Hillyer, J.F.; Bordenstein, S.R. The Mechanism of Cytoplasmic Incompatibility Is Conserved in Wolbachia-Infected Aedes aegypti Mosquitoes Deployed for Arbovirus Control. PLoS Biol. 2024, 22, e3002573. [Google Scholar] [CrossRef] [PubMed]
- Shropshire, J.D.; Hamant, E.; Cooper, B.S. Male Age and Wolbachia Dynamics: Investigating How Fast and Why Bacterial Densities and Cytoplasmic Incompatibility Strengths Vary. mBio 2021, 12, e0299821. [Google Scholar] [CrossRef] [PubMed]
- Namias, A.; Sicard, M.; Weill, M.; Charlat, S. From Wolbachia Genomics to Phenotype: Molecular Models of Cytoplasmic Incompatibility Must Account for the Multiplicity of Compatibility Types. Curr. Opin. Insect Sci. 2022, 49, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, M.; Landmann, F.; Labbé, P.; Justy, F.; Weill, M.; Sicard, M. The Cellular Phenotype of Cytoplasmic Incompatibility in Culex pipiens in the Light of cidB Diversity. PLoS Pathog. 2018, 14, e1007364. [Google Scholar] [CrossRef]
- Ilinsky, Y.Y.; Zakharov, I.K. Cytoplasmic Incompatibility in Drosophila melanogaster Is Caused by Different Wolbachia Genotypes. Russ. J. Genet. Appl. Res. 2011, 1, 458–462. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Townsend, M.; Paton, C.J.; Callahan, A.G.; Hoffmann, A.A. Application of wMelPop Wolbachia Strain to Crash Local Populations of Aedes aegypti. PLoS Neglected Trop. Dis. 2015, 9, e0003930. [Google Scholar] [CrossRef]
- Ant, T.H.; Sinkins, S.P. A Wolbachia Triple-Strain Infection Generates Self-Incompatibility in Aedes albopictus and Transmission Instability in Aedes aegypti. Parasit. Vectors 2018, 11, 295. [Google Scholar] [CrossRef]
- Zeng, Q.; She, L.; Yuan, H.; Luo, Y.; Wang, R.; Mao, W.; Wang, W.; She, Y.; Wang, C.; Shi, M.; et al. A Standalone Incompatible Insect Technique Enables Mosquito Suppression in the Urban Subtropics. Commun. Biol. 2022, 5, 1419. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Turelli, M. Cytoplasmic Incompatibility in Insects. In Influential Passengers; O’Neill, S.L., Hoffmann, A.A., Werren, J.H., Eds.; Oxford University Press: Oxford, UK, 1997; pp. 42–80. ISBN 978-0-19-857786-7. [Google Scholar]
- Dobson, S.L.; Fox, C.W.; Jiggins, F.M. The Effect of Wolbachia-Induced Cytoplasmic Incompatibility on Host Population Size in Natural and Manipulated Systems. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002, 269, 437–445. [Google Scholar] [CrossRef]
- Hughes, G.L.; Rasgon, J.L. Transinfection: A Method to Investigate Wolbachia–Host Interactions and Control Arthropod-borne Disease. Insect Mol. Biol. 2014, 23, 141–151. [Google Scholar] [CrossRef]
- McMeniman, C.J.; Lane, R.V.; Cass, B.N.; Fong, A.W.C.; Sidhu, M.; Wang, Y.-F.; O’Neill, S.L. Stable Introduction of a Life-Shortening Wolbachia Infection into the Mosquito Aedes aegypti. Science 2009, 323, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia Strain Blocks Dengue and Invades Caged Aedes aegypti Populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef]
- Moretti, R.; Marini, F.; Lampazzi, E.; Calvitti, M. On the Suitability of Aedes Vexans to Wolbachia-based Control Strategies. Entomol. Exp. Appl. 2021, 169, 772–778. [Google Scholar] [CrossRef]
- Ant, T.H.; Herd, C.S.; Geoghegan, V.; Hoffmann, A.A.; Sinkins, S.P. The Wolbachia Strain wAu Provides Highly Efficient Virus Transmission Blocking in Aedes aegypti. PLoS Pathog. 2018, 14, e1006815. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Joshi, D.; Dong, Y.; Lu, P.; Zhou, G.; Pan, X.; Xu, Y.; Dimopoulos, G.; Xi, Z. Wolbachia Invades Anopheles stephensi Populations and Induces Refractoriness to Plasmodium Infection. Science 2013, 340, 748–751. [Google Scholar] [CrossRef]
- Calvitti, M.; Moretti, R.; Lampazzi, E.; Bellini, R.; Dobson, S.L. Characterization of a New Aedes albopictus (Diptera: Culicidae)-Wolbachia pipientis (Rickettsiales: Rickettsiaceae) Symbiotic Association Generated by Artificial Transfer of the wPip Strain from Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2010, 47, 179–187. [Google Scholar] [CrossRef]
- Blagrove, M.S.C.; Arias-Goeta, C.; Failloux, A.-B.; Sinkins, S.P. Wolbachia Strain wMel Induces Cytoplasmic Incompatibility and Blocks Dengue Transmission in Aedes albopictus. Proc. Natl. Acad. Sci. USA 2012, 109, 255–260. [Google Scholar] [CrossRef]
- Suh, E.; Mercer, D.R.; Fu, Y.; Dobson, S.L. Pathogenicity of Life-Shortening Wolbachia in Aedes albopictus after Transfer from Drosophila melanogaster. Appl. Environ. Microbiol. 2009, 75, 7783–7788. [Google Scholar] [CrossRef]
- Ant, T.H.; Herd, C.; Louis, F.; Failloux, A.B.; Sinkins, S.P. Wolbachia Transinfections in Culex quinquefasciatus Generate Cytoplasmic Incompatibility. Insect Mol. Biol. 2020, 29, 1–8. [Google Scholar] [CrossRef]
- Pimentel, A.C.; Cesar, C.S.; Martins, M.; Cogni, R. The Antiviral Effects of the Symbiont Bacteria Wolbachia in Insects. Front. Immunol. 2020, 11, 626329. [Google Scholar] [CrossRef]
- Kean, J.; Rainey, S.M.; McFarlane, M.; Donald, C.L.; Schnettler, E.; Kohl, A.; Pondeville, E. Fighting Arbovirus Transmission: Natural and Engineered Control of Vector Competence in Aedes Mosquitoes. Insects 2015, 6, 236–278. [Google Scholar] [CrossRef] [PubMed]
- Ant, T.H.; Mancini, M.V.; McNamara, C.J.; Rainey, S.M.; Sinkins, S.P. Wolbachia-Virus Interactions and Arbovirus Control through Population Replacement in Mosquitoes. Pathog. Glob. Health 2023, 117, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Rainey, S.M.; Geoghegan, V.; Lefteri, D.A.; Ant, T.H.; Martinez, J.; McNamara, C.J.; Kamel, W.; De Laurent, Z.R.; Castello, A.; Sinkins, S.P. Differences in Proteome Perturbations Caused by the Wolbachia Strain wAu Suggest Multiple Mechanisms of Wolbachia-Mediated Antiviral Activity. Sci. Rep. 2023, 13, 11737. [Google Scholar] [CrossRef] [PubMed]
- Vandana, V.; Dong, S.; Sheth, T.; Sun, Q.; Wen, H.; Maldonado, A.; Xi, Z.; Dimopoulos, G. Wolbachia Infection-Responsive Immune Genes Suppress Plasmodium Falciparum Infection in Anopheles stephensi. PLoS Pathog. 2024, 20, e1012145. [Google Scholar] [CrossRef]
- Gu, X.; Ross, P.A.; Rodriguez-Andres, J.; Robinson, K.L.; Yang, Q.; Lau, M.; Hoffmann, A.A. A wMel Wolbachia Variant in Aedes aegypti from Field-collected Drosophila Melanogaster with Increased Phenotypic Stability under Heat Stress. Environ. Microbiol. 2022, 24, 2119–2135. [Google Scholar] [CrossRef]
- Simmons, C.P.; Donald, W.; Tagavi, L.; Tarivonda, L.; Quai, T.; Tavoa, R.; Noran, T.; Manikaoti, E.; Kareaua, L.; Abwai, T.T.; et al. Successful Introgression of wMel Wolbachia into Aedes aegypti Populations in Fiji, Vanuatu and Kiribati. PLoS Neglected Trop. Dis. 2024, 18, e0012022. [Google Scholar] [CrossRef]
- Velez, I.D.; Uribe, A.; Barajas, J.; Uribe, S.; Ángel, S.; Suaza-Vasco, J.D.; Mejia Torres, M.C.; Arbeláez, M.P.; Santacruz-Sanmartin, E.; Duque, L.; et al. Large-Scale Releases and Establishment of wMel Wolbachia in Aedes aegypti Mosquitoes throughout the Cities of Bello, Medellín and Itagüí, Colombia. PLoS Neglected Trop. Dis. 2023, 17, e0011642. [Google Scholar] [CrossRef]
- Ribeiro Dos Santos, G.; Durovni, B.; Saraceni, V.; Souza Riback, T.I.; Pinto, S.B.; Anders, K.L.; Moreira, L.A.; Salje, H. Estimating the Effect of the wMel Release Programme on the Incidence of Dengue and Chikungunya in Rio de Janeiro, Brazil: A Spatiotemporal Modelling Study. Lancet Infect. Dis. 2022, 22, 1587–1595. [Google Scholar] [CrossRef]
- Maciel-de-Freitas, R.; Sauer, F.G.; Kliemke, K.; Garcia, G.A.; Pavan, M.G.; David, M.R.; Schmidt-Chanasit, J.; Hoffmann, A.; Lühken, R. Wolbachia Strains wMel and wAlbB Differentially Affect Aedes aegypti Traits Related to Fecundity. Microbiol. Spectr. 2024, 12, e0012824. [Google Scholar] [CrossRef]
- Mancini, M.V.; Ant, T.H.; Herd, C.S.; Martinez, J.; Murdochy, S.M.; Gingell, D.D.; Mararo, E.; Johnson, P.C.D.; Sinkins, S.P. High Temperature Cycles Result in Maternal Transmission and Dengue Infection Differences Between Wolbachia Strains in Aedes aegypti. mBio 2021, 12, e0025021. [Google Scholar] [CrossRef]
- Ross, P.A.; Axford, J.K.; Yang, Q.; Staunton, K.M.; Ritchie, S.A.; Richardson, K.M.; Hoffmann, A.A. Heatwaves Cause Fluctuations in wMel Wolbachia Densities and Frequencies in Aedes aegypti. PLoS Neglected Trop. Dis. 2020, 14, e0007958. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, K.; Sadanandane, C.; Panneer, D.; Kumar, A.; Rahi, M.; Dinesh, S.; Vijayakumar, B.; Krishnaraja, M.; Subbarao, S.K.; Jambulingam, P. Sensitivity of wMel and wAlbB Wolbachia Infections in Aedes aegypti Puducherry (Indian) Strains to Heat Stress during Larval Development. Parasit. Vectors 2022, 15, 221. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.N.; Beier, J.C.; Devine, G.J.; Hugo, L.E. Heat Sensitivity of wMel Wolbachia during Aedes aegypti Development. PLoS Neglected Trop. Dis. 2016, 10, e0004873. [Google Scholar] [CrossRef]
- Lau, M.-J.; Ross, P.A.; Hoffmann, A.A. Infertility and Fecundity Loss of Wolbachia-Infected Aedes aegypti Hatched from Quiescent Eggs Is Expected to Alter Invasion Dynamics. PLoS Neglected Trop. Dis. 2021, 15, e0009179. [Google Scholar] [CrossRef]
- Ryan, P.A.; Turley, A.P.; Wilson, G.; Hurst, T.P.; Retzki, K.; Brown-Kenyon, J.; Hodgson, L.; Kenny, N.; Cook, H.; Montgomery, B.L.; et al. Establishment of wMel Wolbachia in Aedes aegypti Mosquitoes and Reduction of Local Dengue Transmission in Cairns and Surrounding Locations in Northern Queensland, Australia. Gates Open Res. 2019, 3, 1547. [Google Scholar] [CrossRef]
- Fraser, J.E.; O’Donnell, T.B.; Duyvestyn, J.M.; O’Neill, S.L.; Simmons, C.P.; Flores, H.A. Novel Phenotype of Wolbachia Strain wPip in Aedes aegypti Challenges Assumptions on Mechanisms of Wolbachia-Mediated Dengue Virus Inhibition. PLoS Pathog. 2020, 16, e1008410. [Google Scholar] [CrossRef]
- Frentiu, F.D.; Zakir, T.; Walker, T.; Popovici, J.; Pyke, A.T.; Van Den Hurk, A.; McGraw, E.A.; O’Neill, S.L. Limited Dengue Virus Replication in Field-Collected Aedes aegypti Mosquitoes Infected with Wolbachia. PLoS Neglected Trop. Dis. 2014, 8, e2688. [Google Scholar] [CrossRef]
- Duong Thi Hue, K.; Da Silva Goncalves, D.; Tran Thuy, V.; Thi Vo, L.; Le Thi, D.; Vu Tuyet, N.; Nguyen Thi, G.; Huynh Thi Xuan, T.; Nguyen Minh, N.; Nguyen Thanh, P.; et al. Wolbachia wMel Strain-Mediated Effects on Dengue Virus Vertical Transmission from Aedes aegypti to Their Offspring. Parasit. Vectors 2023, 16, 308. [Google Scholar] [CrossRef]
- Ye, Y.H.; Carrasco, A.M.; Frentiu, F.D.; Chenoweth, S.F.; Beebe, N.W.; Van Den Hurk, A.F.; Simmons, C.P.; O’Neill, S.L.; McGraw, E.A. Wolbachia Reduces the Transmission Potential of Dengue-Infected Aedes aegypti. PLoS Neglected Trop. Dis. 2015, 9, e0003894. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Hue Kien, D.T.; Clapham, H.; Aguas, R.; Trung, V.T.; Bich Chau, T.N.; Popovici, J.; Ryan, P.A.; O’Neill, S.L.; McGraw, E.A.; et al. Modeling the Impact on Virus Transmission of Wolbachia-Mediated Blocking of Dengue Virus Infection of Aedes aegypti. Sci. Transl. Med. 2015, 7, 279ra37. [Google Scholar] [CrossRef]
- Indriani, C.; Tantowijoyo, W.; Rancès, E.; Andari, B.; Prabowo, E.; Yusdi, D.; Ansari, M.R.; Wardana, D.S.; Supriyati, E.; Nurhayati, I.; et al. Reduced Dengue Incidence Following Deployments of Wolbachia-Infected Aedes aegypti in Yogyakarta, Indonesia: A Quasi-Experimental Trial Using Controlled Interrupted Time Series Analysis. Gates Open Res. 2020, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.A.; Allen, S.L.; Ohm, J.R.; Sigle, L.T.; Sebastian, A.; Albert, I.; Chenoweth, S.F.; McGraw, E.A. Selection on Aedes aegypti Alters Wolbachia-Mediated Dengue Virus Blocking and Fitness. Nat. Microbiol. 2019, 4, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Joubert, D.A.; Walker, T.; Carrington, L.B.; De Bruyne, J.T.; Kien, D.H.T.; Hoang, N.L.T.; Chau, N.V.V.; Iturbe-Ormaetxe, I.; Simmons, C.P.; O’Neill, S.L. Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog. 2016, 12, e1005434. [Google Scholar] [CrossRef]
- Flores, H.A.; Taneja De Bruyne, J.; O’Donnell, T.B.; Tuyet Nhu, V.; Thi Giang, N.; Thi Xuan Trang, H.; Thi Thuy Van, H.; Thi Long, V.; Thi Dui, L.; Le Anh Huy, H.; et al. Multiple Wolbachia Strains Provide Comparative Levels of Protection against Dengue Virus Infection in Aedes aegypti. PLoS Pathog. 2020, 16, e1008433. [Google Scholar] [CrossRef]
- Terradas, G.; Allen, S.L.; Chenoweth, S.F.; McGraw, E.A. Family Level Variation in Wolbachia-Mediated Dengue Virus Blocking in Aedes aegypti. Parasit. Vectors 2017, 10, 622. [Google Scholar] [CrossRef]
- Amuzu, H.E.; Simmons, C.P.; McGraw, E.A. Effect of Repeat Human Blood Feeding on Wolbachia Density and Dengue Virus Infection in Aedes aegypti. Parasit. Vectors 2015, 8, 246. [Google Scholar] [CrossRef]
- Carrington, L.B.; Tran, B.C.N.; Le, N.T.H.; Luong, T.T.H.; Nguyen, T.T.; Nguyen, P.T.; Nguyen, C.V.V.; Nguyen, H.T.C.; Vu, T.T.; Vo, L.T.; et al. Field- and Clinically Derived Estimates of Wolbachia-Mediated Blocking of Dengue Virus Transmission Potential in Aedes aegypti Mosquitoes. Proc. Natl. Acad. Sci. USA 2018, 115, 361–366. [Google Scholar] [CrossRef]
- Amuzu, H.E.; McGraw, E.A. Wolbachia-Based Dengue Virus Inhibition Is Not Tissue-Specific in Aedes aegypti. PLoS Neglected Trop. Dis. 2016, 10, e0005145. [Google Scholar] [CrossRef]
- Pacidônio, E.C.; Caragata, E.P.; Alves, D.M.; Marques, J.T.; Moreira, L.A. The Impact of Wolbachia Infection on the Rate of Vertical Transmission of Dengue Virus in Brazilian Aedes aegypti. Parasit. Vectors 2017, 10, 296. [Google Scholar] [CrossRef]
- Audsley, M.D.; Ye, Y.H.; McGraw, E.A. The Microbiome Composition of Aedes aegypti Is Not Critical for Wolbachia-Mediated Inhibition of Dengue Virus. PLoS Neglected Trop. Dis. 2017, 11, e0005426. [Google Scholar] [CrossRef]
- Kho, E.A.; Hugo, L.E.; Lu, G.; Smith, D.D.; Kay, B.H. Effects of Larval Nutrition on Wolbachia-Based Dengue Virus Interference in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2016, 53, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.H.; Carrasco, A.M.; Dong, Y.; Sgrò, C.M.; McGraw, E.A. The Effect of Temperature on Wolbachia-Mediated Dengue Virus Blocking in Aedes aegypti. Am. Soc. Trop. Med. Hyg. 2016, 94, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Caragata, E.P.; Rocha, M.N.; Pereira, T.N.; Mansur, S.B.; Dutra, H.L.C.; Moreira, L.A. Pathogen Blocking in Wolbachia-Infected Aedes aegypti Is Not Affected by Zika and Dengue Virus Co-Infection. PLoS Neglected Trop. Dis. 2019, 13, e0007443. [Google Scholar] [CrossRef]
- Aliota, M.T.; Peinado, S.A.; Velez, I.D.; Osorio, J.E. The wMel Strain of Wolbachia Reduces Transmission of Zika Virus by Aedes aegypti. Sci. Rep. 2016, 6, 28792. [Google Scholar] [CrossRef]
- Caragata, E.P.; Dutra, H.L.C.; Moreira, L.A. Inhibition of Zika Virus by Wolbachia in Aedes aegypti. Microb. Cell 2016, 3, 293–295. [Google Scholar] [CrossRef]
- Dutra, H.L.C.; Rocha, M.N.; Dias, F.B.S.; Mansur, S.B.; Caragata, E.P.; Moreira, L.A. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe 2016, 19, 771–774. [Google Scholar] [CrossRef]
- Tan, C.H.; Wong, P.J.; Li, M.I.; Yang, H.; Ng, L.C.; O’Neill, S.L. wMel Limits Zika and Chikungunya Virus Infection in a Singapore Wolbachia-Introgressed Ae. Aegypti Strain, wMel-Sg. PLoS Neglected Trop. Dis. 2017, 11, e0005496. [Google Scholar] [CrossRef]
- Van Den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.D.; McElroy, K.; Day, A.; Higgs, S.; O’Neill, S.L. Impact of Wolbachia on Infection with Chikungunya and Yellow Fever Viruses in the Mosquito Vector Aedes aegypti. PLoS Neglected Trop. Dis. 2012, 6, e1892. [Google Scholar] [CrossRef]
- Aliota, M.T.; Walker, E.C.; Uribe Yepes, A.; Velez, I.D.; Christensen, B.M.; Osorio, J.E. The wMel Strain of Wolbachia Reduces Transmission of Chikungunya Virus in Aedes aegypti. PLoS Neglected Trop. Dis. 2016, 10, e0004677. [Google Scholar] [CrossRef]
- Rocha, M.N.; Duarte, M.M.; Mansur, S.B.; Silva, B.D.M.E.; Pereira, T.N.; Adelino, T.É.R.; Giovanetti, M.; Alcantara, L.C.J.; Santos, F.M.; Costa, V.R.d.M.; et al. Pluripotency of Wolbachia against Arboviruses: The Case of Yellow Fever. Gates Open Res. 2019, 3, 161. [Google Scholar] [CrossRef]
- Pereira, T.N.; Rocha, M.N.; Sucupira, P.H.F.; Carvalho, F.D.; Moreira, L.A. Wolbachia Significantly Impacts the Vector Competence of Aedes aegypti for Mayaro Virus. Sci. Rep. 2018, 8, 6889. [Google Scholar] [CrossRef] [PubMed]
- Sucupira, P.H.F.; Ferreira, Á.G.A.; Leite, T.H.J.F.; De Mendonça, S.F.; Ferreira, F.V.; Rezende, F.O.; Marques, J.T.; Moreira, L.A. The RNAi Pathway Is Important to Control Mayaro Virus Infection in Aedes aegypti but Not for Wolbachia-Mediated Protection. Viruses 2020, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Gu, X.; Robinson, K.L.; Yang, Q.; Cottingham, E.; Zhang, Y.; Yeap, H.L.; Xu, X.; Endersby-Harshman, N.M.; Hoffmann, A.A. A wAlbB Wolbachia Transinfection Displays Stable Phenotypic Effects across Divergent Aedes aegypti Mosquito Backgrounds. Appl. Environ. Microbiol. 2021, 87, e01264-21. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; Torres-Monzon, J.A.; Koskinioti, P.; Dilrukshi Wijegunawardana, N.D.A.; Liang, X.; Pillwax, G.; Xi, Z.; Bourtzis, K. Aedes aegypti Lines for Combined Sterile Insect Technique and Incompatible Insect Technique Applications: The Importance of Host Genomic Background. Entomol. Exp. Appl. 2020, 168, 560–572. [Google Scholar] [CrossRef]
- Martín-Park, A.; Che-Mendoza, A.; Contreras-Perera, Y.; Pérez-Carrillo, S.; Puerta-Guardo, H.; Villegas-Chim, J.; Guillermo-May, G.; Medina-Barreiro, A.; Delfín-González, H.; Méndez-Vales, R.; et al. Pilot Trial Using Mass Field-Releases of Sterile Males Produced with the Incompatible and Sterile Insect Techniques as Part of Integrated Aedes aegypti Control in Mexico. PLoS Neglected Trop. Dis. 2022, 16, e0010324. [Google Scholar] [CrossRef]
- Liang, X.; Tan, C.H.; Sun, Q.; Zhang, M.; Wong, P.S.J.; Li, M.I.; Mak, K.W.; Martín-Park, A.; Contreras-Perera, Y.; Puerta-Guardo, H.; et al. Wolbachia wAlbB Remains Stable in Aedes aegypti over 15 Years but Exhibits Genetic Background-Dependent Variation in Virus Blocking. PNAS Nexus 2022, 1, pgac203. [Google Scholar] [CrossRef]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The Endosymbiotic Bacterium Wolbachia Induces Resistance to Dengue Virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef]
- Nazni, W.A.; Hoffmann, A.A.; NoorAfizah, A.; Cheong, Y.L.; Mancini, M.V.; Golding, N.; Kamarul, G.M.R.; Arif, M.A.K.; Thohir, H.; NurSyamimi, H.; et al. Establishment of Wolbachia Strain wAlbB in Malaysian Populations of Aedes aegypti for Dengue Control. Curr. Biol. 2019, 29, 4241–4248.e5. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Mancini, M.-V.; Ant, T.H.; Martinez, J.; Kamarul, G.M.R.; Nazni, W.A.; Hoffmann, A.A.; Sinkins, S.P. Wolbachia Strain wAlbB Maintains High Density and Dengue Inhibition Following Introduction into a Field Population of Aedes aegypti. Phil. Trans. R. Soc. B 2021, 376, 20190809. [Google Scholar] [CrossRef]
- Joubert, D.A.; O’Neill, S.L. Comparison of Stable and Transient Wolbachia Infection Models in Aedes aegypti to Block Dengue and West Nile Viruses. PLoS Neglected Trop. Dis. 2017, 11, e0005275. [Google Scholar] [CrossRef]
- Chouin-Carneiro, T.; Ant, T.H.; Herd, C.; Louis, F.; Failloux, A.B.; Sinkins, S.P. Wolbachia Strain wAlbA Blocks Zika Virus Transmission in Aedes aegypti. Med. Vet. Entomol. 2020, 34, 116–119. [Google Scholar] [CrossRef]
- McMeniman, C.J.; O’Neill, S.L. A Virulent Wolbachia Infection Decreases the Viability of the Dengue Vector Aedes aegypti during Periods of Embryonic Quiescence. PLoS Neglected Trop. Dis. 2010, 4, e748. [Google Scholar] [CrossRef]
- Yeap, H.L.; Mee, P.; Walker, T.; Weeks, A.R.; O’Neill, S.L.; Johnson, P.; Ritchie, S.A.; Richardson, K.M.; Doig, C.; Endersby, N.M.; et al. Dynamics of the “Popcorn” Wolbachia Infection in Outbred Aedes aegypti Informs Prospects for Mosquito Vector Control. Genetics 2011, 187, 583–595. [Google Scholar] [CrossRef]
- Ross, P.A.; Endersby, N.M.; Yeap, H.L.; Hoffmann, A.A. Larval Competition Extends Developmental Time and Decreases Adult Size of wMelPop Wolbachia-Infected Aedes aegypti. Am. Soc. Trop. Med. Hyg. 2014, 91, 198–205. [Google Scholar] [CrossRef]
- Turley, A.P.; Moreira, L.A.; O’Neill, S.L.; McGraw, E.A. Wolbachia Infection Reduces Blood-Feeding Success in the Dengue Fever Mosquito, Aedes aegypti. PLoS Neglected Trop. Dis. 2009, 3, e516. [Google Scholar] [CrossRef]
- Ross, P.A.; Wiwatanaratanabutr, I.; Axford, J.K.; White, V.L.; Endersby-Harshman, N.M.; Hoffmann, A.A. Wolbachia Infections in Aedes aegypti Differ Markedly in Their Response to Cyclical Heat Stress. PLoS Pathog. 2017, 13, e1006006. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Geoghegan, V.; Stainton, K.; Rainey, S.M.; Ant, T.H.; Dowle, A.A.; Larson, T.; Hester, S.; Charles, P.D.; Thomas, B.; Sinkins, S.P. Perturbed Cholesterol and Vesicular Trafficking Associated with Dengue Blocking in Wolbachia-Infected Aedes aegypti Cells. Nat. Commun. 2017, 8, 526. [Google Scholar] [CrossRef]
- Fraser, J.E.; De Bruyne, J.T.; Iturbe-Ormaetxe, I.; Stepnell, J.; Burns, R.L.; Flores, H.A.; O’Neill, S.L. Novel Wolbachia-Transinfected Aedes aegypti Mosquitoes Possess Diverse Fitness and Vector Competence Phenotypes. PLoS Pathog. 2017, 13, e1006751. [Google Scholar] [CrossRef]
- Ruang-Areerate, T.; Kittayapong, P. Wolbachia Transinfection in Aedes aegypti: A Potential Gene Driver of Dengue Vectors. Proc. Natl. Acad. Sci. USA 2006, 103, 12534–12539. [Google Scholar] [CrossRef]
- Scussel, S.; Gaudillat, B.; Esnault, J.; Lejarre, Q.; Duployer, M.; Lebon, C.; Benlali, A.; Mavingui, P.; Tortosa, P.; Cattel, J. Combining Transinfected Wolbachia and a Genetic Sexing Strain to Control Aedes albopictus in Laboratory-Controlled Conditions. Proc. Biol. Sci. 2024, 291, 20240429. [Google Scholar] [CrossRef]
- Moretti, R.; Yen, P.-S.; Houé, V.; Lampazzi, E.; Desiderio, A.; Failloux, A.-B.; Calvitti, M. Combining Wolbachia-Induced Sterility and Virus Protection to Fight Aedes albopictus-Borne Viruses. PLoS Neglected Trop. Dis. 2018, 12, e0006626. [Google Scholar] [CrossRef]
- Blagrove, M.S.C.; Arias-Goeta, C.; Di Genua, C.; Failloux, A.-B.; Sinkins, S.P. A Wolbachia wMel Transinfection in Aedes albopictus Is Not Detrimental to Host Fitness and Inhibits Chikungunya Virus. PLoS Neglected Trop. Dis. 2013, 7, e2152. [Google Scholar] [CrossRef]
- Xi, Z.; Khoo, C.C.H.; Dobson, S.L. Interspecific Transfer of Wolbachia into the Mosquito Disease Vector Aedes albopictus. Proc. R. Soc. B. 2006, 273, 1317–1322. [Google Scholar] [CrossRef]
- Andrews, E.S.; Fu, Y.; Calvitti, M.; Dobson, S.L. Interspecific Transfer of a Wolbachia Infection Into Aedes albopictus (Diptera: Culicidae) Yields a Novel Phenotype Capable of Rescuing a Superinfection. J. Med. Entomol. 2014, 51, 1192–1198. [Google Scholar] [CrossRef]
- Mancini, M.V.; Herd, C.S.; Ant, T.H.; Murdochy, S.M.; Sinkins, S.P. Wolbachia Strain wAu Efficiently Blocks Arbovirus Transmission in Aedes albopictus. PLoS Neglected Trop. Dis. 2020, 14, e0007926. [Google Scholar] [CrossRef]
- Fu, Y.; Gavotte, L.; Mercer, D.R.; Dobson, S.L. Artificial Triple Wolbachia Infection in Aedes albopictus Yields a New Pattern of Unidirectional Cytoplasmic Incompatibility. Appl. Environ. Microbiol. 2010, 76, 5887–5891. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and Sterile Insect Techniques Combined Eliminate Mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef]
- Suh, E.; Fu, Y.; Mercer, D.R.; Dobson, S.L. Interaction of Wolbachia and Bloodmeal Type in Artificially Infected Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2016, 53, 1156–1162. [Google Scholar] [CrossRef]
- Brelsfoard, C.L.; Séchan, Y.; Dobson, S.L. Interspecific Hybridization Yields Strategy for South Pacific Filariasis Vector Elimination. PLoS Neglected Trop. Dis. 2008, 2, e129. [Google Scholar] [CrossRef]
- Bian, G.; Zhou, G.; Lu, P.; Xi, Z. Replacing a Native Wolbachia with a Novel Strain Results in an Increase in Endosymbiont Load and Resistance to Dengue Virus in a Mosquito Vector. PLoS Neglected Trop. Dis. 2013, 7, e2250. [Google Scholar] [CrossRef]
- Chambers, E.W.; Hapairai, L.; Peel, B.A.; Bossin, H.; Dobson, S.L. Male Mating Competitiveness of a Wolbachia-Introgressed Aedes polynesiensis Strain under Semi-Field Conditions. PLoS Neglected Trop. Dis. 2011, 5, e1271. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.S.; Crain, P.R.; Fu, Y.; Howe, D.K.; Dobson, S.L. Reactive Oxygen Species Production and Brugia Pahangi Survivorship in Aedes polynesiensis with Artificial Wolbachia Infection Types. PLoS Pathog. 2012, 8, e1003075. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Seidl, C.M.; Ipsaro, I.J.; Garrison, C.E.; Fabbri, G.; Howell, P.I.; McGowan, A.G.; White, B.J.; Mitchell, S.N. Transinfection of Wolbachia wAlbB into Culex quinquefasciatus Mosquitoes Does Not Alter Vector Competence for Hawaiian Avian Malaria (Plasmodium relictum GRW4). PLoS Pathog. 2024, 20, e1012052. [Google Scholar] [CrossRef]
- Yen, P.-S.; Failloux, A.-B. A Review: Wolbachia-Based Population Replacement for Mosquito Control Shares Common Points with Genetically Modified Control Approaches. Pathogens 2020, 9, 404. [Google Scholar] [CrossRef]
- Turelli, M.; Katznelson, A.; Ginsberg, P.S. Why Wolbachia-Induced Cytoplasmic Incompatibility Is so Common. Proc. Natl. Acad. Sci. USA 2022, 119, e2211637119. [Google Scholar] [CrossRef]
- Garcia, G.D.A.; Sylvestre, G.; Aguiar, R.; da Costa, G.B.; Martins, A.J.; Lima, J.B.P.; Petersen, M.T.; Lourenço-de-Oliveira, R.; Shadbolt, M.F.; Rašić, G.; et al. Matching the Genetics of Released and Local Aedes aegypti Populations Is Critical to Assure Wolbachia Invasion. PLoS Neglected Trop. Dis. 2019, 13, e0007023. [Google Scholar] [CrossRef]
- Adams, K.L.; Abernathy, D.G.; Willett, B.C.; Selland, E.K.; Itoe, M.A.; Catteruccia, F. Wolbachia cifB Induces Cytoplasmic Incompatibility in the Malaria Mosquito Vector. Nat. Microbiol. 2021, 6, 1575–1582. [Google Scholar] [CrossRef]
- McNamara, C.J.; Ant, T.H.; Harvey-Samuel, T.; White-Cooper, H.; Martinez, J.; Alphey, L.; Sinkins, S.P. Transgenic Expression of Cif Genes from Wolbachia Strain wAlbB Recapitulates Cytoplasmic Incompatibility in Aedes aegypti. Nat. Commun. 2024, 15, 869. [Google Scholar] [CrossRef]
- Alphey, L.; Benedict, M.; Bellini, R.; Clark, G.G.; Dame, D.A.; Service, M.W.; Dobson, S.L. Sterile-Insect Methods for Control of Mosquito-Borne Diseases: An Analysis. Vector-Borne Zoonotic Dis. 2010, 10, 295–311. [Google Scholar] [CrossRef]
- Wang, G.-H.; Gamez, S.; Raban, R.R.; Marshall, J.M.; Alphey, L.; Li, M.; Rasgon, J.L.; Akbari, O.S. Combating Mosquito-Borne Diseases Using Genetic Control Technologies. Nat. Commun. 2021, 12, 4388. [Google Scholar] [CrossRef]
- Caragata, E.P.; Dutra, H.L.C.; Sucupira, P.H.F.; Ferreira, A.G.A.; Moreira, L.A. Wolbachia as Translational Science: Controlling Mosquito-Borne Pathogens. Trends Parasitol. 2021, 37, 1050–1067. [Google Scholar] [CrossRef] [PubMed]
- Alphey, L. Sex or Poison? Genetic Pest Management in the 21st Century. BMC Biol. 2023, 21, 289. [Google Scholar] [CrossRef]
- Knipling, E.F. Possibilities of Insect Control or Eradication Through the Use of Sexually Sterile Males. J. Econ. Entomol. 1955, 48, 459–462. [Google Scholar] [CrossRef]
- Oliva, C.F.; Benedict, M.Q.; Collins, C.M.; Baldet, T.; Bellini, R.; Bossin, H.; Bouyer, J.; Corbel, V.; Facchinelli, L.; Fouque, F.; et al. Sterile Insect Technique (SIT) against Aedes Species Mosquitoes: A Roadmap and Good Practice Framework for Designing, Implementing and Evaluating Pilot Field Trials. Insects 2021, 12, 191. [Google Scholar] [CrossRef]
- Helinski, M.E.; Parker, A.G.; Knols, B.G. Radiation Biology of Mosquitoes. Malar. J. 2009, 8, S6. [Google Scholar] [CrossRef]
- Bourtzis, K.; Vreysen, M.J.B. Sterile Insect Technique (SIT) and Its Applications. Insects 2021, 12, 638. [Google Scholar] [CrossRef]
- Smidler, A.L.; Marrogi, E.; Kauffman, J.; Paton, D.G.; Westervelt, K.A.; Church, G.M.; Esvelt, K.M.; Shaw, W.R.; Catteruccia, F. CRISPR-Mediated Germline Mutagenesis for Genetic Sterilization of Anopheles Gambiae Males. Sci. Rep. 2024, 14, 4057. [Google Scholar] [CrossRef]
- Kandul, N.P.; Liu, J.; Sanchez, C.H.M.; Wu, S.L.; Marshall, J.M.; Akbari, O.S. Transforming Insect Population Control with Precision Guided Sterile Males with Demonstration in Flies. Nat. Commun. 2019, 10, 84. [Google Scholar] [CrossRef]
- Li, M.; Yang, T.; Bui, M.; Gamez, S.; Wise, T.; Kandul, N.P.; Liu, J.; Alcantara, L.; Lee, H.; Edula, J.R.; et al. Suppressing Mosquito Populations with Precision Guided Sterile Males. Nat. Commun. 2021, 12, 5374. [Google Scholar] [CrossRef]
- Phuc, H.K.; Andreasen, M.H.; Burton, R.S.; Vass, C.; Epton, M.J.; Pape, G.; Fu, G.; Condon, K.C.; Scaife, S.; Donnelly, C.A.; et al. Late-Acting Dominant Lethal Genetic Systems and Mosquito Control. BMC Biol. 2007, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Spinner, S.A.M.; Barnes, Z.H.; Puinean, A.M.; Gray, P.; Dafa’alla, T.; Phillips, C.E.; Nascimento de Souza, C.; Frazon, T.F.; Ercit, K.; Collado, A.; et al. New Self-Sexing Aedes aegypti Strain Eliminates Barriers to Scalable and Sustainable Vector Control for Governments and Communities in Dengue-Prone Environments. Front. Bioeng. Biotechnol. 2022, 10, 975786. [Google Scholar] [CrossRef] [PubMed]
- Hammond, A.; Galizi, R.; Kyrou, K.; Simoni, A.; Siniscalchi, C.; Katsanos, D.; Gribble, M.; Baker, D.; Marois, E.; Russell, S.; et al. A CRISPR-Cas9 Gene Drive System Targeting Female Reproduction in the Malaria Mosquito Vector Anopheles gambiae. Nat. Biotechnol. 2016, 34, 78–83. [Google Scholar] [CrossRef]
- Brady, O.J.; Godfray, H.C.J.; Tatem, A.J.; Gething, P.W.; Cohen, J.M.; McKenzie, F.E.; Perkins, T.A.; Reiner, R.C.; Tusting, L.S.; Sinka, M.E.; et al. Vectorial Capacity and Vector Control: Reconsidering Sensitivity to Parameters for Malaria Elimination. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 107–117. [Google Scholar] [CrossRef]
- Zardini, A.; Menegale, F.; Gobbi, A.; Manica, M.; Guzzetta, G.; d’Andrea, V.; Marziano, V.; Trentini, F.; Montarsi, F.; Caputo, B.; et al. Estimating the Potential Risk of Transmission of Arboviruses in the Americas and Europe: A Modelling Study. Lancet Planet Health 2024, 8, e30–e40. [Google Scholar] [CrossRef]
- Focks, D.A.; Brenner, R.J.; Hayes, J.; Daniels, E. Transmission Thresholds for Dengue in Terms of Aedes aegypti Pupae per Person with Discussion of Their Utility in Source Reduction Efforts. Am. J. Trop. Med. Hyg. 2000, 62, 11–18. [Google Scholar] [CrossRef]
- Gómez-Vargas, W.; Ríos-Tapias, P.A.; Marin-Velásquez, K.; Giraldo-Gallo, E.; Segura-Cardona, A.; Arboleda, M. Density of Aedes aegypti and Dengue Virus Transmission Risk in Two Municipalities of Northwestern Antioquia, Colombia. PLoS ONE 2024, 19, e0295317. [Google Scholar] [CrossRef]
- Benedict, M.Q.; Knols, B.G.; Bossin, H.C.; Howell, P.I.; Mialhe, E.; Caceres, C.; Robinson, A.S. Colonisation and Mass Rearing: Learning from Others. Malar. J. 2009, 8, S4. [Google Scholar] [CrossRef]
- Ross, P.A.; Endersby-Harshman, N.M.; Hoffmann, A.A. A Comprehensive Assessment of Inbreeding and Laboratory Adaptation in Aedes aegypti Mosquitoes. Evol. Appl. 2019, 12, 572–586. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Addison, M.F.; Terblanche, J.S. Mass-Rearing of Insects for Pest Management: Challenges, Synergies and Advances from Evolutionary Physiology. Crop Prot. 2012, 38, 87–94. [Google Scholar] [CrossRef]
- Liu, N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Contreras, B.; Adelman, Z.N.; Chae, K. Evaluating the Mating Competency of Genetically Modified Male Mosquitoes in Laboratory Conditions. Front. Trop. Dis. 2023, 4, 1106671. [Google Scholar] [CrossRef]
- Pinto, S.B.; Riback, T.I.S.; Sylvestre, G.; Costa, G.; Peixoto, J.; Dias, F.B.S.; Tanamas, S.K.; Simmons, C.P.; Dufault, S.M.; Ryan, P.A.; et al. Effectiveness of Wolbachia-Infected Mosquito Deployments in Reducing the Incidence of Dengue and Other Aedes-Borne Diseases in Niterói, Brazil: A Quasi-Experimental Study. PLoS Neglected Trop. Dis. 2021, 15, e0009556. [Google Scholar] [CrossRef]
- Gomes, F.M.; Barillas-Mury, C. Infection of Anopheline Mosquitoes with Wolbachia: Implications for Malaria Control. PLoS Pathog. 2018, 14, e1007333. [Google Scholar] [CrossRef]
- Axford, J.K.; Ross, P.A.; Yeap, H.L.; Callahan, A.G.; Hoffmann, A.A. Fitness of wAlbB Wolbachia Infection in Aedes aegypti: Parameter Estimates in an Outcrossed Background and Potential for Population Invasion. Am. Soc. Trop. Med. Hyg. 2016, 94, 507–516. [Google Scholar] [CrossRef]
- Farnesi, L.C.; Belinato, T.A.; Gesto, J.S.M.; Martins, A.J.; Bruno, R.V.; Moreira, L.A. Embryonic Development and Egg Viability of wMel-Infected Aedes aegypti. Parasit. Vectors 2019, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Turley, A.P.; Zalucki, M.P.; O’Neill, S.L.; McGraw, E.A. Transinfected Wolbachia Have Minimal Effects on Male Reproductive Success in Aedes aegypti. Parasit. Vectors 2013, 6, 36. [Google Scholar] [CrossRef]
- Cholvi, M.; Trelis, M.; Bueno-Marí, R.; Khoubbane, M.; Gil, R.; Marcilla, A.; Moretti, R. Wolbachia Infection through Hybridization to Enhance an Incompatible Insect Technique-Based Suppression of Aedes albopictus in Eastern Spain. Insects 2024, 15, 206. [Google Scholar] [CrossRef]
- Calvitti, M.; Marini, F.; Desiderio, A.; Puggioli, A.; Moretti, R. Wolbachia Density and Cytoplasmic Incompatibility in Aedes albopictus: Concerns with Using Artificial Wolbachia Infection as a Vector Suppression Tool. PLoS ONE 2015, 10, e0121813. [Google Scholar] [CrossRef]
- Stone, C.M. Transient Population Dynamics of Mosquitoes during Sterile Male Releases: Modelling Mating Behaviour and Perturbations of Life History Parameters. PLoS ONE 2013, 8, e76228. [Google Scholar] [CrossRef]
- White, S.M.; Rohani, P.; Sait, S.M. Modelling Pulsed Releases for Sterile Insect Techniques: Fitness Costs of Sterile and Transgenic Males and the Effects on Mosquito Dynamics. J. Appl. Ecol. 2010, 47, 1329–1339. [Google Scholar] [CrossRef]
- Papathanos, P.A.; Bourtzis, K.; Tripet, F.; Bossin, H.; Virginio, J.F.; Capurro, M.L.; Pedrosa, M.C.; Guindo, A.; Sylla, L.; Coulibaly, M.B.; et al. A Perspective on the Need and Current Status of Efficient Sex Separation Methods for Mosquito Genetic Control. Parasit. Vectors 2018, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Lampazzi, E.; Damiani, C.; Fabbri, G.; Lombardi, G.; Pioli, C.; Desiderio, A.; Serrao, A.; Calvitti, M. Increased Biting Rate and Decreased Wolbachia Density in Irradiated Aedes Mosquitoes. Parasit. Vectors 2022, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Ching, N.L. Wolbachia-Mediated Sterility Suppresses Aedes aegypti Populations in the Urban Tropics 2021. MedRxiv 2021. [Google Scholar] [CrossRef]
- Moretti, R.; Marzo, G.A.; Lampazzi, E.; Calvitti, M. Cytoplasmic Incompatibility Management to Support Incompatible Insect Technique against Aedes albopictus. Parasit. Vectors 2018, 11, 649. [Google Scholar] [CrossRef]
- Lombardi, G.; Lampazzi, E.; Calvitti, M. Incompatible Insect Technique: Insights on Potential Outcomes of Releasing Contaminant Females: A Proof of Concept under Semi-field Conditions. Pest Manag. Sci. 2024, 80, 5342–5352. [Google Scholar] [CrossRef]
- Malfacini, M.; Puggioli, A.; Balestrino, F.; Carrieri, M.; Dindo, M.L.; Bellini, R. Aedes albopictus Sterile Male Production: Influence of Strains, Larval Diet and Mechanical Sexing Tools. Insects 2022, 13, 899. [Google Scholar] [CrossRef]
- Mamai, W.; Bueno-Masso, O.; Wallner, T.; Nikièma, S.A.; Meletiou, S.; Deng, L.; Balestrino, F.; Yamada, H.; Bouyer, J. Efficiency Assessment of a Novel Automatic Mosquito Pupae Sex Separation System in Support of Area-Wide Male-Based Release Strategies. Sci. Rep. 2024, 14, 9170. [Google Scholar] [CrossRef]
- Morán-Aceves, B.M.; Marina, C.F.; Dor, A.; Liedo, P.; Toledo, J. Sex Separation of Aedes Spp. Mosquitoes for Sterile Insect Technique Application: A Review. Entomol. Exp. Appl. 2021, 169, 918–927. [Google Scholar] [CrossRef]
- Gong, J.-T.; Mamai, W.; Wang, X.; Zhu, J.; Li, Y.; Liu, J.; Tang, Q.; Huang, Y.; Zhang, J.; Zhou, J.; et al. Upscaling the Production of Sterile Male Mosquitoes with an Automated Pupa Sex Sorter. Sci. Robot. 2024, 9, eadj6261. [Google Scholar] [CrossRef]
- Lutrat, C.; Giesbrecht, D.; Marois, E.; Whyard, S.; Baldet, T.; Bouyer, J. Sex Sorting for Pest Control: It’s Raining Men! Trends Parasitol. 2019, 35, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Lutrat, C.; Burckbuchler, M.; Olmo, R.P.; Beugnon, R.; Fontaine, A.; Akbari, O.S.; Argilés-Herrero, R.; Baldet, T.; Bouyer, J.; Marois, E. Combining Two Genetic Sexing Strains Allows Sorting of Non-Transgenic Males for Aedes Genetic Control. Commun. Biol. 2023, 6, 646. [Google Scholar] [CrossRef] [PubMed]
- Marois, E. Sorting Mosquito Larvae with a COPAS Machine. Cold Spring Harb. Protoc. 2024, 2024, pdb.prot108307. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Nikolouli, K.; Duran De La Fuente, L.; Misbah-ul-Haq, M.; Carvalho, D.O.; Bourtzis, K. Introgression of the Aedes aegypti Red-Eye Genetic Sexing Strains Into Different Genomic Backgrounds for Sterile Insect Technique Applications. Front. Bioeng. Biotechnol. 2022, 10, 821428. [Google Scholar] [CrossRef]
- Mysore, K.; Hapairai, L.K.; Li, P.; Roethele, J.B.; Sun, L.; Igiede, J.; Misenti, J.K.; Duman-Scheel, M. A Functional Requirement for Sex-Determination M/m Locus Region lncRNA Genes in Aedes aegypti Female Larvae. Sci. Rep. 2021, 11, 10657. [Google Scholar] [CrossRef]
- Figueiredo Prates, L.H.; Fiebig, J.; Schlosser, H.; Liapi, E.; Rehling, T.; Lutrat, C.; Bouyer, J.; Sun, Q.; Wen, H.; Xi, Z.; et al. Challenges of Robust RNAi-Mediated Gene Silencing in Aedes Mosquitoes. Int. J. Mol. Sci. 2024, 25, 5218. [Google Scholar] [CrossRef]
- Crawford, J.E.; Clarke, D.W.; Criswell, V.; Desnoyer, M.; Cornel, D.; Deegan, B.; Gong, K.; Hopkins, K.C.; Howell, P.; Hyde, J.S.; et al. Efficient Production of Male Wolbachia-Infected Aedes aegypti Mosquitoes Enables Large-Scale Suppression of Wild Populations. Nat. Biotechnol. 2020, 38, 482–492. [Google Scholar] [CrossRef]
- Burt, A. Heritable Strategies for Controlling Insect Vectors of Disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130432. [Google Scholar] [CrossRef]
- Benedict, M.Q. Sterile Insect Technique: Lessons from the Past. J. Med. Entomol. 2021, 58, 1974–1979. [Google Scholar] [CrossRef]
- Andreo, V.; Porcasi, X.; Guzman, C.; Lopez, L.; Scavuzzo, C.M. Spatial Distribution of Aedes aegypti Oviposition Temporal Patterns and Their Relationship with Environment and Dengue Incidence. Insects 2021, 12, 919. [Google Scholar] [CrossRef]
- Dye, C. Models for the Population Dynamics of the Yellow Fever Mosquito, Aedes aegypti. J. Anim. Ecol. 1984, 53, 247–268. [Google Scholar] [CrossRef]
- Bier, E. Gene Drives Gaining Speed. Nat. Rev. Genet. 2022, 23, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-H.; Hoffmann, A.; Champer, J. Gene Drive and Symbiont Technologies for Control of Mosquito-Borne Diseases. Ann. Rev. Entomol. 2024, 70, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.; Windbichler, N.; Wenger, E.A.; Bever, C.A.; Selvaraj, P. Population Replacement Gene Drive Characteristics for Malaria Elimination in a Range of Seasonal Transmission Settings: A Modelling Study. Malar. J. 2022, 21, 226. [Google Scholar] [CrossRef]
- Adolfi, A.; Gantz, V.M.; Jasinskiene, N.; Lee, H.-F.; Hwang, K.; Terradas, G.; Bulger, E.A.; Ramaiah, A.; Bennett, J.B.; Emerson, J.J.; et al. Efficient Population Modification Gene-Drive Rescue System in the Malaria Mosquito Anopheles stephensi. Nat. Commun. 2020, 11, 5553. [Google Scholar] [CrossRef]
- Carballar-Lejarazú, R.; Dong, Y.; Pham, T.B.; Tushar, T.; Corder, R.M.; Mondal, A.; Sánchez, C.H.M.; Lee, H.-F.; Marshall, J.M.; Dimopoulos, G.; et al. Dual Effector Population Modification Gene-Drive Strains of the African Malaria Mosquitoes, Anopheles gambiae and Anopheles coluzzii. Proc. Natl. Acad. Sci. USA 2023, 120, e2221118120. [Google Scholar] [CrossRef]
- Connolly, J.B.; Burt, A.; Christophides, G.; Diabate, A.; Habtewold, T.; Hancock, P.A.; James, A.A.; Kayondo, J.K.; Lwetoijera, D.W.; Manjurano, A.; et al. Considerations for First Field Trials of Low-Threshold Gene Drive for Malaria Vector Control. Malar. J. 2024, 23, 156. [Google Scholar] [CrossRef]
- Wang, G.-H.; Du, J.; Chu, C.Y.; Madhav, M.; Hughes, G.L.; Champer, J. Symbionts and Gene Drive: Two Strategies to Combat Vector-Borne Disease. Trends Genet. 2022, 38, 708–723. [Google Scholar] [CrossRef]
- Dalla Benetta, E.; López-Denman, A.J.; Li, H.-H.; Masri, R.A.; Brogan, D.J.; Bui, M.; Yang, T.; Li, M.; Dunn, M.; Klein, M.J.; et al. Engineered Antiviral Sensor Targets Infected Mosquitoes. CRISPR J. 2023, 6, 543–556. [Google Scholar] [CrossRef]
- Liu, W.-L.; Hsu, C.-W.; Chan, S.-P.; Yen, P.-S.; Su, M.P.; Li, J.-C.; Li, H.-H.; Cheng, L.; Tang, C.-K.; Ko, S.-H.; et al. Transgenic Refractory Aedes aegypti Lines Are Resistant to Multiple Serotypes of Dengue Virus. Sci. Rep. 2021, 11, 23865. [Google Scholar] [CrossRef]
- Buchman, A.; Gamez, S.; Li, M.; Antoshechkin, I.; Li, H.-H.; Wang, H.-W.; Chen, C.-H.; Klein, M.J.; Duchemin, J.-B.; Paradkar, P.N.; et al. Engineered Resistance to Zika Virus in Transgenic Aedes aegypti Expressing a Polycistronic Cluster of Synthetic Small RNAs. Proc. Natl. Acad. Sci. USA 2019, 116, 3656–3661. [Google Scholar] [CrossRef] [PubMed]
- Gantz, V.M.; Jasinskiene, N.; Tatarenkova, O.; Fazekas, A.; Macias, V.M.; Bier, E.; James, A.A. Highly Efficient Cas9-Mediated Gene Drive for Population Modification of the Malaria Vector Mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. USA 2015, 112, E6736–E6743. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, T.; Kandul, N.P.; Bui, M.; Gamez, S.; Raban, R.; Bennett, J.; Sánchez, C.H.M.; Lanzaro, G.C.; Schmidt, H.; et al. Development of a Confinable Gene Drive System in the Human Disease Vector Aedes aegypti. eLife 2020, 9, e51701. [Google Scholar] [CrossRef]
- Anderson, M.A.E.; Gonzalez, E.; Edgington, M.P.; Ang, J.X.D.; Purusothaman, D.-K.; Shackleford, L.; Nevard, K.; Verkuijl, S.A.N.; Harvey-Samuel, T.; Leftwich, P.T.; et al. A Multiplexed, Confinable CRISPR/Cas9 Gene Drive Can Propagate in Caged Aedes aegypti Populations. Nat. Commun. 2024, 15, 729. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.B.; Smithyman, R.; O’Neill, S.L.; Moreira, L.A. How to Engage Communities on a Large Scale? Lessons from World Mosquito Program in Rio de Janeiro, Brazil. Gates Open Res. 2020, 4, 109. [Google Scholar] [CrossRef]
- Schairer, C.E.; Triplett, C.; Akbari, O.S.; Bloss, C.S. California Residents’ Perceptions of Gene Drive Systems to Control Mosquito-Borne Disease. Front. Bioeng. Biotechnol. 2022, 10, 848707. [Google Scholar] [CrossRef]
- Ross, P.A.; Turelli, M.; Hoffmann, A.A. Evolutionary Ecology of Wolbachia Releases for Disease Control. Annu. Rev. Genet. 2019, 53, 93–116. [Google Scholar] [CrossRef]
- Schmidt, T.L.; Barton, N.H.; Rašić, G.; Turley, A.P.; Montgomery, B.L.; Iturbe-Ormaetxe, I.; Cook, P.E.; Ryan, P.A.; Ritchie, S.A.; Hoffmann, A.A.; et al. Local Introduction and Heterogeneous Spatial Spread of Dengue-Suppressing Wolbachia through an Urban Population of Aedes aegypti. PLoS Biol. 2017, 15, e2001894. [Google Scholar] [CrossRef]
- Hien, N.T.; Anh, D.D.; Le, N.H.; Yen, N.T.; Phong, T.V.; Nam, V.S.; Duong, T.N.; Nguyen, N.B.; Huong, D.T.T.; Hung, L.Q.; et al. Environmental Factors Influence the Local Establishment of Wolbachia in Aedes aegypti Mosquitoes in Two Small Communities in Central Vietnam. Gates Open Res. 2021, 5, 147. [Google Scholar] [CrossRef]
- Schmidt, T.L.; Filipović, I.; Hoffmann, A.A.; Rašić, G. Fine-Scale Landscape Genomics Helps Explain the Slow Spatial Spread of Wolbachia through the Aedes aegypti Population in Cairns, Australia. Heredity 2018, 120, 386–395. [Google Scholar] [CrossRef]
- Hien, T.H.; Nguyen, H.L.; Nguyen, T.Y.; Vu, S.N.; Tran, N.D.; Le, T.N.; Vien, Q.M.; Bui, T.C.; Le, H.T.; Kutcher, S.; et al. Field Evaluation of the Establishment Potential of wMelPop Wolbachia in Australia and Vietnam for Dengue Control. Parasit. Vectors 2015, 8, 563. [Google Scholar] [CrossRef]
- National Environmental Agency Risk Assessment for the Use of Male Wolbachia-Carrying Aedes aegypti for Suppression of the Aedes aegypti Mosquito Population. Available online: https://www.nea.gov.sg/docs/default-source/project-wolbachia/2016-08-24-risk-assessment-for-the-use-of-wolbachia-carrying-aedes-males.pdf (accessed on 21 October 2024).
- Popovici, J.; Moreira, L.A.; Poinsignon, A.; Iturbe-Ormaetxe, I.; McNaughton, D.; O’Neill, S.L. Assessing Key Safety Concerns of a Wolbachia-Based Strategy to Control Dengue Transmission by Aedes Mosquitoes. Mem. Inst. Oswaldo Cruz 2010, 105, 957–964. [Google Scholar] [CrossRef] [PubMed]
- CDC. Mosquitoes with Wolbachia. Available online: https://www.cdc.gov/mosquitoes/mosquito-control/mosquitoes-with-wolbachia.html (accessed on 21 October 2024).
- World Mosquito Program. Our Wolbachia Method. Available online: https://www.worldmosquitoprogram.org/en/work/wolbachia-method (accessed on 22 October 2024).
- Buchori, D.; Mawan, A.; Nurhayati, I.; Aryati, A.; Kusnanto, H.; Hadi, U.K. Risk Assessment on the Release of Wolbachia-Infected Aedes aegypti in Yogyakarta, Indonesia. Insects 2022, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How Many Species Are Infected with Wolbachia?—A Statistical Analysis of Current Data: Wolbachia Infection Rates. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef]
- Werren, J.H. Biology of Wolbachia. Annu. Rev. Entomol. 1997, 42, 587–609. [Google Scholar] [CrossRef]
- Fallon, A.M. Growth and Maintenance of Wolbachia in Insect Cell Lines. Insects 2021, 12, 706. [Google Scholar] [CrossRef]
- Yeap, H.L.; Rašić, G.; Endersby-Harshman, N.M.; Lee, S.F.; Arguni, E.; Le Nguyen, H.; Hoffmann, A.A. Mitochondrial DNA Variants Help Monitor the Dynamics of Wolbachia Invasion into Host Populations. Heredity 2016, 116, 265–276. [Google Scholar] [CrossRef]
- Lau, M.-J.; Schmidt, T.L.; Yang, Q.; Chung, J.; Sankey, L.; Ross, P.A.; Hoffmann, A.A. Genetic Stability of Aedes aegypti Populations Following Invasion by wMel Wolbachia. BMC Genom. 2021, 22, 894. [Google Scholar] [CrossRef]
- Pavan, M.G.; Garcia, G.A.; David, M.R.; Maciel-de-Freitas, R. The Double-Edged Sword Effect of Expanding Wolbachia Deployment in Dengue Endemic Settings. Lancet Reg. Health—Am. 2023, 27, 100610. [Google Scholar] [CrossRef]
- Endersby, N.M.; Hoffmann, A.A. Effect of Wolbachia on Insecticide Susceptibility in Lines of Aedes aegypti. Bull. Entomol. Res. 2013, 103, 269–277. [Google Scholar] [CrossRef]
- Salje, H.; Jiggins, F.M. Risks of Releasing Imperfect Wolbachia Strains for Arbovirus Control. Lancet Microbe 2024, 5, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Edenborough, K.M.; Flores, H.A.; Simmons, C.P.; Fraser, J.E. Using Wolbachia to Eliminate Dengue: Will the Virus Fight Back? J. Virol. 2021, 95, e0220320. [Google Scholar] [CrossRef] [PubMed]
- Dainty, K.R.; Hawkey, J.; Judd, L.M.; Pacidônio, E.C.; Duyvestyn, J.M.; Gonçalves, D.S.; Lin, S.Y.; O’Donnell, T.B.; O’Neill, S.L.; Simmons, C.P.; et al. wMel Wolbachia Genome Remains Stable after 7 Years in Australian Aedes aegypti Field Populations. Microb. Genom. 2021, 7, 000641. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yang, Q.; Hoffmann, A.A.; Ritchie, S.A.; Van Den Hurk, A.F.; Warrilow, D. Wolbachia Genome Stability and mtDNA Variants in Aedes aegypti Field Populations Eight Years after Release. iScience 2020, 23, 101572. [Google Scholar] [CrossRef]
- Ross, P.A.; Robinson, K.L.; Yang, Q.; Callahan, A.G.; Schmidt, T.L.; Axford, J.K.; Coquilleau, M.P.; Staunton, K.M.; Townsend, M.; Ritchie, S.A.; et al. A Decade of Stability for wMel Wolbachia in Natural Aedes aegypti Populations. PLoS Pathog. 2022, 18, e1010256. [Google Scholar] [CrossRef]
- Martinez, J.; Ross, P.A.; Gu, X.; Ant, T.H.; Murdochy, S.M.; Tong, L.; da Silva Filipe, A.; Hoffmann, A.A.; Sinkins, S.P. Genomic and Phenotypic Comparisons Reveal Distinct Variants of Wolbachia Strain wAlbB. Appl. Environ. Microbiol. 2022, 88, e0141222. [Google Scholar] [CrossRef]
- Tantowijoyo, W.; Tanamas, S.K.; Nurhayati, I.; Setyawan, S.; Budiwati, N.; Fitriana, I.; Ernesia, I.; Wardana, D.S.; Supriyati, E.; Arguni, E.; et al. Aedes aegypti Abundance and Insecticide Resistance Profiles in the Applying Wolbachia to Eliminate Dengue Trial. PLoS Neglected Trop. Dis. 2022, 16, e0010284. [Google Scholar] [CrossRef]
- Bansal, S.; Lim, J.T.; Chong, C.-S.; Dickens, B.; Ng, Y.; Deng, L.; Lee, C.; Tan, L.Y.; Kakani, E.G.; Yoong, Y.; et al. Effectiveness of Wolbachia-Mediated Sterility Coupled with Sterile Insect Technique to Suppress Adult Aedes aegypti Populations in Singapore: A Synthetic Control Study. Lancet Planet. Health 2024, 8, e617–e628. [Google Scholar] [CrossRef]
- De Barro, P.J.; Murphy, B.; Jansen, C.C.; Murray, J. The Proposed Release of the Yellow Fever Mosquito, Aedes aegypti Containing a Naturally Occurring Strain of Wolbachia pipientis, a Question of Regulatory Responsibility. J. Verbr. Lebensm. 2011, 6, 33–40. [Google Scholar] [CrossRef]
- Murphy, B.; Jansen, C.; Murray, J.; De Barro, P. Risk Analysis on the Australian Release of Aedes aegypti (L.) (Diptera: Culicidae) Containing Wolbachia. Available online: https://www.worldmosquitoprogram.org/en/learn/scientific-publications/risk-analysis-australian-release-aedes-aegypti-l-diptera-culicidae (accessed on 22 October 2024).
- Naegeli, H.; Bresson, J.; Dalmay, T.; Dewhurst, I.C.; Epstein, M.M.; Guerche, P.; Hejatko, J.; Moreno, F.J.; Mullins, E.; Nogué, F.; et al. Adequacy and Sufficiency Evaluation of Existing EFSA Guidelines for the Molecular Characterisation, Environmental Risk Assessment and Post-market Environmental Monitoring of Genetically Modified Insects Containing Engineered Gene Drives. EFS2 2020, 18, e06297. [Google Scholar] [CrossRef]
- US EPA Pesticide Registration Manual: Chapter 12—Applying for an Experimental Use Permit. Available online: https://www.epa.gov/pesticide-registration/pesticide-registration-manual-chapter-12-applying-experimental-use-permit (accessed on 22 October 2024).
- European Commission Regulation on the Supply and Use of Biocidal Products. Available online: https://health.ec.europa.eu/biocides/regulation_en (accessed on 22 October 2024).
- Environmental Protection Agency Final Registration Decision of the New Active Ingredient Wolbachia pipientis ZAP (wPip) Strain in Aedes albopictus. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2016-0205-0034 (accessed on 22 October 2024).
- Dobson, S.L.; Bordenstein, S.R.; Rose, R.I. Wolbachia Mosquito Control: Regulated. Science 2016, 352, 526–527. [Google Scholar] [CrossRef] [PubMed]
- National Environmental Agency. Wolbachia-Aedes Mosquito Suppression Strategy. Available online: https://www.nea.gov.sg (accessed on 22 October 2024).
- European Union. Commission Implementing Decision (EU) 2018/1623 of 29 October 2018 Pursuant to Article 3(3) of Regulation (EU) No 528/2012 of the European Parliament and of the Council on Mosquitoes Non-Naturally Infected with Wolbachia Used for Vector Control Purposes (Text with EEA Relevance); European Union: Brussels, Belgium, 2018; Volume 271, Available online: https://eur-lex.europa.eu/legal-content/EN/PIN/?uri=CELEX:32018D1623 (accessed on 31 January 2025).
- Organizacion Panamericana de la Salud Uso de Wolbachia en las Américas, Para el Control de Vectores Responsables de Enfermedades de Interés en Salud Pública. Análisis de Información y Posición de la OPS—OPS/OMS|Organización Panamericana de la Salud. Available online: https://www.paho.org/es/documentos/uso-wolbachia-americas-para-control-vectores-responsables-enfermedades-interes-salud (accessed on 22 October 2024).
- Organización Panamericana de la Salud Evaluación de Las Estrategias Innovadoras Para El Control de Aedes aegypti: Desafíos Para Su Introducción y Evaluación Del Impacto; Organización Panamericana de la Salud: Washington, DC, USA, 2019; ISBN 978-92-75-32096-9.
- Consultas. Agência Nacional de Vigilância Sanitária. Available online: https://consultas.anvisa.gov.br/#/ (accessed on 31 January 2025).
- Consulta de Processos. Brazilian Institute of the Environment and Renewable Natural Resources. Available online: https://www.gov.br/ibama/pt-br/assuntos/quimicos-e-biologicos/consulta-de-processos/consulta-de-processos (accessed on 31 January 2025).
- Ministério Da Agricultura e Pecuária. Available online: https://www.gov.br/agricultura/pt-br (accessed on 31 January 2025).
- Comissão Nacional de Ética em Pesquisa. Available online: https://www.gov.br/conselho-nacional-de-saude/pt-br/acesso-a-informacao/sobre-o-conselho/camaras-tecnicas-e-comissoes/conep/comissao-nacional-de-etica-em-pesquisa (accessed on 31 January 2025).
- Bartumeus, F.; Costa, G.B.; Eritja, R.; Kelly, A.H.; Finda, M.; Lezaun, J.; Okumu, F.; Quinlan, M.M.; Thizy, D.C.; Toé, L.P.; et al. Sustainable Innovation in Vector Control Requires Strong Partnerships with Communities. PLoS Neglected Trop. Dis. 2019, 13, e0007204. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Vector Control Response 2017–2030. Available online: https://www.who.int/publications/i/item/9789241512978 (accessed on 22 October 2024).
- Vélez, I.D. Credibility Gain Challenges to Province Innovations. El Reto de Ganar Credibilidad Para Poder Innovar Desde La Provincia. Biomedica 2023, 43, 153–154. [Google Scholar] [CrossRef]
- WMP. Global Progress. Vietnam. Available online: https://www.worldmosquitoprogram.org/en/global-progress/vietnam (accessed on 14 January 2025).
- Liew, C.; Soh, L.T.; Chen, I.; Ng, L.C. Public Sentiments towards the Use of Wolbachia-Aedes Technology in Singapore. BMC Public Health 2021, 21, 1417. [Google Scholar] [CrossRef]
- Abdullah, Z. Project Wolbachia to Expand to Five More Sites in S’pore to Lower Dengue Risk. The Straits Times. 3 October 2024. Available online: https://www.straitstimes.com/singapore/project-wolbachia-to-expand-to-five-more-sites-in-s-pore-to-lower-dengue-risk (accessed on 31 January 2025).
- Liew, C.; Soh, L.T.; Chen, I.; Li, X.; Sim, S.; Ng, L.C. Community Engagement for Wolbachia-Based Aedes aegypti Population Suppression for Dengue Control: The Singapore Experience. In Area-Wide Integrated Pest Management; CRC Press: Boca Raton, FL, USA, 2021; pp. 747–761. ISBN 978-1-003-16923-9. [Google Scholar]
- Laven, H. Eradication of Culex Pipiens Fatigans through Cytoplasmic Incompatibility. Nature 1967, 216, 383–384. [Google Scholar] [CrossRef]
- Caputo, B.; Moretti, R.; Virgillito, C.; Manica, M.; Lampazzi, E.; Lombardi, G.; Serini, P.; Pichler, V.; Beebe, N.W.; Della Torre, A.; et al. A Bacterium against the Tiger: Further Evidence of the Potential of Noninundative Releases of Males with Manipulated Wolbachia Infection in Reducing Fertility of Aedes albopictus Field Populations in Italy. Pest Manag. Sci. 2023, 79, 3167–3176. [Google Scholar] [CrossRef]
- Caputo, B.; Moretti, R.; Manica, M.; Serini, P.; Lampazzi, E.; Bonanni, M.; Fabbri, G.; Pichler, V.; Della Torre, A.; Calvitti, M. A Bacterium against the Tiger: Preliminary Evidence of Fertility Reduction after Release of Aedes albopictus Males with Manipulated Wolbachia Infection in an Italian Urban Area. Pest Manag. Sci. 2020, 76, 1324–1332. [Google Scholar] [CrossRef]
- Mains, J.W.; Brelsfoard, C.L.; Rose, R.I.; Dobson, S.L. Female Adult Aedes albopictus Suppression by Wolbachia-Infected Male Mosquitoes. Sci. Rep. 2016, 6, 33846. [Google Scholar] [CrossRef]
- O’Connor, L.; Plichart, C.; Sang, A.C.; Brelsfoard, C.L.; Bossin, H.C.; Dobson, S.L. Open Release of Male Mosquitoes Infected with a Wolbachia Biopesticide: Field Performance and Infection Containment. PLoS Neglected Trop. Dis. 2012, 6, e1797. [Google Scholar] [CrossRef]
- Mains, J.W.; Kelly, P.H.; Dobson, K.L.; Petrie, W.D.; Dobson, S.L. Localized Control of Aedes aegypti (Diptera: Culicidae) in Miami, FL, via Inundative Releases of Wolbachia-Infected Male Mosquitoes. J. Med. Entomol. 2019, 56, 1296–1303. [Google Scholar] [CrossRef]
- Lim, J.T.; Bansal, S.; Chong, C.S.; Dickens, B.; Ng, Y.; Deng, L.; Lee, C.; Tan, L.Y.; Chain, G.; Ma, P.; et al. Efficacy of Wolbachia-Mediated Sterility to Reduce the Incidence of Dengue: A Synthetic Control Study in Singapore. Lancet Microbe 2024, 5, e422–e432. [Google Scholar] [CrossRef] [PubMed]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I.; et al. Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue. N. Engl. J. Med. 2021, 384, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Calvitti, M.; Moretti, R.; Skidmore, A.R.; Dobson, S.L. Wolbachia Strain wPip Yields a Pattern of Cytoplasmic Incompatibility Enhancing a Wolbachia-Based Suppression Strategy against the Disease Vector Aedes albopictus. Parasit. Vectors 2012, 5, 254. [Google Scholar] [CrossRef]
- Moretti, R.; Calvitti, M. Issues with Combining Incompatible and Sterile Insect Techniques. Nature 2021, 590, E1–E2. [Google Scholar] [CrossRef]
- Lim, J.T.; Mailepessov, D.; Chong, C.-S.; Dickens, B.; Lai, Y.L.; Ng, Y.; Deng, L.; Lee, C.; Tan, L.Y.; Chain, G.; et al. Assessing Wolbachia-Mediated Sterility for Dengue Control: Emulation of a Cluster-Randomized Target Trial in Singapore. J. Travel Med. 2024, 31, taae103. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Crawford, J.E.; Adams, L.E.; Brown, G.; Ryff, K.R.; Delorey, M.; Ruiz-Valcarcel, J.; Nazario, N.; Borrero, N.; Miranda, J.; et al. Incompatible Aedes aegypti Male Releases as an Intervention to Reduce Mosquito Population—A Field Trial in Puerto Rico. PLoS Neglected Trop. Dis. 2025, 19, e0012839. [Google Scholar] [CrossRef]
- Lozano, S.; Pritts, K.; Duguma, D.; Fredregill, C.; Connelly, R. Independent Evaluation of Wolbachia Infected Male Mosquito Releases for Control of Aedes aegypti in Harris County, Texas, Using a Bayesian Abundance Estimator. PLoS Neglected Trop. Dis. 2022, 16, e0010907. [Google Scholar] [CrossRef]
- Beebe, N.W.; Pagendam, D.; Trewin, B.J.; Boomer, A.; Bradford, M.; Ford, A.; Liddington, C.; Bondarenco, A.; De Barro, P.J.; Gilchrist, J.; et al. Releasing Incompatible Males Drives Strong Suppression across Populations of Wild and Wolbachia-Carrying Aedes aegypti in Australia. Proc. Natl. Acad. Sci. USA 2021, 118, e2106828118. [Google Scholar] [CrossRef]
- Debug Project. Debug Project 2024. Available online: https://blog.debug.com/2024/ (accessed on 31 January 2025).
- Kittayapong, P.; Ninphanomchai, S.; Limohpasmanee, W.; Chansang, C.; Chansang, U.; Mongkalangoon, P. Combined Sterile Insect Technique and Incompatible Insect Technique: The First Proof-of-Concept to Suppress Aedes aegypti Vector Populations in Semi-Rural Settings in Thailand. PLoS Neglected Trop. Dis. 2019, 13, e0007771. [Google Scholar] [CrossRef]
- Verily. Research, Care and Health Financing. Alphabet Precision Health Company. Available online: https://verily.com/home (accessed on 31 January 2025).
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful Establishment of Wolbachia in Aedes Populations to Suppress Dengue Transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Iturbe-Ormaetxe, I.; Callahan, A.G.; Phillips, B.L.; Billington, K.; Axford, J.K.; Montgomery, B.; Turley, A.P.; O’Neill, S.L. Stability of the wMel Wolbachia Infection Following Invasion into Aedes aegypti Populations. PLoS Neglected Trop. Dis. 2014, 8, e3115. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.L.; Ryan, P.A.; Turley, A.P.; Wilson, G.; Retzki, K.; Iturbe-Ormaetxe, I.; Dong, Y.; Kenny, N.; Paton, C.J.; Ritchie, S.A.; et al. Scaled Deployment of Wolbachia to Protect the Community from Dengue and Other Aedes-Transmitted Arboviruses. Gates Open Res. 2018, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- World Mosquito Program. Available online: https://www.worldmosquitoprogram.org/en/home (accessed on 12 December 2024).
- Nota Informativa No 28/2023-CGARB/DEDT/SVSA/MS—Ministério Da Saúde. Brazil. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/estudos-e-notas-informativas/2023/nota-informativa-no-28-2023-cgarb-dedt-svsa-ms/view (accessed on 31 January 2025).
- Tantowijoyo, W.; Andari, B.; Arguni, E.; Budiwati, N.; Nurhayati, I.; Fitriana, I.; Ernesia, I.; Daniwijaya, E.W.; Supriyati, E.; Yusdiana, D.H.; et al. Stable Establishment of wMel Wolbachia in Aedes aegypti Populations in Yogyakarta, Indonesia. PLoS Neglected Trop. Dis. 2020, 14, e0008157. [Google Scholar] [CrossRef]
- Gesto, J.S.M.; Pinto, S.B.; Dias, F.B.S.; Peixoto, J.; Costa, G.; Kutcher, S.; Montgomery, J.; Green, B.R.; Anders, K.L.; Ryan, P.A.; et al. Large-Scale Deployment and Establishment of Wolbachia Into the Aedes aegypti Population in Rio de Janeiro, Brazil. Front. Microbiol. 2021, 12, 711107. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Ahmad, N.W.; Keong, W.M.; Ling, C.Y.; Ahmad, N.A.; Golding, N.; Tierney, N.; Jelip, J.; Putit, P.W.; Mokhtar, N.; et al. Introduction of Aedes aegypti Mosquitoes Carrying wAlbB Wolbachia Sharply Decreases Dengue Incidence in Disease Hotspots. iScience 2024, 27, 108942. [Google Scholar] [CrossRef]
- Velez, I.D.; Tanamas, S.K.; Arbelaez, M.P.; Kutcher, S.C.; Duque, S.L.; Uribe, A.; Zuluaga, L.; Martínez, L.; Patiño, A.C.; Barajas, J.; et al. Reduced Dengue Incidence Following City-Wide wMel Wolbachia Mosquito Releases throughout Three Colombian Cities: Interrupted Time Series Analysis and a Prospective Case-Control Study. PLoS Neglected Trop. Dis. 2023, 17, e0011713. [Google Scholar] [CrossRef]
- Chow, J.Y.; Bansal, S.; Dickens, B.S.L.; Ma, P.; Hoffmann, A.; Cheong, Y.L.; Ahmad, N.W.; Lim, J.T. Assessing the Direct and Spillover Protective Effectiveness of Wolbachia-Mediated Introgression to Combat Dengue. EBioMedicine 2024, 110, 105456. [Google Scholar] [CrossRef]
- Cavany, S.; Huber, J.H.; Wieler, A.; Tran, Q.M.; Alkuzweny, M.; Elliott, M.; España, G.; Moore, S.M.; Perkins, T.A. Does Ignoring Transmission Dynamics Lead to Underestimation of the Impact of Interventions against Mosquito-Borne Disease? BMJ Glob. Health 2023, 8, e012169. [Google Scholar] [CrossRef]
- Indriani, C.; Tanamas, S.K.; Khasanah, U.; Ansari, M.R.; Rubangi; Tantowijoyo, W.; Ahmad, R.A.; Dufault, S.M.; Jewell, N.P.; Utarini, A.; et al. Impact of Randomised wMel Wolbachia Deployments on Notified Dengue Cases and Insecticide Fogging for Dengue Control in Yogyakarta City. Glob. Health Action 2023, 16, 2166650. [Google Scholar] [CrossRef]
- Collins, M.H.; Potter, G.E.; Hitchings, M.D.T.; Butler, E.; Wiles, M.; Kennedy, J.K.; Pinto, S.B.; Teixeira, A.B.M.; Casanovas-Massana, A.; Rouphael, N.G.; et al. EVITA Dengue: A Cluster-Randomized Controlled Trial to EValuate the Efficacy of Wolbachia-InfecTed Aedes aegypti Mosquitoes in Reducing the Incidence of Arboviral Infection in Brazil. Trials 2022, 23, 185. [Google Scholar] [CrossRef]
- Gesto, J.S.M.; Ribeiro, G.S.; Rocha, M.N.; Dias, F.B.S.; Peixoto, J.; Carvalho, F.D.; Pereira, T.N.; Moreira, L.A. Reduced Competence to Arboviruses Following the Sustainable Invasion of Wolbachia into Native Aedes aegypti from Southeastern Brazil. Sci. Rep 2021, 11, 10039. [Google Scholar] [CrossRef] [PubMed]
- Innisfail Mozzie Project. CSIRO. Available online: https://research.csiro.au/mozzieproject/ (accessed on 31 January 2025).
- BugOut. Green VI 2019. Available online: https://greenvi.org/bugout/ (accessed on 31 January 2025).
- BugOut Program. Verily. Available online: https://verily.com/perspectives/bugout-wolbachia-launches-in-the-british-virgin-islands (accessed on 31 January 2025).
- World Mosquito Program in Australia: Combating Mosquito-Borne Diseases with Wolbachia. Available online: https://www.worldmosquitoprogram.org/en/global-progress/australia (accessed on 14 January 2025).
- Sohail, A.; Anders, K.L.; McGuinness, S.L.; Leder, K. The Epidemiology of Imported and Locally Acquired Dengue in Australia, 2012–2022. J. Travel Med. 2024, 31, taae014. [Google Scholar] [CrossRef] [PubMed]
- WMP. Global Progress. New Caledonia. Available online: https://www.worldmosquitoprogram.org/en/global-progress/new-caledonia (accessed on 14 January 2025).
- WMP. Global Progress. Fiji. Available online: https://www.worldmosquitoprogram.org/en/global-progress/fiji (accessed on 14 January 2025).
- WMP. Global Progress. Vanuatu. Available online: https://www.worldmosquitoprogram.org/en/global-progress/vanuatu (accessed on 14 January 2025).
- WMP. Global Progress. Kiribati. Available online: https://www.worldmosquitoprogram.org/en/global-progress/kiribati (accessed on 14 January 2025).
- Durovni, B.; Saraceni, V.; Eppinghaus, A.; Riback, T.I.S.; Moreira, L.A.; Jewell, N.P.; Dufault, S.M.; O’Neill, S.L.; Simmons, C.P.; Tanamas, S.K.; et al. The Impact of Large-Scale Deployment of Wolbachia Mosquitoes on Dengue and Other Aedes-Borne Diseases in Rio de Janeiro and Niterói, Brazil: Study Protocol for a Controlled Interrupted Time Series Analysis Using Routine Disease Surveillance Data. F1000Res 2019, 8, 1328. [Google Scholar] [CrossRef] [PubMed]
- WMP. Global Progress. Brazil. Available online: https://www.worldmosquitoprogram.org/en/global-progress/brazil (accessed on 17 January 2025).
- WMP. Global Progress. Colombia. Available online: https://www.worldmosquitoprogram.org/en/global-progress/colombia (accessed on 17 January 2025).
- Calle-Tobón, A.; Rojo-Ospina, R.; Zuluaga, S.; Giraldo-Muñoz, J.F.; Cadavid, J.M. Evaluation of Wolbachia Infection in Aedes aegypti Suggests Low Prevalence and Highly Heterogeneous Distribution in Medellín, Colombia. Acta Trop. 2024, 260, 107423. [Google Scholar] [CrossRef]
- WMP. Global Progress. Mexico. Available online: https://www.worldmosquitoprogram.org/en/global-progress/mexico (accessed on 14 January 2025).
- WMP. Global Progress. Honduras. Available online: https://www.worldmosquitoprogram.org/en/global-progress/honduras (accessed on 14 January 2025).
- WMP. Global Progress. El Salvador. Available online: https://www.worldmosquitoprogram.org/en/global-progress/el-salvador (accessed on 14 January 2025).
- WMP. Global Progress. Sri Lanka. Available online: https://www.worldmosquitoprogram.org/en/global-progress/sri-lanka (accessed on 14 January 2025).
- WMP. Global Progress. Laos. Available online: https://www.worldmosquitoprogram.org/en/global-progress/laos (accessed on 14 January 2025).
- Cheong, Y.L.; Nazni, W.A.; Lee, H.L.; NoorAfizah, A.; MohdKhairuddin, I.C.; Kamarul, G.M.R.; Nizam, N.M.N.; Arif, M.A.K.; NurZatilAqmar, Z.M.; Irwan, S.M.; et al. Spatial Distribution and Long-Term Persistence of Wolbachia-Infected Aedes aegypti in the Mentari Court, Malaysia. Insects 2023, 14, 373. [Google Scholar] [CrossRef]
- Chow, J.Y.; Geng, L.; Bansal, S.; Dickens, B.S.L.; Ng, L.C.; Hoffmann, A.A.; Lim, J.T. Evaluating Quasi-Experimental Approaches for Estimating Epidemiological Efficacy of Non-Randomised Field Trials: Applications in Wolbachia Interventions for Dengue. BMC Med. Res. Methodol. 2024, 24, 170. [Google Scholar] [CrossRef]
- Duman-Scheel, M.; Eggleson, K.K.; Achee, N.L.; Grieco, J.P.; Hapairai, L.K. Mosquito Control Practices and Perceptions: An Analysis of Economic Stakeholders during the Zika Epidemic in Belize, Central America. PLoS ONE 2018, 13, e0201075. [Google Scholar] [CrossRef]
- Abidemi, A.; Fatmawati; Peter, O.J. An Optimal Control Model for Dengue Dynamics with Asymptomatic, Isolation, and Vigilant Compartments. Decis. Anal. J. 2024, 10, 100413. [Google Scholar] [CrossRef]
- Baitharu, I.; Shroff, S.; Naik, P.P.; Sahu, J.K. Environmental Management and Sustainable Control of Mosquito Vector: Challenges and Opportunities. In Molecular Identification of Mosquito Vectors and Their Management; Barik, T.K., Ed.; Springer Singapore: Singapore, 2020; pp. 129–147. ISBN 978-981-15-9455-7. [Google Scholar]
- Brühl, C.A.; Després, L.; Frör, O.; Patil, C.D.; Poulin, B.; Tetreau, G.; Allgeier, S. Environmental and Socioeconomic Effects of Mosquito Control in Europe Using the Biocide Bacillus thuringiensis Subsp. Israelensis (Bti). Sci. Total Environ. 2020, 724, 137800. [Google Scholar] [CrossRef]
- Rayhan Sarker, M.; Mithun Ali, S.; Kumar Paul, S.; Haque Munim, Z. Measuring Sustainability Performance Using an Integrated Model. Measurement 2021, 184, 109931. [Google Scholar] [CrossRef]
- World Health Organization. Norms, Standards and Processes Underpinning Development of WHO Recommendations on Vector Control. Available online: https://www.who.int/publications/i/item/9789240017382 (accessed on 31 October 2024).
- World Health Organization. Dengue Guidelines, for Diagnosis, Treatment, Prevention and Control. Available online: https://www.who.int/publications/i/item/9789241547871 (accessed on 31 October 2024).
- Shepard, D.S.; Suaya, J.A.; Halstead, S.B.; Nathan, M.B.; Gubler, D.J.; Mahoney, R.T.; Wang, D.N.C.; Meltzer, M.I. Cost-Effectiveness of a Pediatric Dengue Vaccine. Vaccine 2004, 22, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Shepard, D.S.; Coudeville, L.; Halasa, Y.A.; Zambrano, B.; Dayan, G.H. Economic Impact of Dengue Illness in the Americas. Am. Soc. Trop. Med. Hyg. 2011, 84, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Baly, A.; Toledo, M.E.; Boelaert, M.; Reyes, A.; Vanlerberghe, V.; Ceballos, E.; Carvajal, M.; Maso, R.; La Rosa, M.; Denis, O.; et al. Cost Effectiveness of Aedes aegypti Control Programmes: Participatory versus Vertical. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 578–586. [Google Scholar] [CrossRef]
- Alphey, N.; Alphey, L.; Bonsall, M.B. A Model Framework to Estimate Impact and Cost of Genetics-Based Sterile Insect Methods for Dengue Vector Control. PLoS ONE 2011, 6, e25384. [Google Scholar] [CrossRef]
- Suaya, J.A.; Shepard, D.S.; Chang, M.; Caram, M.; Hoyer, S.; Socheat, D.; Chantha, N.; Nathan, M.B. Cost-effectiveness of Annual Targeted Larviciding Campaigns in Cambodia against the Dengue Vector Aedes aegypti. Tropical Med. Int. Health 2007, 12, 1026–1036. [Google Scholar] [CrossRef]
- Pepin, K.M.; Marques-Toledo, C.; Scherer, L.; Morais, M.M.; Ellis, B.; Eiras, A.E. Cost-Effectiveness of Novel System of Mosquito Surveillance and Control, Brazil. Emerg. Infect. Dis. 2013, 19, 542–550. [Google Scholar] [CrossRef]
- Carvalho, S.A.; Da Silva, S.O.; Charret, I.D.C. Mathematical Modeling of Dengue Epidemic: Control Methods and Vaccination Strategies. Theory Biosci. 2019, 138, 223–239. [Google Scholar] [CrossRef]
- Ho, S.H.; Lim, J.T.; Ong, J.; Hapuarachchi, H.C.; Sim, S.; Ng, L.C. Singapore’s 5 Decades of Dengue Prevention and Control—Implications for Global Dengue Control. PLoS Neglected Trop. Dis. 2023, 17, e0011400. [Google Scholar] [CrossRef]
- Knerer, G.; Currie, C.S.M.; Brailsford, S.C. The Economic Impact and Cost-Effectiveness of Combined Vector-Control and Dengue Vaccination Strategies in Thailand: Results from a Dynamic Transmission Model. PLoS Neglected Trop. Dis. 2020, 14, e0008805. [Google Scholar] [CrossRef]
- Zimmermann, I.R.; Alves Fernandes, R.R.; Santos Da Costa, M.G.; Pinto, M.; Peixoto, H.M. Simulation-Based Economic Evaluation of the Wolbachia Method in Brazil: A Cost-Effective Strategy for Dengue Control. Lancet Reg. Health—Am. 2024, 35, 100783. [Google Scholar] [CrossRef]
- Brady, O.J.; Kharisma, D.D.; Wilastonegoro, N.N.; O’Reilly, K.M.; Hendrickx, E.; Bastos, L.S.; Yakob, L.; Shepard, D.S. The Cost-Effectiveness of Controlling Dengue in Indonesia Using wMel Wolbachia Released at Scale: A Modelling Study. BMC Med. 2020, 18, 186. [Google Scholar] [CrossRef]
- Soh, S.; Ho, S.H.; Seah, A.; Ong, J.; Dickens, B.S.; Tan, K.W.; Koo, J.R.; Cook, A.R.; Tan, K.B.; Sim, S.; et al. Economic Impact of Dengue in Singapore from 2010 to 2020 and the Cost-Effectiveness of Wolbachia Interventions. PLoS Glob. Public Health 2021, 1, e0000024. [Google Scholar] [CrossRef] [PubMed]
- Knerer, G.; Currie, C.S.M.; Brailsford, S.C. Reducing Dengue Fever Cases at the Lowest Budget: A Constrained Optimization Approach Applied to Thailand. BMC Public Health 2021, 21, 807. [Google Scholar] [CrossRef]
- Turner, H.C.; Quyen, D.L.; Dias, R.; Huong, P.T.; Simmons, C.P.; Anders, K.L. An Economic Evaluation of Wolbachia Deployments for Dengue Control in Vietnam. PLoS Neglected Trop. Dis. 2023, 17, e0011356. [Google Scholar] [CrossRef]
- Ogunlade, S.T.; Meehan, M.T.; Adekunle, A.I.; McBryde, E.S. A Systematic Review of Mathematical Models of Dengue Transmission and Vector Control: 2010–2020. Viruses 2023, 15, 254. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Ponti, L. Analysis of Invasive Insects: Links to Climate Change. In Invasive Species and Global Climate Change; Ziska, L.H., Dukes, J.S., Eds.; CABI: Wallingford, UK, 2014; pp. 45–61. ISBN 978-1-78064-164-5. [Google Scholar]
- Mordecai, E.A.; Ryan, S.J.; Caldwell, J.M.; Shah, M.M.; LaBeaud, A.D. Climate Change Could Shift Disease Burden from Malaria to Arboviruses in Africa. Lancet Planet. Health 2020, 4, e416–e423. [Google Scholar] [CrossRef]
- Kearney, M.; Porter, W.P.; Williams, C.; Ritchie, S.; Hoffmann, A.A. Integrating Biophysical Models and Evolutionary Theory to Predict Climatic Impacts on Species’ Ranges: The Dengue Mosquito Aedes aegypti in Australia. Funct. Ecol. 2009, 23, 528–538. [Google Scholar] [CrossRef]
- Jia, P.; Lu, L.; Chen, X.; Chen, J.; Guo, L.; Yu, X.; Liu, Q. A Climate-Driven Mechanistic Population Model of Aedes albopictus with Diapause. Parasit. Vectors 2016, 9, 175. [Google Scholar] [CrossRef]
- Pasquali, S.; Mariani, L.; Calvitti, M.; Moretti, R.; Ponti, L.; Chiari, M.; Sperandio, G.; Gilioli, G. Development and Calibration of a Model for the Potential Establishment and Impact of Aedes albopictus in Europe. Acta Trop. 2020, 202, 105228. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Ponti, L.; Neteler, M.; Cure, J.R.; Kenmore, P.E.; Simmons, G. Physiologically Based Demographic Model/GIS Analyses of Thirteen Invasive Species in Africa: Why the Biology Matters. BioRxive 2024. [Google Scholar] [CrossRef]
- Tjaden, N.B.; Caminade, C.; Beierkuhnlein, C.; Thomas, S.M. Mosquito-Borne Diseases: Advances in Modelling Climate-Change Impacts. Trends Parasitol. 2018, 34, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, P.F.; Artigas, P.; Lorenzo-Morales, J.; Bargues, M.D.; Mas-Coma, S. Ecological Niche Modelling Approaches: Challenges and Applications in Vector-Borne Diseases. Trop. Med. 2023, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.P.; Ponti, L.; Gilioli, G. Climate Change Effects on Plant-Pest-Natural Enemy Interactions; Series on Climate Change Impacts, Adaptation, and Mitigation; Imperial College Press: London, UK, 2010; Volume 1, pp. 209–237. ISBN 978-1-84816-655-4. [Google Scholar]
- Dennington, N.L.; Grossman, M.K.; Ware-Gilmore, F.; Teeple, J.L.; Johnson, L.R.; Shocket, M.S.; McGraw, E.A.; Thomas, M.B. Phenotypic Adaptation to Temperature in the Mosquito Vector, Aedes aegypti. Glob. Change Biol. 2024, 30, e17041. [Google Scholar] [CrossRef] [PubMed]
- Paz, S. Climate Change: A Driver of Increasing Vector-Borne Disease Transmission in Non-Endemic Areas. PLoS Med. 2024, 21, e1004382. [Google Scholar] [CrossRef]
- Laporta, G.Z.; Potter, A.M.; Oliveira, J.F.A.; Bourke, B.P.; Pecor, D.B.; Linton, Y.-M. Global Distribution of Aedes aegypti and Aedes albopictus in a Climate Change Scenario of Regional Rivalry. Insects 2023, 14, 49. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Lai, Z.; Zhou, T.; Jia, Z.; Gu, J.; Wu, K.; Chen, X.-G. Temperature Increase Enhances Aedes albopictus Competence to Transmit Dengue Virus. Front. Microbiol. 2017, 8, 2337. [Google Scholar] [CrossRef]
- Anoopkumar, A.N.; Aneesh, E.M. A Critical Assessment of Mosquito Control and the Influence of Climate Change on Mosquito-Borne Disease Epidemics. Environ. Dev. Sustain. 2022, 24, 8900–8929. [Google Scholar] [CrossRef]
- Robert, M.A.; Stewart-Ibarra, A.M.; Estallo, E.L. Climate Change and Viral Emergence: Evidence from Aedes-Borne Arboviruses. Curr. Opin. Virol. 2020, 40, 41–47. [Google Scholar] [CrossRef]
- Liu-Helmersson, J.; Stenlund, H.; Wilder-Smith, A.; Rocklöv, J. Vectorial Capacity of Aedes aegypti: Effects of Temperature and Implications for Global Dengue Epidemic Potential. PLoS ONE 2014, 9, e89783. [Google Scholar] [CrossRef]
- Ross, P.; Hoffmann, A. Continued Susceptibility of the wMel Wolbachia Infection in Aedes aegypti to Heat Stress Following Field Deployment and Selection. Insects 2018, 9, 78. [Google Scholar] [CrossRef]
- Ross, P.A.; Elfekih, S.; Collier, S.; Klein, M.J.; Lee, S.S.; Dunn, M.; Jackson, S.; Zhang, Y.; Axford, J.K.; Gu, X.; et al. Developing Wolbachia-Based Disease Interventions for an Extreme Environment. PLoS Pathog. 2023, 19, e1011117. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Hoffmann, A.A. Limits on Modelling the Thermal Sensitivity of Wolbachia. Nat. Clim. Chang. 2024, 14, 803–804. [Google Scholar] [CrossRef]
- Lau, M.-J.; Ross, P.A.; Endersby-Harshman, N.M.; Hoffmann, A.A. Impacts of Low Temperatures on Wolbachia (Rickettsiales: Rickettsiaceae)-Infected Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Ware-Gilmore, F.; Sgrò, C.M.; Xi, Z.; Dutra, H.L.C.; Jones, M.J.; Shea, K.; Hall, M.D.; Thomas, M.B.; McGraw, E.A. Microbes Increase Thermal Sensitivity in the Mosquito Aedes aegypti, with the Potential to Change Disease Distributions. PLoS Neglected Trop. Dis. 2021, 15, e0009548. [Google Scholar] [CrossRef]
- Tajudeen, Y.A.; Oladunjoye, I.O.; Mustapha, M.O.; Mustapha, S.T.; Ajide-Bamigboye, N.T. Tackling the Global Health Threat of Arboviruses: An Appraisal of the Three Holistic Approaches to Health. Health Promot. Perspect. 2021, 11, 371–381. [Google Scholar] [CrossRef]
- FAO; UNEP; WHO; WOAH. One Health Joint Plan of Action, 2022–2026; FAO; UNEP; WHO; World Organisation for Animal Health (WOAH): Rome, Italy, 2022; ISBN 978-92-5-136957-9. [Google Scholar]
- Balakrishnan, V.S. WHO Launches Global Initiative for Arboviral Diseases. Lancet Microbe 2022, 3, e407. [Google Scholar] [CrossRef]
- WHO. Global Arbovirus Initiative. Available online: https://www.who.int/news-room/events/detail/2022/03/31/default-calendar/global-arbovirus-initiative (accessed on 31 January 2025).
- World Health Organization Eighteenth Meeting of the WHO Vector Control Advisory Group. Available online: https://www.who.int/publications/i/item/9789240077300 (accessed on 5 November 2024).
- Vita, S.; Lalle, E.; Caputi, P.; Faraglia, F.; D’Abramo, A.; Bordi, L.; De Carli, G.; Sberna, G.; Giancola, M.L.; Maffongelli, G.; et al. Dengue Fever as Autochthonous Infectious Disease in Italy: Epidemiological, Clinical and Virological Characteristics. Travel Med. Infect. Dis. 2024, 62, 102762. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control and European Food Safety Authority. Aedes aegypti-Current Known Distribution: May 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/aedes-aegypti-current-known-distribution-may-2024 (accessed on 8 November 2024).
- Dobson, S.L. Reversing Wolbachia-Based Population Replacement. Trends Parasitol. 2003, 19, 128–133. [Google Scholar] [CrossRef]
- Hughes, G.L.; Koga, R.; Xue, P.; Fukatsu, T.; Rasgon, J.L. Wolbachia Infections Are Virulent and Inhibit the Human Malaria Parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011, 7, e1002043. [Google Scholar] [CrossRef]
- Chrostek, E.; Gerth, M. Is Anopheles gambiae a Natural Host of Wolbachia ? mBio 2019, 10, e00784-19. [Google Scholar] [CrossRef]
- Kache, P.A.; Santos-Vega, M.; Stewart-Ibarra, A.M.; Cook, E.M.; Seto, K.C.; Diuk-Wasser, M.A. Bridging Landscape Ecology and Urban Science to Respond to the Rising Threat of Mosquito-Borne Diseases. Nat. Ecol. Evol. 2022, 6, 1601–1616. [Google Scholar] [CrossRef] [PubMed]
- WHO Ending the Neglect to Attain the Sustainable Development Goals: A Rationale for Continued Investment in Tackling Neglected Tropical Diseases 2021–2030. Available online: https://www.who.int/publications/i/item/9789240052932 (accessed on 31 January 2025).
- Gilioli, G.; Groppi, M.; Vesperoni, M.P.; Baumgärtner, J.; Gutierrez, A.P. An Epidemiological Model of East Coast Fever in African Livestock. Ecolo. Model. 2009, 220, 1652–1662. [Google Scholar] [CrossRef]
- Gilioli, G.; Mariani, L. Sensitivity of Anopheles gambiae Population Dynamics to Meteo-Hydrological Variability: A Mechanistic Approach. Malar. J. 2011, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.P.; Gilioli, G.; Baumgärtner, J. Ecosocial Consequences and Policy Implications of Disease Management in East African Agropastoral Systems. Proc. Natl. Acad. Sci. USA 2009, 106, 13136–13141. [Google Scholar] [CrossRef]
- Rasgon, J.L. Using Predictive Models to Optimize Wolbachia-Based Strategies for Vector-Borne Disease Control. Adv. Exp. Med. Biol. 2008, 627, 114–125. [Google Scholar] [CrossRef]
- Pagendam, D.E.; Trewin, B.J.; Snoad, N.; Ritchie, S.A.; Hoffmann, A.A.; Staunton, K.M.; Paton, C.; Beebe, N. Modelling the Wolbachia Incompatible Insect Technique: Strategies for Effective Mosquito Population Elimination. BMC Biol. 2020, 18, 161. [Google Scholar] [CrossRef]
- Matsufuji, T.; Seirin-Lee, S. The Optimal Strategy of Incompatible Insect Technique (IIT) Using Wolbachia and the Application to Malaria Control. J. Theor. Biol. 2023, 569, 111519. [Google Scholar] [CrossRef]
- Cardona-Salgado, D.; Campo-Duarte, D.E.; Sepulveda-Salcedo, L.S.; Vasilieva, O. Wolbachia-Based Biocontrol for Dengue Reduction Using Dynamic Optimization Approach. Appl. Math. Model. 2020, 82, 125–149. [Google Scholar] [CrossRef]
- Dye, D.; Cain, J.W. Efficacy of Wolbachia-Based Mosquito Control: Predictions of a Spatially Discrete Mathematical Model. PLoS ONE 2024, 19, e0297964. [Google Scholar] [CrossRef]
- Bruzzone, O.A.; Utgés, M.E. Analysis of the Invasion of a City by Aedes aegypti via Mathematical Models and Bayesian Statistics. Theor. Ecol. 2022, 15, 65–80. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Ponti, L.; Hoddle, M.; Almeida, R.P.P.; Irvin, N.A. Geographic Distribution and Relative Abundance of the Invasive Glassy-Winged Sharpshooter: Effects of Temperature and Egg Parasitoids. Environ. Entomol. 2011, 40, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.P.; Ponti, L. Prospective Analysis of the Geographic Distribution and Relative Abundance of Asian Citrus Psyllid (Hemiptera: Liviidae) and Citrus Greening Disease in North America and the Mediterranean Basin. Fla. Entomol. 2013, 96, 1375–1391. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Ellis, C.K. Applied Population Ecology: A Supply-Demand Approach; Wiley: New York, NY, USA, 1996; ISBN 978-0-471-13586-9. [Google Scholar]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The Global Distribution of the Arbovirus Vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Toko, M.; Neuenschwander, P.; Yaninek, J.S.; Ortega-Beltran, A.; Fanou, A.; Zinsou, V.; Wydra, K.D.; Hanna, R.; Fotso, A.; Douro-Kpindou, O. Identifying and Managing Plant Health Risks for Key African Crops: Cassava. In Critical Issues in Plant Health: 50 Years of Research in African Agriculture; Burleigh Dodds Series in Agricultural Science; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 139–171. ISBN 978-1-78676-232-0. [Google Scholar]
- Dhandapani, R.K.; Gurusamy, D.; Howell, J.L.; Palli, S.R. Development of CS-TPP-dsRNA Nanoparticles to Enhance RNAi Efficiency in the Yellow Fever Mosquito, Aedes aegypti. Sci. Rep. 2019, 9, 8775. [Google Scholar] [CrossRef]
- Sasmita, H.I.; Ernawan, B.; Sadar, M.; Nasution, I.A.; Indarwatmi, M.; Tu, W.-C.; Neoh, K.-B. Assessment of Packing Density and Transportation Effect on Sterilized Pupae and Adult Aedes aegypti (Diptera: Culicidae) in Non-Chilled Conditions. Acta Trop. 2022, 226, 106243. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Joubert, D.A.; Kaeser, S.; Dowd, C.; Germann, J.; Khalid, A.; Denton, J.A.; Retski, K.; Tavui, A.; Simmons, C.P.; et al. Field Deployment of Wolbachia-Infected Aedes aegypti Using Uncrewed Aerial Vehicle. Sci. Robot. 2024, 9, eadk7913. [Google Scholar] [CrossRef]
- Branda, F.; Cella, E.; Scarpa, F.; Slavov, S.N.; Bevivino, A.; Moretti, R.; Degafu, A.L.; Pecchia, L.; Rizzo, A.; Defilippo, F.; et al. Wolbachia-Based Approaches to Controlling Mosquito-Borne Viral Threats: Innovations, AI Integration, and Future Directions in the Context of Climate Change. Viruses 2024, 16, 1868. [Google Scholar] [CrossRef]
- Sparks Our Goal: Expanding Access to Wolbachia. Available online: https://www.sparking-joy.com/en/about (accessed on 31 January 2025).
- Balatsos, G.; Karras, V.; Puggioli, A.; Balestrino, F.; Bellini, R.; Papachristos, D.P.; Milonas, P.G.; Papadopoulos, N.T.; Malfacini, M.; Carrieri, M.; et al. Sterile Insect Technique (SIT) Field Trial Targeting the Suppression of Aedes albopictus in Greece. Parasite 2024, 31, 17. [Google Scholar] [CrossRef]
- Gato, R.; Menéndez, Z.; Rodríguez, M.; Gutiérrez-Bugallo, G.; Del Carmen Marquetti, M. Advancing the Art of Mosquito Control: The Journey of the Sterile Insect Technique against Aedes aegypti in Cuba. Infect. Dis. Poverty 2024, 13, 61. [Google Scholar] [CrossRef]
- Lees, R.S.; Gilles, J.R.; Hendrichs, J.; Vreysen, M.J.; Bourtzis, K. Back to the Future: The Sterile Insect Technique against Mosquito Disease Vectors. Curr. Opin. Insect Sci. 2015, 10, 156–162. [Google Scholar] [CrossRef]
- Sinkins, S.P.; O’Neill, S.O. Wolbachia as a Vehicle to Modify Insect Populations. In Insect Transgenesis; Handler, A., James, A., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 271–287. ISBN 978-0-8493-2028-6. [Google Scholar]
- Thomas, D.D.; Donnelly, C.A.; Wood, R.J.; Alphey, L.S. Insect Population Control Using a Dominant, Repressible, Lethal Genetic System. Science 2000, 287, 2474–2476. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.F.; McKemey, A.R.; Nimmo, D.; Curtis, Z.; Black, I.; Morgan, S.A.; Oviedo, M.N.; Lacroix, R.; Naish, N.; Morrison, N.I.; et al. Successful Suppression of a Field Mosquito Population by Sustained Release of Engineered Male Mosquitoes. Nat. Biotechnol. 2012, 30, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; McKemey, A.R.; Garziera, L.; Lacroix, R.; Donnelly, C.A.; Alphey, L.; Malavasi, A.; Capurro, M.L. Suppression of a Field Population of Aedes aegypti in Brazil by Sustained Release of Transgenic Male Mosquitoes. PLoS Neglected Trop. Dis. 2015, 9, e0003864. [Google Scholar] [CrossRef]
- Galizi, R.; Doyle, L.A.; Menichelli, M.; Bernardini, F.; Deredec, A.; Burt, A.; Stoddard, B.L.; Windbichler, N.; Crisanti, A. A Synthetic Sex Ratio Distortion System for the Control of the Human Malaria Mosquito. Nat. Commun. 2014, 5, 3977. [Google Scholar] [CrossRef]
- Simoni, A.; Hammond, A.M.; Beaghton, A.K.; Galizi, R.; Taxiarchi, C.; Kyrou, K.; Meacci, D.; Gribble, M.; Morselli, G.; Burt, A.; et al. A Male-Biased Sex-Distorter Gene Drive for the Human Malaria Vector Anopheles gambiae. Nat. Biotechnol. 2020, 38, 1054–1060. [Google Scholar] [CrossRef]
- Facchinelli, L.; North, A.R.; Collins, C.M.; Menichelli, M.; Persampieri, T.; Bucci, A.; Spaccapelo, R.; Crisanti, A.; Benedict, M.Q. Large-Cage Assessment of a Transgenic Sex-Ratio Distortion Strain on Populations of an African Malaria Vector. Parasit. Vectors 2019, 12, 70. [Google Scholar] [CrossRef]
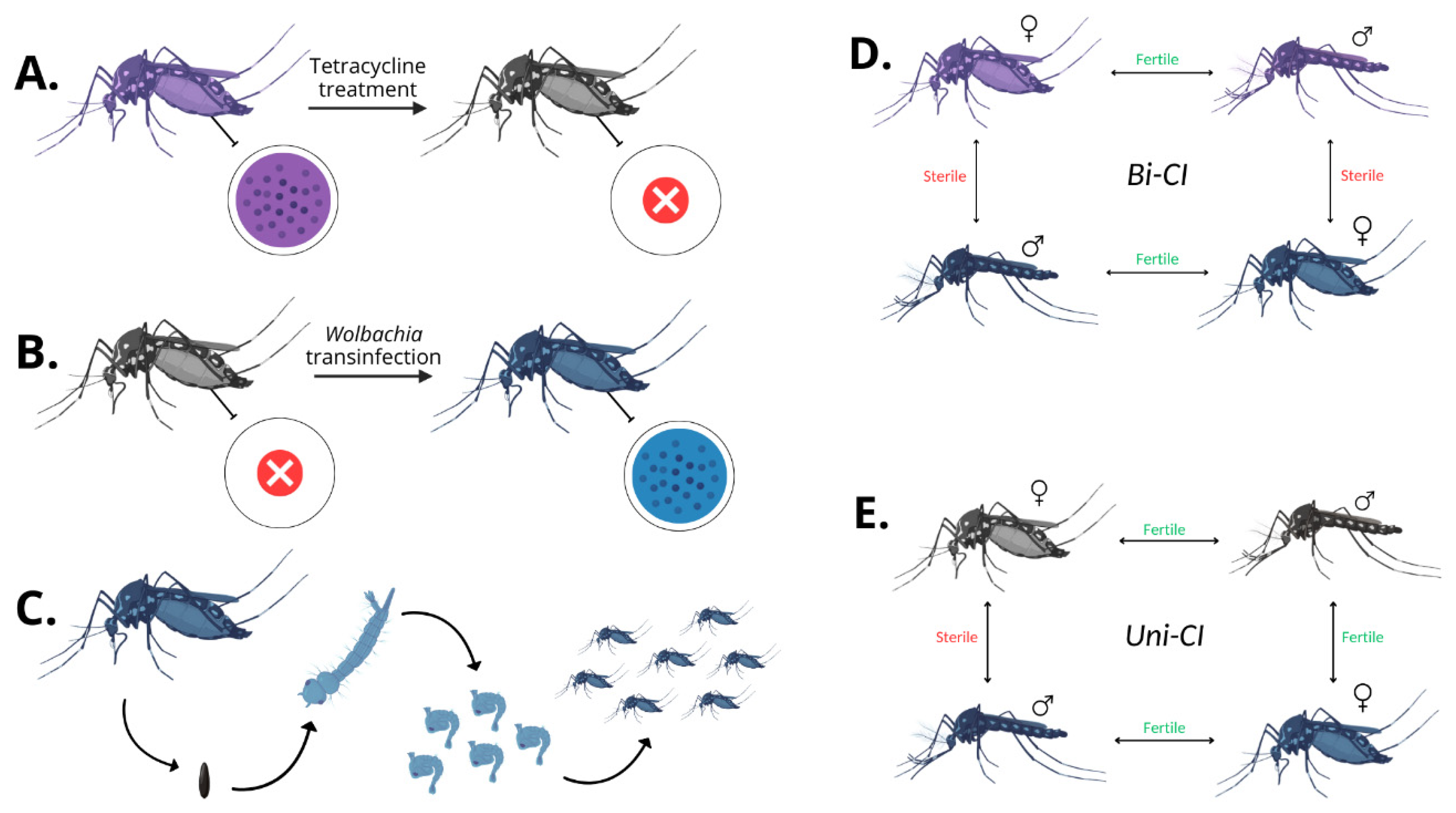


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, R.; Lim, J.T.; Ferreira, A.G.A.; Ponti, L.; Giovanetti, M.; Yi, C.J.; Tewari, P.; Cholvi, M.; Crawford, J.; Gutierrez, A.P.; et al. Exploiting Wolbachia as a Tool for Mosquito-Borne Disease Control: Pursuing Efficacy, Safety, and Sustainability. Pathogens 2025, 14, 285. https://doi.org/10.3390/pathogens14030285
Moretti R, Lim JT, Ferreira AGA, Ponti L, Giovanetti M, Yi CJ, Tewari P, Cholvi M, Crawford J, Gutierrez AP, et al. Exploiting Wolbachia as a Tool for Mosquito-Borne Disease Control: Pursuing Efficacy, Safety, and Sustainability. Pathogens. 2025; 14(3):285. https://doi.org/10.3390/pathogens14030285
Chicago/Turabian StyleMoretti, Riccardo, Jue Tao Lim, Alvaro Gil Araujo Ferreira, Luigi Ponti, Marta Giovanetti, Chow Jo Yi, Pranav Tewari, Maria Cholvi, Jacob Crawford, Andrew Paul Gutierrez, and et al. 2025. "Exploiting Wolbachia as a Tool for Mosquito-Borne Disease Control: Pursuing Efficacy, Safety, and Sustainability" Pathogens 14, no. 3: 285. https://doi.org/10.3390/pathogens14030285
APA StyleMoretti, R., Lim, J. T., Ferreira, A. G. A., Ponti, L., Giovanetti, M., Yi, C. J., Tewari, P., Cholvi, M., Crawford, J., Gutierrez, A. P., Dobson, S. L., & Ross, P. A. (2025). Exploiting Wolbachia as a Tool for Mosquito-Borne Disease Control: Pursuing Efficacy, Safety, and Sustainability. Pathogens, 14(3), 285. https://doi.org/10.3390/pathogens14030285









