Molecular Characterization and Antimicrobial Resistance Evaluation of Listeria monocytogenes Strains from Food and Human Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. L. monocytogenes from Food Samples
2.2. L. monocytogenes from Clinical Samples
2.3. Molecular Investigations, Bioinformatic and Cluster Analyses
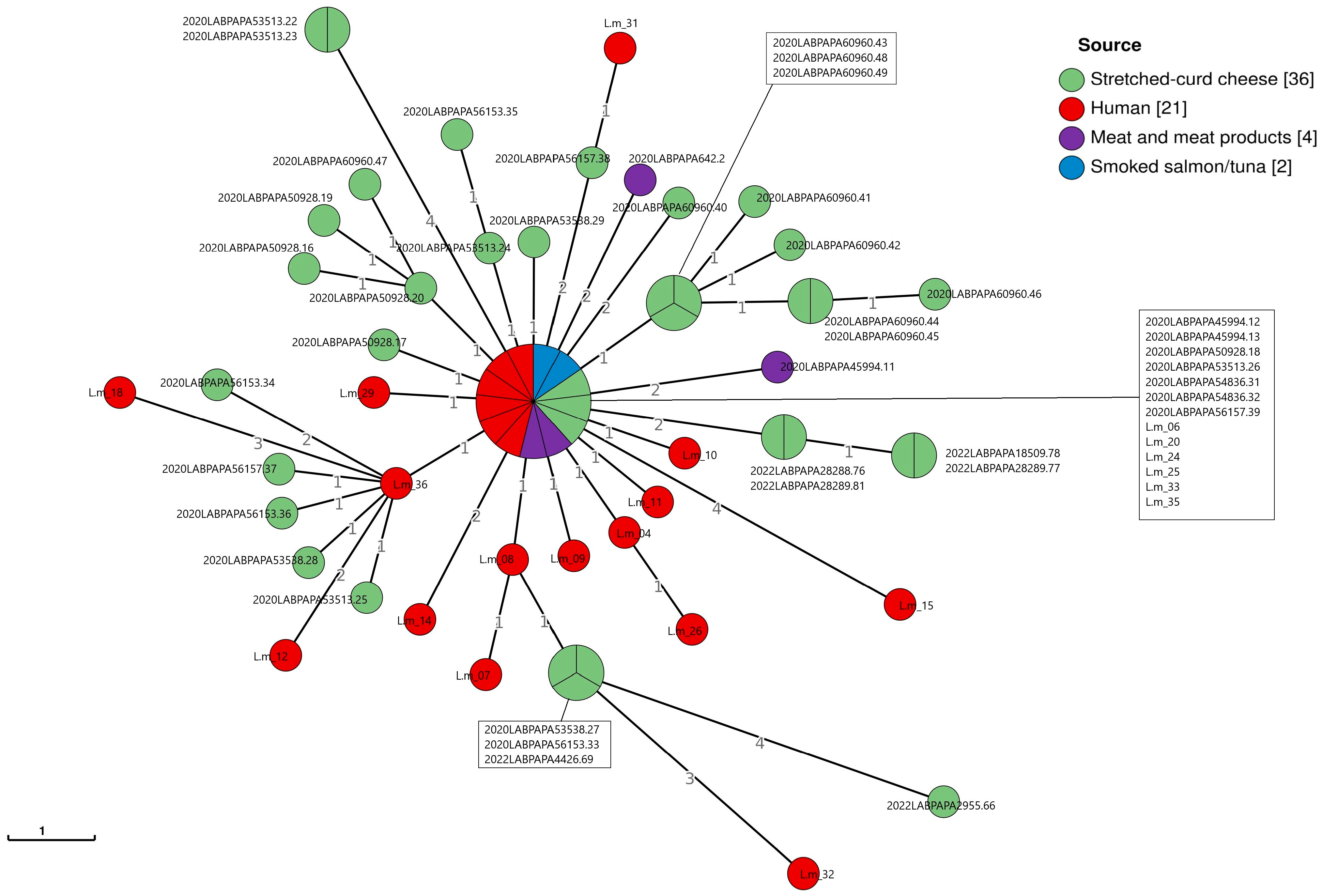
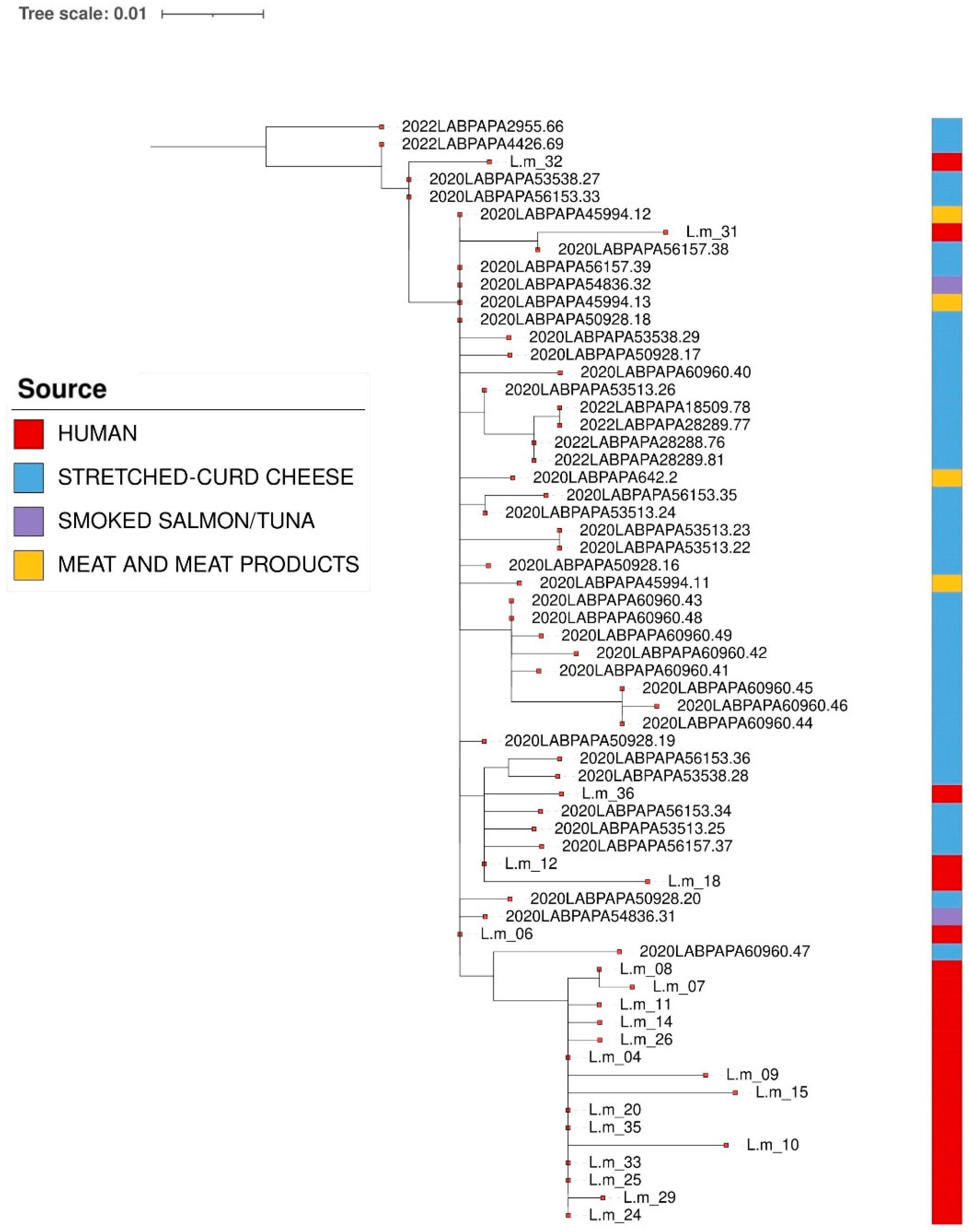
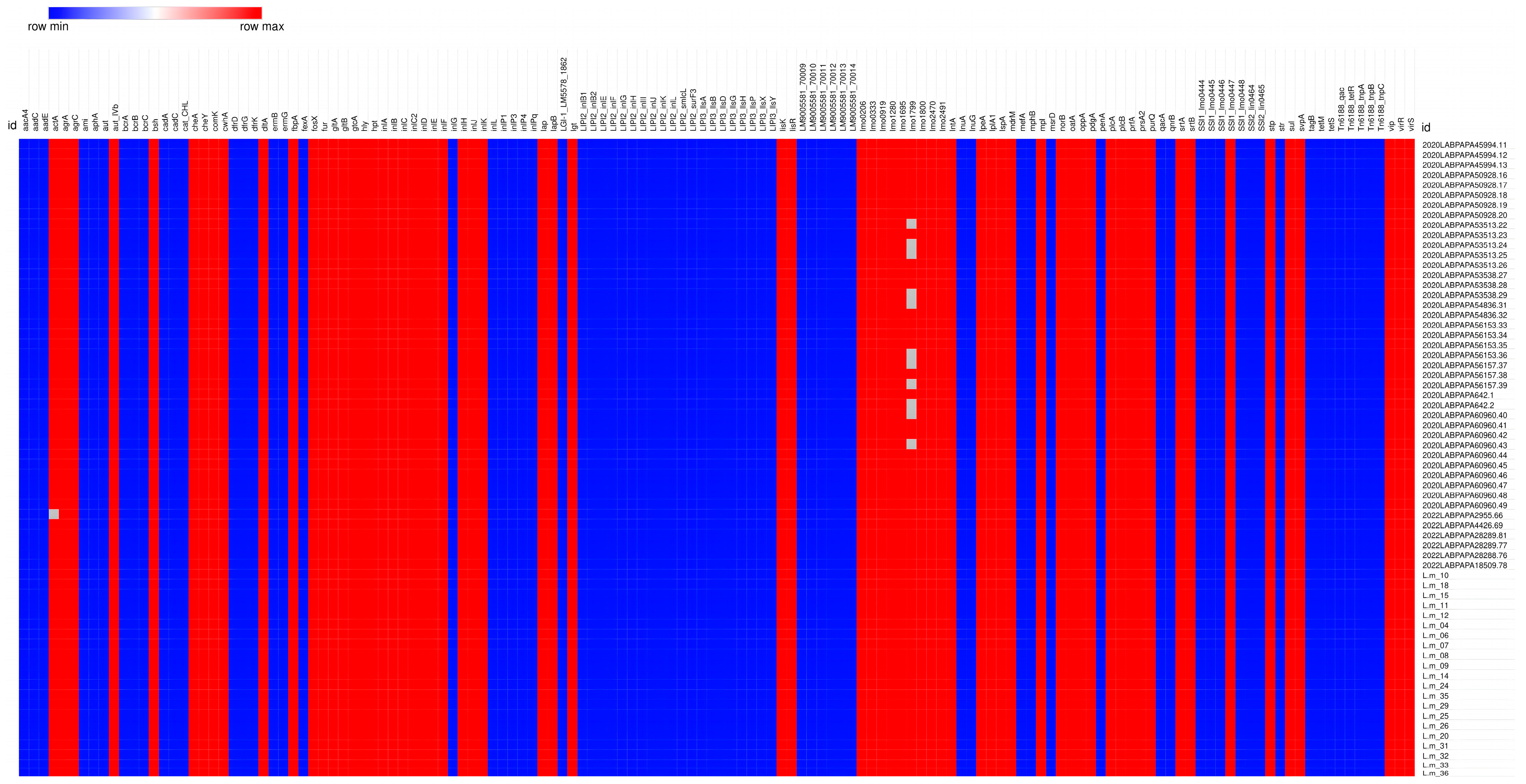
3. Results
3.1. L. monocytogenes from Food Samples
3.2. L. monocytogenes from Clinical Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amagliani, G.; Blasi, G.; Scuota, S.; Duranti, A.; Fisichella, S.; Gattuso, A.; Gianfranceschi, M.V.; Schiavano, G.F.; Brandi, G.; Pomilio, F.; et al. Detection and Virulence Characterization of Listeria monocytogenes Strains in Ready-to-Eat Products. Foodborne Pathog. Dis. 2021, 18, 675–682. [Google Scholar] [CrossRef]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes Persistence in Food-Associated Environments: Epidemiology, Strain Characteristics, and Implications for Public Health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernandez-Escamez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; et al. Scientific Opinion on the Listeria monocytogenes Contamination of Ready-to-Eat Foods and the Risk for Human Health in the EU. EFSA J. 2018, 16, 5134. [Google Scholar] [CrossRef]
- Lundén, J.; Autio, T.; Markkula, A.; Hellström, S.; Korkeala, H. Adaptive and Cross-Adaptive Responses of Persistent and Non-Persistent Listeria monocytogenes Strains to Disinfectants. Int. J. Food Microbiol. 2003, 82, 265–272. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2023 Zoonoses Report. EFS2 2024, 22, e9106. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Threats and Outbreaks of Listeriosis. 2024. Available online: https://www.ecdc.europa.eu/en/listeriosis/threats-and-outbreaks (accessed on 24 August 2024).
- Eurostat (European Commission). Population Structure and Ageing. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=population_structure_and_ageing (accessed on 9 September 2024).
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Koutsoumanis, K.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. The Public Health Risk Posed by Listeria monocytogenes in Frozen Fruit and Vegetables Including Herbs, Blanched during Processing. EFSA J. 2020, 18, 6092. [Google Scholar] [CrossRef]
- Quereda, J.J.; Morón-García, A.; Palacios-Gorba, C.; Dessaux, C.; García-del Portillo, F.; Pucciarelli, M.G.; Ortega, A.D. Pathogenicity and Virulence of Listeria monocytogenes: A Trip from Environmental to Medical Microbiology. Virulence 2021, 12, 2509–2545. [Google Scholar] [CrossRef]
- Weindl, L.; Frank, E.; Ullrich, U.; Heurich, M.; Kleta, S.; Ellerbroek, L.; Gareis, M. Listeria monocytogenes in Different Specimens from Healthy Red Deer and Wild Boars. Foodborne Pathog. Dis. 2016, 13, 391–397. [Google Scholar] [CrossRef]
- Hellström, S.; Kiviniemi, K.; Autio, T.; Korkeala, H. Listeria monocytogenes Is Common in Wild Birds in Helsinki Region and Genotypes Are Frequently Similar with Those Found along the Food Chain. J. Appl. Microbiol. 2008, 104, 883–888. [Google Scholar] [CrossRef]
- Gu, Y.; Liang, X.; Huang, Z.; Yang, Y. Outbreak of Listeria monocytogenes in Pheasants. Poult. Sci. 2015, 94, 2905–2908. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, J.; Ma, L.; Li, L.; Zhang, W.; Li, J. Identification of Fish Source Vibrio alginolyticus and Evaluation of Its Bacterial Ghosts Vaccine Immune Effects. MicrobiologyOpen 2018, 7, e00576. [Google Scholar] [CrossRef]
- Iwu, C.D.; Okoh, A.I. Characterization of Antibiogram Fingerprints in Listeria monocytogenes Recovered from Irrigation Water and Agricultural Soil Samples. PLoS ONE 2020, 15, e0228956. [Google Scholar] [CrossRef] [PubMed]
- Schoder, D.; Schmalwieser, A.; Szakmary-Brändle, K.; Stessl, B.; Wagner, M. Urban Prevalence of Listeria spp. and Listeria monocytogenes in Public Lavatories and on Shoe Soles of Facility Patrons in the European Capital City Vienna. Zoonoses Public Health 2015, 62, 179–186. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). Collaboration Agreement on the Management and Sharing of Molecular Typing Data of Isolates from Human, Food, Feed, Animal, and the Related En-Vironment for Public Health Purposes. 2022. Available online: https://www.efsa.europa.eu/sites/default/files/2022-06/collaboration-agreement-molecular-typing-EFSA-ECDC-WGS-DataCollection.pdf (accessed on 23 September 2024).
- ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. ISO: Geneva, Switzerland, 2017.
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Available online: https://www.eucast.org/ (accessed on 22 October 2024).
- CLSI. Performance Standards for Antimicrobial Susceptibility: Supplement M100, 31st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021; ISBN 978-1-68440-105-5. [Google Scholar]
- Andriyanov, P.A.; Zhurilov, P.A.; Liskova, E.A.; Karpova, T.I.; Sokolova, E.V.; Yushina, Y.K.; Zaiko, E.V.; Bataeva, D.S.; Voronina, O.L.; Psareva, E.K.; et al. Antimicrobial Resistance of Listeria monocytogenes Strains Isolated from Humans, Animals, and Food Products in Russia in 1950–1980, 2000–2005, and 2018–2021. Antibiotics 2021, 10, 1206. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, V.; Gomes, J.P.; Coelho, A.; Batista, R.; Saraiva, C.; Esteves, A.; Martins, Â.; Contente, D.; Diaz-Formoso, L.; et al. Listeria monocytogenes from Food Products and Food Associated Environments: Antimicrobial Resistance, Genetic Clustering and Biofilm Insights. Antibiotics 2024, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Torresi, M.; Ruolo, A.; Acciari, V.A.; Ancora, M.; Blasi, G.; Cammà, C.; Centorame, P.; Centorotola, G.; Curini, V.; Guidi, F.; et al. A Real-Time PCR Screening Assay for Rapid Detection of Listeria monocytogenes Outbreak Strains. Foods 2020, 9, 67. [Google Scholar] [CrossRef]
- Chiarini, A.; Palmeri, A.; Amato, T.; Immordino, R.; Distefano, S.; Giammanco, A. Detection of Bacterial and Yeast Species with the Bactec 9120 Automated System with Routine Use of Aerobic, Anaerobic, and Fungal Media. J. Clin. Microbiol. 2008, 46, 4029–4033. [Google Scholar] [CrossRef]
- D’agostino, M.; Wagner, M.; Vazquez-Boland, J.A.; Kuchta, T.; Karpiskova, R.; Hoorfar, J.; Novella, S.; Scortti, M.; Ellison, J.; Murray, A.; et al. A Validated PCR-Based Method to Detect Listeria monocytogenes Using Raw Milk as a Food Model—Towards an International Standard. J. Food Prot. 2004, 67, 1646–1655. [Google Scholar] [CrossRef]
- Kérouanton, A.; Marault, M.; Petit, L.; Grout, J.; Dao, T.T.; Brisabois, A. Evaluation of a Multiplex PCR Assay as an Alternative Method for Listeria monocytogenes Serotyping. J. Microbiol. Methods 2010, 80, 134–137. [Google Scholar] [CrossRef]
- Doumith, M.; Jacquet, C.; Gerner-Smidt, P.; Graves, L.M.; Loncarevic, S.; Mathisen, T.; Morvan, A.; Salcedo, C.; Torpdahl, M.; Vazquez, J.A.; et al. Multicenter Validation of a Multiplex PCR Assay for Differentiating the Major Listeria monocytogenes Serovars 1/2a, 1/2b, 1/2c, and 4b: Toward an International Standard. J. Food Prot. 2005, 68, 2648–2650. [Google Scholar] [CrossRef]
- Centorotola, G.; Ziba, M.W.; Cornacchia, A.; Chiaverini, A.; Torresi, M.; Guidi, F.; Cammà, C.; Bowa, B.; Mtonga, S.; Magambwa, P.; et al. Listeria monocytogenes in Ready to Eat Meat Products from Zambia: Phenotypical and Genomic Characterization of Isolates. Front. Microbiol. 2023, 14, 1228726. [Google Scholar] [CrossRef]
- ISO 23418:2022; Microbiology of the Food Chain-Whole Genome Sequencing for Typing and Genomic Characterization of Bacteria-General Requirements and Guidance. ISO: Geneva, Switzerland, 2022.
- Moura, A.; Criscuolo, A.; Pouseele, H.; Maury, M.M.; Leclercq, A.; Tarr, C.; Björkman, J.T.; Dallman, T.; Reimer, A.; Enouf, V.; et al. Whole Genome-Based Population Biology and Epidemiological Surveillance of Listeria monocytogenes. Nat. Microbiol. 2016, 2, 16185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Alikhan, N.-F.; Sergeant, M.J.; Luhmann, N.; Vaz, C.; Francisco, A.P.; Carriço, J.A.; Achtman, M. GrapeTree: Visualization of Core Genomic Relationships among 100,000 Bacterial Pathogens. Genome Res. 2018, 28, 1395–1404. [Google Scholar] [CrossRef]
- Davis, S.; Pettengill, J.B.; Luo, Y.; Payne, J.; Shpuntoff, A.; Rand, H.; Strain, E. CFSAN SNP Pipeline: An Automated Method for Constructing SNP Matrices from next-Generation Sequence Data. PeerJ Comput. Sci. 2015, 1, e20. [Google Scholar] [CrossRef]
- Tricoli, M.R.; Massaro, C.; Arrigo, I.; Diquattro, O.; Di Bernardo, F.; Galia, E.; Palermo, M.; Fasciana, T.; Giammanco, A. Characterization of Listeria monocytogenes Strains Isolated in Palermo (Sicily and Italy) during the Years 2018–2020 from Severe Cases of Listeriosis. Antibiotics 2024, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Maury, M.M.; Tsai, Y.-H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria monocytogenes Hypervirulence by Harnessing Its Biodiversity. Nat. Genet. 2016, 48, 308–313. [Google Scholar] [CrossRef]
- Vázquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Domínguez-Bernal, G.; Goebel, W.; González-Zorn, B.; Wehland, J.; Kreft, J. Listeria Pathogenesis and Molecular Virulence Determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef]
- Avila-Novoa, M.G.; González-Torres, B.; González-Gómez, J.P.; Guerrero-Medina, P.J.; Martínez-Chávez, L.; Martínez-Gonzáles, N.E.; Chaidez, C.; Gutiérrez-Lomelí, M. Genomic Insights into Listeria monocytogenes: Organic Acid Interventions for Biofilm Prevention and Control. Int. J. Mol. Sci. 2023, 24, 13108. [Google Scholar] [CrossRef]
- Lee, S.; Parsons, C.; Chen, Y.; Dungan, R.S.; Kathariou, S. Contrasting Genetic Diversity of Listeria Pathogenicity Islands 3 and 4 Harbored by Nonpathogenic Listeria spp. Appl. Environ. Microbiol. 2023, 89, e02097-22. [Google Scholar] [CrossRef]
- Parsons, C.; Lee, S.; Jayeola, V.; Kathariou, S. Novel Cadmium Resistance Determinant in Listeria monocytogenes. Appl. Environ. Microbiol. 2017, 83, e02580-16. [Google Scholar] [CrossRef]
- Lebrun, M.; Audurier, A.; Cossart, P. Plasmid-Borne Cadmium Resistance Genes in Listeria monocytogenes Are Present on Tn5422, a Novel Transposon Closely Related to Tn917. J. Bacteriol. 1994, 176, 3049–3061. [Google Scholar] [CrossRef] [PubMed]
- Dutta, V.; Elhanafi, D.; Kathariou, S. Conservation and Distribution of the Benzalkonium Chloride Resistance Cassette bcrABC in Listeria monocytogenes. Appl. Environ. Microbiol. 2013, 79, 6067–6074. [Google Scholar] [CrossRef]
- Ryan, S.; Begley, M.; Hill, C.; Gahan, C.G.M. A Five-Gene Stress Survival Islet (SSI-1) That Contributes to the Growth of Listeria monocytogenes in Suboptimal Conditions: Stress Survival Islet in L. monocytogenes. J. Appl. Microbiol. 2010, 109, 984–995. [Google Scholar] [CrossRef]
- Goh, Y.-X.; Anupoju, S.M.B.; Nguyen, A.; Zhang, H.; Ponder, M.; Krometis, L.-A.; Pruden, A.; Liao, J. Evidence of Horizontal Gene Transfer and Environmental Selection Impacting Antibiotic Resistance Evolution in Soil-Dwelling Listeria. Nat. Commun. 2024, 15, 10034. [Google Scholar] [CrossRef]
- Chesneau, O.; Ligeret, H.; Hosan-Aghaie, N.; Morvan, A.; Dassa, E. Molecular Analysis of Resistance to Streptogramin A Compounds Conferred by the Vga Proteins of Staphylococci. Antimicrob. Agents Chemother. 2005, 49, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.epicentro.iss.it/listeria/ (accessed on 2 November 2023).
- Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 22 November 2023).
- Lachtara, B.; Wieczorek, K.; Osek, J. Antimicrobial Resistance of Listeria monocytogenes Serogroups IIa and IVb from Food and Food-Production Environments in Poland. J. Vet. Res. 2023, 67, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Tirloni, E.; Centorotola, G.; Pomilio, F.; Torresi, M.; Bernardi, C.; Stella, S. Listeria monocytogenes in Ready-to-Eat (RTE) Delicatessen Foods: Prevalence, Genomic Characterization of Isolates and Growth Potential. Int. J. Food Microbiol. 2024, 410, 110515. [Google Scholar] [CrossRef]
- Kayode, A.J.; Okoh, A.I. Assessment of Multidrug-Resistant Listeria monocytogenes in Milk and Milk Product and One Health Perspective. PLoS ONE 2022, 17, e0270993. [Google Scholar] [CrossRef]
- Dos Reis, J.O.; Vieira, B.S.; Cunha Neto, A.; Castro, V.S.; Figueiredo, E.E.D.S. Antimicrobial Resistance of Listeria monocytogenes from Animal Foods to First- and Second-Line Drugs in the Treatment of Listeriosis from 2008 to 2021: A Systematic Review and Meta-Analysis. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 1351983. [Google Scholar] [CrossRef]
- Truong, H.; Garmyn, D.; Gal, L.; Fournier, C.; Sevellec, Y.; Jeandroz, S.; Piveteau, P. Plants as a Realized Niche for Listeria monocytogenes. Microbiol. Open 2021, 10, e1255. [Google Scholar] [CrossRef]
- Ramos, B.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Biopreservation Approaches to Reduce Listeria monocytogenes in Fresh Vegetables. Food Microbiol. 2020, 85, 103282. [Google Scholar] [CrossRef] [PubMed]
- Wartha, S.; Bretschneider, N.; Dangel, A.; Hobmaier, B.; Hörmansdorfer, S.; Huber, I.; Murr, L.; Pavlovic, M.; Sprenger, A.; Wenning, M.; et al. Genetic Characterization of Listeria from Food of Non-Animal Origin Products and from Producing and Processing Companies in Bavaria, Germany. Foods 2023, 12, 1120. [Google Scholar] [CrossRef] [PubMed]
- Segerman, B.; Skarin, H.; Ástvaldsson, Á. Guidance Document for Cluster Analysis of Whole Genome Sequence Data; Inter EURLs Working Group on NGS (NEXT GENERATION SEQUENCING): Bilthoven, The Netherlands, 2024. [Google Scholar]
- Alvarez-Molina, A.; Cobo-Díaz, J.F.; López, M.; Prieto, M.; De Toro, M.; Alvarez-Ordóñez, A. Unraveling the Emergence and Population Diversity of Listeria monocytogenes in a Newly Built Meat Facility through Whole Genome Sequencing. Int. J. Food Microbiol. 2021, 340, 109043. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Baltar, A.; Pérez-Boto, D.; Medina, M.; Montiel, R. Genomic Diversity and Characterization of Listeria monocytogenes from Dry-Cured Ham Processing Plants. Food Microbiol. 2021, 99, 103779. [Google Scholar] [CrossRef]
- Moura, A.; Leclercq, A.; Vales, G.; Tessaud-Rita, N.; Bracq-Dieye, H.; Thouvenot, P.; Madec, Y.; Charlier, C.; Lecuit, M. Phenotypic and Genotypic Antimicrobial Resistance of Listeria monocytogenes: An Observational Study in France. Lancet Reg. Health—Eur. 2024, 37, 100800. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Zakrzewski, A.J.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Antimicrobial Resistance and Virulence Characterization of Listeria monocytogenes Strains Isolated from Food and Food Processing Environments. Pathogens 2022, 11, 1099. [Google Scholar] [CrossRef]
- Noll, M.; Kleta, S.; Al Dahouk, S. Antibiotic Susceptibility of 259 Listeria monocytogenes Strains Isolated from Food, Food-Processing Plants and Human Samples in Germany. J. Infect. Public Health 2018, 11, 572–577. [Google Scholar] [CrossRef]
- Baquero, F.; Lanza, V.F.; Duval, M.; Coque, T.M. Ecogenetics of Antibiotic Resistance in Listeria monocytogenes. Mol. Microbiol. 2020, 113, 570–579. [Google Scholar] [CrossRef]
- Allen, K.J.; Wałecka-Zacharska, E.; Chen, J.C.; Katarzyna, K.-P.; Devlieghere, F.; Van Meervenne, E.; Osek, J.; Wieczorek, K.; Bania, J. Listeria monocytogenes—An Examination of Food Chain Factors Potentially Contributing to Antimicrobial Resistance. Food Microbiol. 2016, 54, 178–189. [Google Scholar] [CrossRef]
- Srinivasan, V.; Nam, H.M.; Nguyen, L.T.; Tamilselvam, B.; Murinda, S.E.; Oliver, S.P. Prevalence of Antimicrobial Resistance Genes in Listeria monocytogenes Isolated from Dairy Farms. Foodborne Pathog. Dis. 2005, 2, 201–211. [Google Scholar] [CrossRef]
- Morvan, A.; Moubareck, C.; Leclercq, A.; Hervé-Bazin, M.; Bremont, S.; Lecuit, M.; Courvalin, P.; Le Monnier, A. Antimicrobial Resistance of Listeria monocytogenes Strains Isolated from Humans in France. Antimicrob. Agents Chemother. 2010, 54, 2728–2731. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh, S.; Pourmand, M.R.; Hasanvand, S.; Pirjani, R.; Afshar, D.; Noori, M.; Soltan Dallal, M.M. Antimicrobial Susceptibility, Serotyping, and Molecular Characterization of Antibiotic Resistance Genes in Listeria monocytogenes Isolated from Pregnant Women with a History of Abortion. Iran. J. Public Health 2021, 50, 170–179. [Google Scholar] [CrossRef] [PubMed]


| Determination | Target Gene | Amplicon Size | References |
|---|---|---|---|
| Species L. monocytogenes | prfA | 274 bp | [24,25] |
| Serogroup | lmo0737 | 691 bp | [26] |
| Serogroup | lmo1118 | 906 bp | [26] |
| Serogroup | ORF2819 | 471 bp | [26] |
| Serogroup | ORF2110 | 597 bp | [26] |
| Type of Food | No. of Isolates | Year | Serogroup | Clonal Complex (CC) | Sequence Type (ST) | |
|---|---|---|---|---|---|---|
| Dairy products | Ricotta cheese/ ricotta cream | 5 1 | 2020 | IIa | CC199 | ST199 |
| 2 1 | 2020 | IVb | CC6 | ST6 | ||
| Stretched-curd cheese | 31 2 | 2020 | IVb | CC2 | ST2 | |
| 3 2 | 2022 | IVb | CC2 | ST2 | ||
| 2 | 2022 | IVb | CC2 | ST2 | ||
| 1 | 2022 | IIa | CC204 | ST204 | ||
| Meat and meat products | 1 | 2020 | IVb | CC2 | ST67 | |
| 4 | 2020 | IVb | CC2 | ST2 | ||
| 1 | 2020 | IIa | CC14 | ST14 | ||
| 2 | 2020 | IIa | CC155 | ST155 | ||
| 1 | 2020 | IVb | CC6 | ST6 | ||
| 1 | 2021 | IIc | CC9 | ST9 | ||
| 2 | 2021 | IVb | CC1 | ST1 | ||
| 2 | 2021 | IIa | CC475 | ST504 | ||
| 1 | 2022 | IIc | CC9 | ST763 | ||
| 2 | 2022 | IIa | CC121 | ST121 | ||
| 1 | 2022 | IIa | CC14 | ST399 | ||
| 3 | 2022 | IIa | CC8 | ST8 | ||
| Fish products | seafood salad | 3 | 2020 | IIa | CC8 | ST8 |
| 1 | 2021 | |||||
| 1 | 2020 | IIb | CC426 | ST426 | ||
| 2 | 2022 | |||||
| 1 | 2023 | |||||
| 1 | 2022 | IIa | CC121 | ST121 | ||
| smoked salmon/ tuna | 2 | 2020 | IIb | CC3 | ST3 | |
| 1 | 2020 | IIa | CC7 | ST7 | ||
| 1 | 2021 | |||||
| 2 | 2020 | IVb | CC2 | ST2 | ||
| 1 | 2022 | IIb | CC87 | ST87 | ||
| 1 | 2022 | IIa | CC155 | ST155 | ||
| Vegetables | 3 | 2020 | IVb | CC1 | ST1 | |
| 1 | 2023 | |||||
| 1 | 2022 | IVb | CC6 | ST6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castello, A.; Alio, V.; Torresi, M.; Centorotola, G.; Chiaverini, A.; Pomilio, F.; Arrigo, I.; Giammanco, A.; Fasciana, T.; Ortoffi, M.F.; et al. Molecular Characterization and Antimicrobial Resistance Evaluation of Listeria monocytogenes Strains from Food and Human Samples. Pathogens 2025, 14, 294. https://doi.org/10.3390/pathogens14030294
Castello A, Alio V, Torresi M, Centorotola G, Chiaverini A, Pomilio F, Arrigo I, Giammanco A, Fasciana T, Ortoffi MF, et al. Molecular Characterization and Antimicrobial Resistance Evaluation of Listeria monocytogenes Strains from Food and Human Samples. Pathogens. 2025; 14(3):294. https://doi.org/10.3390/pathogens14030294
Chicago/Turabian StyleCastello, Annamaria, Vincenzina Alio, Marina Torresi, Gabriella Centorotola, Alexandra Chiaverini, Francesco Pomilio, Ignazio Arrigo, Anna Giammanco, Teresa Fasciana, Marco Francesco Ortoffi, and et al. 2025. "Molecular Characterization and Antimicrobial Resistance Evaluation of Listeria monocytogenes Strains from Food and Human Samples" Pathogens 14, no. 3: 294. https://doi.org/10.3390/pathogens14030294
APA StyleCastello, A., Alio, V., Torresi, M., Centorotola, G., Chiaverini, A., Pomilio, F., Arrigo, I., Giammanco, A., Fasciana, T., Ortoffi, M. F., Gattuso, A., Oliveri, G., Cardamone, C., & Costa, A. (2025). Molecular Characterization and Antimicrobial Resistance Evaluation of Listeria monocytogenes Strains from Food and Human Samples. Pathogens, 14(3), 294. https://doi.org/10.3390/pathogens14030294






