Albaconazole Polymeric Nanocapsules for Treating Trypanosoma cruzi Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
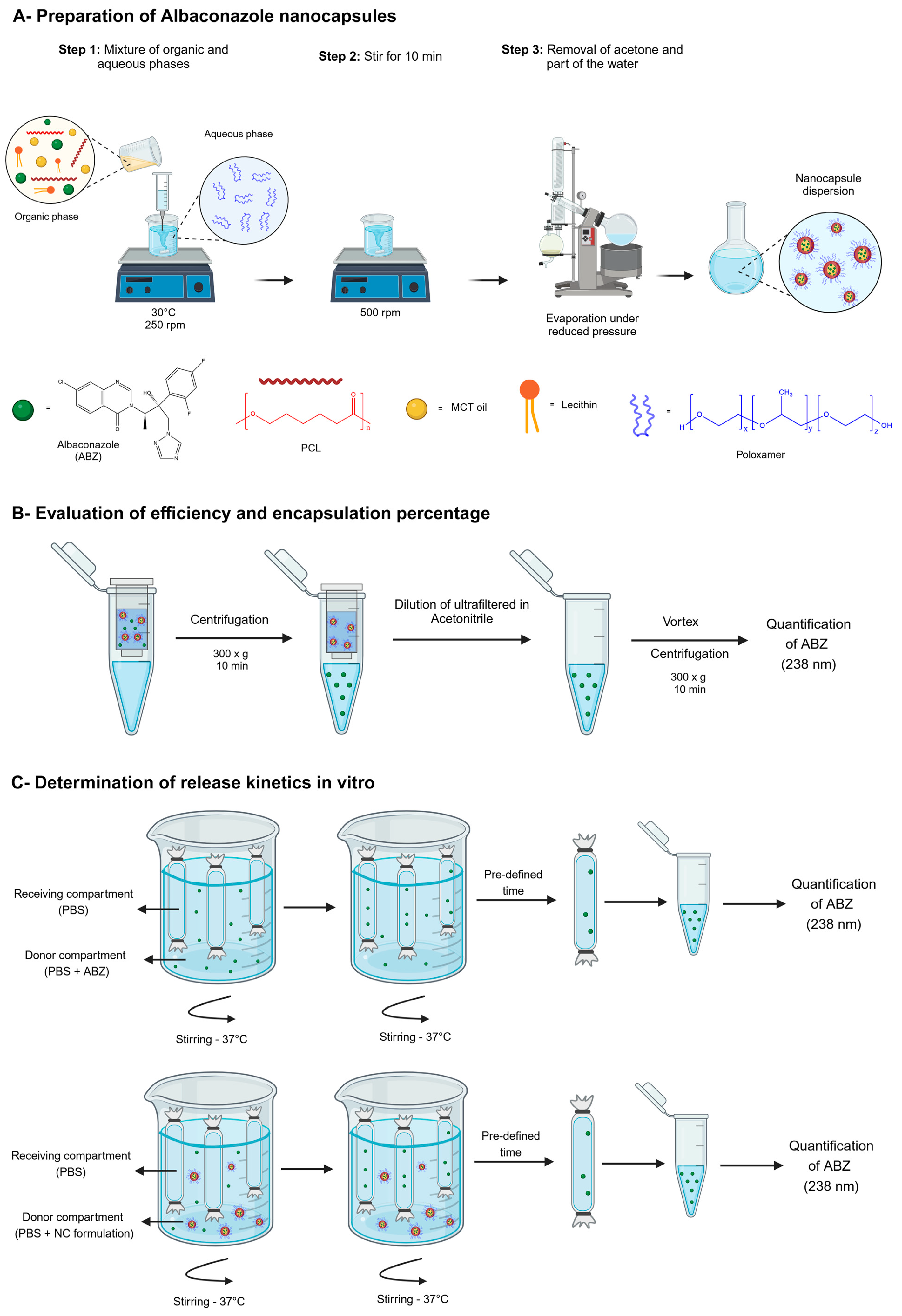
2.2. Preparation of ABZ Nanocapsules and Solutions
2.3. ABZ Quantification by Ultraviolet Spectrometry
2.4. Polymeric Nanocapsule Characterization
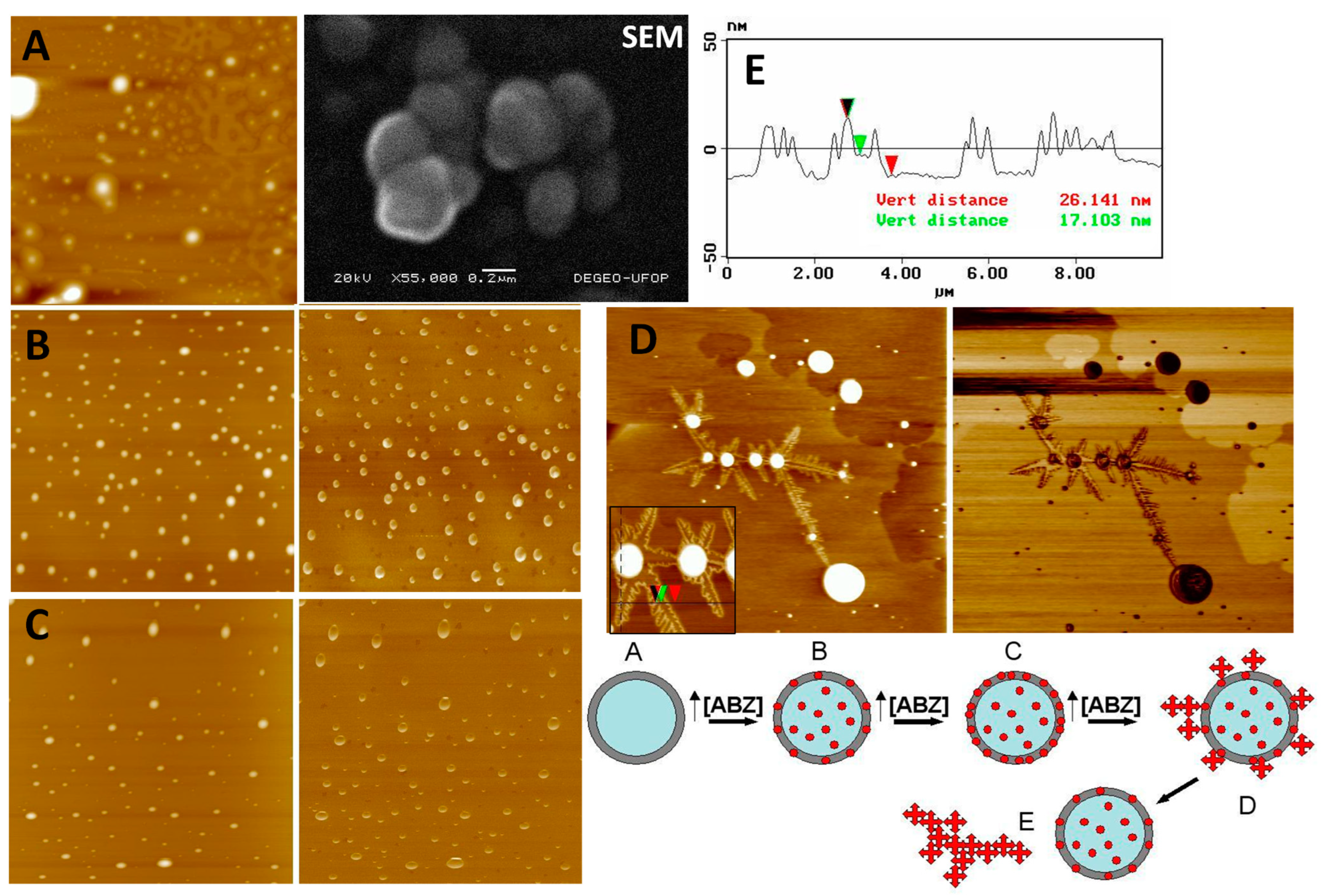
2.5. Atomic Force Microscopy and Scanning Electron Microscopy Analysis
2.6. Evaluation of Efficiency and Encapsulation Percentages
2.7. Determination of ABZ Release In Vitro
2.8. In Vitro Activity of Albaconazole Nanocapsules (Epimastigote Inhibition Assay)
2.9. Trypanosoma cruzi Strain and Evolutionary Forms
2.10. Experimental Animals and Ethics
2.11. Maximal Tolerated Albaconazole Dose in Healthy Mice
2.12. Efficacy Evaluation in the Acute Phase in Infected Mice
2.13. Treatment
2.14. Statistical Analysis
3. Results
3.1. Nanocapsule Characterization
3.2. Albaconazole Release Profiles
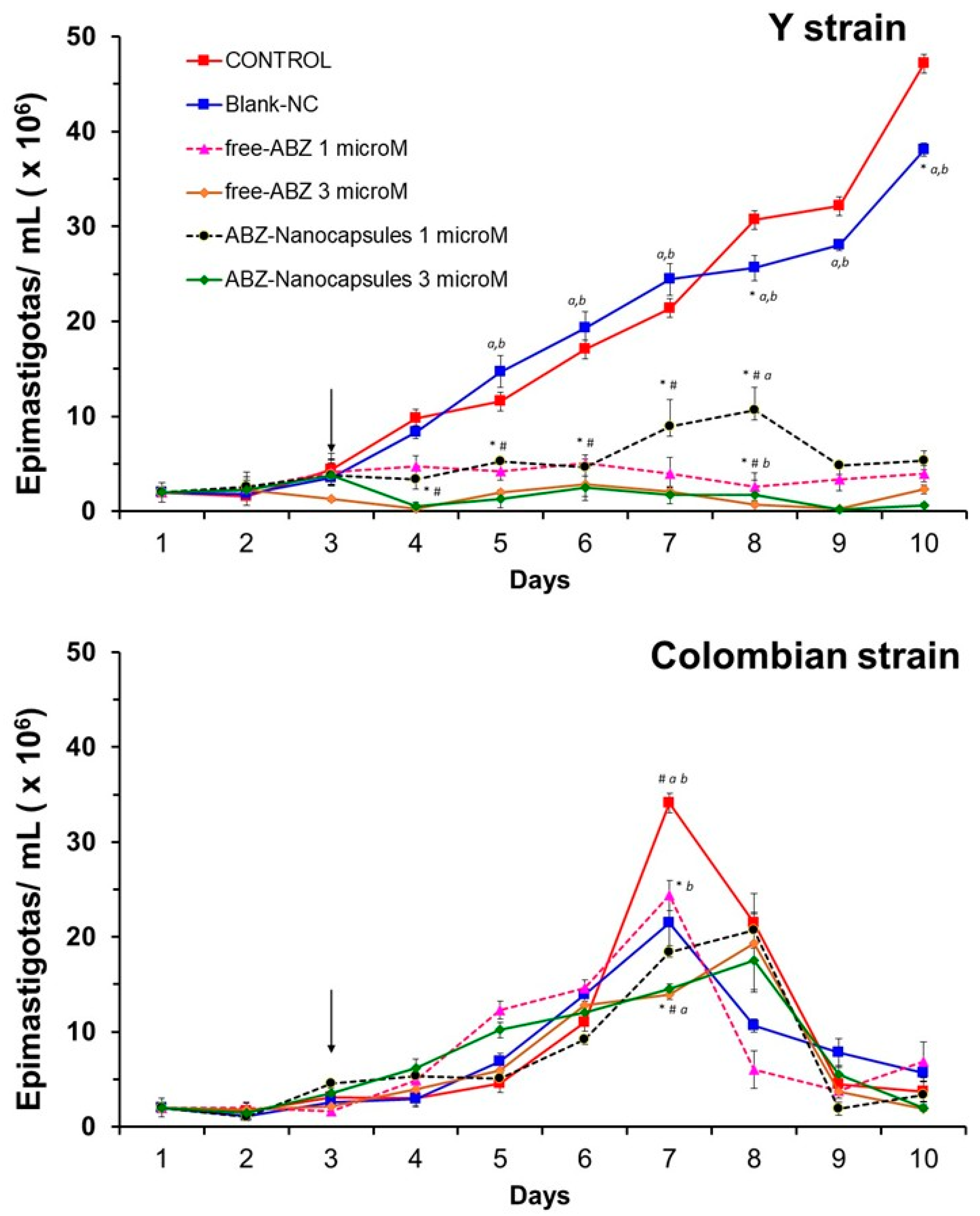
3.3. Albaconazole Activity In Vitro Against T. cruzi Epimastigotes
3.4. General Toxicity Evaluation by Intravenous Route
3.5. In Vivo Efficacy in Infected Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PAHO/WHO—Pan American Health Organization. Chagas Disease in the Americas for Public Health Workers-PAHO/WHO. 2022. Available online: https://www.who.int/campaigns/world-chagas-disease-day/2022 (accessed on 10 January 2025).
- PAHO/WHO. PAHO/WHO: Guidelines for the Diagnosis and Treatment of Chagas Disease; PAHO/WHO: Washington, DC, USA, 2019; ISBN 978-92-75-12043-9. [Google Scholar]
- Brindley, P.J.; Hotez, P.J.; Kamhawi, S. Revisiting What Constitutes a Neglected Tropical Disease? PLoS Negl. Trop. Dis. 2025, 19, e0012794. [Google Scholar] [CrossRef] [PubMed]
- Torchelsen, F.K.V.D.S.; Mazzeti, A.L.; Mosqueira, V.C.F. Drugs in Preclinical and Early Clinical Development for the Treatment of Chagas’s Disease: The Current Status. Expert Opin. Investig. Drugs 2024, 33, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Cançado, J.R. Long term evaluation of etiological treatment of chagas disease with benznidazole. Rev. Inst. Med. Trop. São Paulo 2002, 44, 29–37. [Google Scholar] [CrossRef]
- Andrade, A.L.S.S.; Martelli, C.M.T.; Oliveira, R.M.; Silva, S.A.; Aires, A.I.S.; Soussumi, L.M.T.; Covas, D.T.; Silva, L.S.; Andrade, J.G.; Travassos, L.R.; et al. Short report: Benznidazole efficacy among Trypanosoma cruzi-infected adolescents after a six-year follow-up. Am. J. Trop. Med. Hyg. 2004, 71, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Sosa Estani, S.; Segura, E.L. Treatment of Trypanosoma cruzi infection in the undetermined phase. Experience and current guidelines of treatment in Argentina. Mem. Inst. Oswaldo Cruz 1999, 94, 363–365. [Google Scholar] [CrossRef]
- Yun, O.; Lima, M.A.; Ellman, T.; Chambi, W.; Castillo, S.; Flevaud, L.; Roddy, P.; Parreno, F.; Albajar, V.P.; Palma, P.P. Feasibility, drug safety, and effectiveness of etiological treatment programs for chagas disease in honduras, guatemala, and bolivia: 10-year experience of medecins sans frontieres. PLoS Negl. Trop. Dis. 2009, 3, e488. [Google Scholar] [CrossRef] [PubMed]
- Viotti, R.; Vigliano, C.; Armenti, H.; Segura, E. Treatment of chronic Chagas disease with benznidazole: Clinical and serological evolution of patients with long-term follow-up. Am. Heart J. 1994, 127, 151–162. [Google Scholar] [CrossRef]
- Duschak, V.G. Targets and patented drugs for chemotherapy of Chagas disease in the last 15 years-period. Recent Pat. Anti-Infect. Drug Discov. 2016, 11, 74–173. [Google Scholar] [CrossRef]
- Buckner, F.S.; Urbina, J.A. Recent developments in sterol 14-demethylase inhibitors for Chagas disease. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 236–242. [Google Scholar] [CrossRef]
- Bartrolí, J.; Turmo, E.; Algueró, M.; Boncompte, E.; Vericat, M.L.; Conte, L.; Ramis, J.; Merlos, M.; García-Rafanell, J.; Forn, J. New azole antifungals. 3. Synthesis and antifungal activity of 3-substituted-4(3H)-quinazolinones. J. Med. Chem. 1998, 41, 1869–1882. [Google Scholar] [CrossRef]
- Sorbera, L.A.; Bartroli, J. Castañer. Drugs Future 2003, 28, 529–537. [Google Scholar]
- Seyedmousavi, S.; Rafati, H.; Ilkit, M.; Tolooe, A.; Hedayati, M.T.; Verweij, P. Systemic Antifungal Agents: Current Status and Projected Future Developments. In Human Fungal Pathogen Identification; Methods in Molecular Biology; Lion, T., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1508. [Google Scholar] [CrossRef]
- Bartroli, J.; Merlos, M.; Sisniega, H. Overview of albaconazole. Eur. Infect. Dis. 2011, 5, 88–91. [Google Scholar]
- Guillon, R.; Pagniez, F.; Picot, C.; Hédou, D.; Tonnerre, A.; Chosson, E.; Duflos, M.; Besson, T.; Logé, C.; Le Pape, P. Discovery of a Novel Broad-Spectrum Antifungal Agent Derived from Albaconazole. ACS Med. Chem. Lett. 2013, 4, 288–292. [Google Scholar] [CrossRef]
- Dietz, A.J.; Barnard, J.C.; van Rossem, K. A randomized, double-blind, multiple-dose, placebo-controlled, dose escalation study with a 3-cohort parallel group design to investigate the tolerability and pharmacokinetics of albaconazole in healthy subjects. Clin. Pharmacol. Drug Dev. 2014, 3, 25–33. [Google Scholar] [CrossRef] [PubMed]
- van Rossem, K.; Lowe, J.A. A Phase I, randomized, open-label crossover study to evaluate the safety and pharmacokinetics of 400 mg albaconazole administered to healthy participants as a tablet formulation versus a capsule formulation. Clin. Pharmacol. Adv. Appl. 2013, 5, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sigurgeirsson, B.; van Rossem, K.; Malahias, S.; Raterink, K. A phase II, randomized, double-blind, placebo-controlled, parallel group, dose-ranging study to investigate the efficacy and safety of 4 dose regimens of oral albaconazole in patients with distal subungual onychomycosis. J. Am. Acad. Dermatol. 2013, 69, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Schell, W.A.; Wills, E.A.; Toffaletti, D.L.; Boyce, M.; Benjamin, D.K., Jr.; Bartroli, J.; Perfect, J.R. In vitro and in vivo efficacies of the new triazole albaconazole against Cryptococcus neoformans. Antimicrob. Agents Chemother. 2004, 48, 384–387. [Google Scholar] [CrossRef]
- Capilla, J.; Ortoneda, M.; Pastor, F.J.; Guarro, J. In vitro antifungal activities of the new triazole UR-9825 against clinically important filamentous fungi. Antimicrob. Agents Chemother. 2001, 45, 2635–2637. [Google Scholar] [CrossRef]
- Urbina, J.A.; Lira, R.; Visbal, G.; Bartroli, J. In vitro antiproliferative effects and mechanism of action of the new triazole derivative UR-9825 against the protozoan parasite Trypanosoma (Schizotrypanum) cruzi. Antimicrob. Agents Chemother. 2000, 44, 2498–2502. [Google Scholar] [CrossRef]
- Guedes, P.M.; Urbina, J.A.; Lana, M.; Afonso, L.C.; Veloso, V.M.; Tafuri, W.L.; Machado-Coelho, G.L.; Chiari, E.; Bahia, M.T. Activity of the new triazole derivative albaconazole against Trypanosoma (Schizotrypanum) cruzi in dog hosts. Antimicrob. Agents Chemother. 2004, 48, 4286–4292. [Google Scholar] [CrossRef]
- Girmenia, C. New generation azole antifungals in clinical investigation. Exp. Opin. Investig. Drugs 2009, 18, 1279–1295. [Google Scholar] [CrossRef] [PubMed]
- Aperis, G.; Mylonakis, G. Newer triazole antifungal agents: Pharmacology, spectrum, clinical efficacy and limitations. Expert Opin. Investig. Drugs 2006, 15, 579–602. [Google Scholar] [CrossRef] [PubMed]
- Pasqualotto, A.C.; Thiele, K.O.; Goldani, L.Z. Novel triazole antifungal drugs: Focus on isavuconazole, ravuconazole and albaconazole. Curr. Opin. Investig. Drugs 2010, 11, 165–174. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20112166 (accessed on 10 December 2024).
- Fávero, M.L.D.; Bonetti, A.F.; Domingos, E.L.; Tonin, F.S.; Pontarolo, R. Oral antifungal therapies for toenail onychomycosis: A systematic review with network meta-analysis toenail mycosis: Network meta-analysis. J. Dermatol. Treat. 2022, 33, 121–130. [Google Scholar] [CrossRef]
- Bartroli, X.; Uriach, J. A clinical multicenter study comparing efficacy and tolerability between five single oral doses of albaconazole and fluconazole 150 mg single dose in acute vulvovaginal candidiasis. In Proceedings of the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy: Abs M-722, Washington, DC, USA, 16–19 December 2005. [Google Scholar]
- Girmenia, C.; Finolezzi, E. New-generation triazole antifungal drugs: Review of the Phase II and III trials. Clin. Investig. 2011, 1, 1577–1594. [Google Scholar] [CrossRef]
- Branquinho, R.T.; Roy, J.; Farah, C.; Garcia, G.M.; Aimond, F.; Le Guennec, J.-Y.; Saude-Guimarães, D.A.; Grabe-Guimaraes, A.; Mosqueira, V.C.F.; de Lana, M.; et al. Biodegradable Polymeric Nanocapsules Prevent Cardiotoxicity of Anti-Trypanosomal Lychnopholide. Sci. Rep. 2017, 7, 44998. [Google Scholar] [CrossRef]
- Branquinho, R.T.; Pound-Lana, G.; Marques Milagre, M.; Saúde-Guimarães, D.A.; Vilela, J.M.C.; Spangler Andrade, M.; de Lana, M.; Mosqueira, V.C.F. Increased Body Exposure to New Anti-Trypanosomal Through Nanoencapsulation. Sci. Rep. 2017, 7, 8429. [Google Scholar] [CrossRef]
- Branquinho, R.T.; de Mello, C.G.C.; Oliveira, M.T.; Reis, L.E.S.; de Vieira, P.M.A.; Saúde-Guimarães, D.A.; Mosqueira, V.C.F.; de Lana, M. Lychnopholide in Poly(d,l-Lactide)- block -Polyethylene Glycol Nanocapsules Cures Infection with a Drug-Resistant Trypanosoma cruzi Strain at Acute and Chronic Phases. Antimicrob. Agents Chemother. 2020, 64, 1–13. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Mazzeti, A.L.; Bahia, M.T. Chapter 9—Nanomedicines against Chagas disease. In Applications of Nanobiotechnology for Neglected Tropical Diseases, 1st ed.; Formiga, F.R., Inamuddin, Severino, P., Eds.; Academic Press: London, UK, 2021; pp. 169–189. [Google Scholar] [CrossRef]
- Siqueira, R.P.; Milagre, M.M.; de Oliveira, M.A.; Branquinho, R.T.; Torchelsen, F.K.V.; de Lana, M.; Machado, M.G.C.; Andrade, M.S.; Bahia, M.T.; Mosqueira, V.C.F. In vitro interaction of polyethylene glycol-block-poly(D,L-lactide) nanocapsule devices with host cardiomyoblasts and Trypanosoma cruzi-infective forms. Parasitol. Res. 2022, 121, 2861–2874. [Google Scholar] [CrossRef]
- Okamoto, J.; Fukunami, M.; Kioka, H. Frequent premature ventricular contractions induced by itraconazole. Circ. J. 2007, 71, 1323–1325. [Google Scholar] [CrossRef]
- Philips, J.A.; Marty, F.M.; Stone, R.M.; Koplan, B.A.; Katz, J.T.; Baden, L.R. Torsades de pointes associated with voriconazole use. Transpl. Infect. Dis. 2007, 9, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Tholakanahalli, V.N.; Potti, A.; Hanley, J.F.; Merliss, A.D. Fluconazole-induced torsade de pointes. Ann. Pharmacother. 2001, 35, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Leite, E.A.; Grabe-Guimarães, A.; Guimarães, H.N.; Machado-Coelho, G.L.L.; Barratt, G.; Mosqueira, V.C.F. Cardiotoxicity reduction induced by halofantrine entrapped in nanocapsule devices. Life Sci. 2007, 80, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Moreira Souza, A.C.; Grabe-Guimarães, A.; Cruz, J.D.S.; Santos-Miranda, A.; Farah, C.; Teixeira Oliveira, L.; Lucas, A.; Aimond, F.; Sicard, P.; Mosqueira, V.C.F.; et al. Mechanisms of artemether toxicity on single cardiomyocytes and protective effect of nanoencapsulation. Br. J. Pharmacol. 2020, 177, 4448–4463. [Google Scholar] [CrossRef]
- de Sousa, D.R.T.; de Oliveira Guerra, J.A.; Ortiz, J.V.; do Nascimento Couceiro, K.; da Silva e Silva, M.R.H.; Jorge Brandão, A.R.; Guevara, E.; Arcanjo, A.R.L.; de Oliveira Júnior, E.F.; Smith-Doria, S.; et al. Acute Chagas disease associated with ingestion of contaminated food in Brazilian western Amazon. Trop. Med. Int. Health 2023, 28, 541–550. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- ANVISA; National Health Surveillance Agency Ministry of Health, BRAZIL. Resolution RDC n° 166, of July 24, 2017. Provides Criteria for the Validation of Analytical Methods. Official Gazette of the Federative Republic of Brazil, Brasília, DF, Section 1 25 July. 2017. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2017/rdc0166_24_07_2017.pdf (accessed on 20 July 2018).
- Santos-Magalhaes, N.S.; Fessi, H.; Puisieux, F.; Benita, S.; Seiller, M. In vitro release kinetic examination and comparative evaluation between submicron emulsion and polylactic acid nanocapsules of clofibride. J. Microencapsul. 1995, 12, 195–205. [Google Scholar] [CrossRef]
- Zingales, B.; Andrade, S.; Briones, M.; Campbell, D.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.; Ma-chado, C.; et al. A New Consensus for Trypanosoma cruzi Intraspecific Nomenclature: Second Revision Meeting Recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz 2009, 104, 1051–1054. [Google Scholar] [CrossRef]
- Zingales, B.; Miles, M.A.; Moraes, C.B.; Luquetti, A.; Guhl, F.; Schijman, A.G.; Ribeiro, I. Drug discovery for chagas disease should consider Trypanosoma cruzi strain diversity. Mem. Inst. Oswaldo Cruz 2014, 109, 828–833. [Google Scholar] [CrossRef]
- Filardi, L.S.; Brener, Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas’ disease. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 755–759. [Google Scholar] [CrossRef]
- Brener, Z. Therapeutic activity and criterion of cure in mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo 1962, 4, 89–396. [Google Scholar]
- Romanha, A.J.; Castro, S.L.; De Soeiro, M.D.N.C.; Lannes-Vieira, J.; Ribeiro, I.; Talvani, A.; Bourdin, B.; Blum, B.; Olivieri, B.; Zani, C.; et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem. Inst. Oswaldo Cruz 2010, 105, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Chiari, E.; Dias, J.C.P.; Lana, M.; Chiari, C.A. Hemocultures for the parasitological diagnosis of human chronic Chagas’ disease. Rev. Soc. Bras. Med. Trop. 1989, 22, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Leite, E.A.; Vilela, J.M.C.; Mosqueira, V.C.F.; Andrade, M.S. Poly-Caprolactone Nanocapsules Morphological Features by Atomic Force Microscopy. Microsc. Microanal. 2005, 11 (Suppl. S3), 48–51. [Google Scholar] [CrossRef]
- Veloso, V.M.; Carneiro, C.M.; Toledo, M.J.O.; Lana, M.; Chiari, E.; Tafuri, W.L.; Bahia, M.T. Variation in Susceptibility to Benznidazole in Isolates Derived from Trypanosoma cruzi Parental Strains. Mem. Inst. Oswaldo Cruz 2001, 96, 1005–1011. [Google Scholar] [CrossRef]
- Cao, X.; Sun, Z.; Cao, Y.; Wang, R.; Cai, T.; Chu, W.; Hu, W.; Yang, Y. Design, Synthesis, and Structure-Activity Relationship Studies of Novel Fused Heterocycles-Linked Triazoles with Good Activity and Water Solubility. J. Med. Chem. 2014, 57, 3687–3706. [Google Scholar] [CrossRef]
- Torrico, F.; Gascon, J.; Ortiz, L.; Alonso-Vega, C.; Pinazo, M.-J.; Schijman, A.; Almeida, I.C.; Alves, F.; Strub-Wourgaft, N.; Ribeiro, I. E1224 Study Group. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: A proof-of-concept, randomised, placebo-controlled trial. Lancet Infect. Dis. 2018, 18, 419–430. [Google Scholar] [CrossRef]
- Bartroli, J.; Turmo, E.; Algueró, M.; Boncompte, E.; Vericat, M.L.; Conte, L.; Ramis, J.; García-Rafanell, J.; Forn, J. UR-9825: A New Triazole Derivative with Potent, Broad-Spectrum Antifungal Activity. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), 1997, abstr. E-67, September, Toronto, Canada. American Soc. for Microbiology, Washington, DC. Available online: https://search.worldcat.org/pt/title/633698851 (accessed on 20 July 2018).
- Trindade, I.C.; Pound-Lana, G.; Perera, D.G.S.; De Oliveira, L.A.M.; Andrade, M.S.; Vilela, J.M.C.; Postacchini, B.B.; Mosqueira, V.C.F. Mechanisms of interaction of biodegradable polyester nanocapsules with non-phagocytic cells. Eur. J. Pharm. Sci. 2018, 124, 89–104. [Google Scholar] [CrossRef]
- Chen, C.K.; Leung, S.S.; Guilbert, C.; Jacobson, M.P.; McKerrow, J.H.; Podust, L.M. Structural Characterization of CYP51 from Trypanosoma cruzi and Trypanosoma brucei Bound to the Antifungal Drugs Posaconazole and Fluconazole. PLoS Negl. Trop. Dis. 2010, 4, e651. [Google Scholar] [CrossRef]
- Dumoulin, P.; Vollrath, J.; Tomko, S.S.; Burleigh, B. Glutamine metabolism modulates azole susceptibility in Trypanosoma cruzi amastigotes. eLife 2020, 9, e60226. [Google Scholar] [CrossRef]
- Souza, A.C.M.; Mosqueira, V.C.F.; Silveira, A.P.A.; Antunes, L.R.; Richard, S.; Guimarães, H.N.; Grabe-Guimarães, A. Reduced cardiotoxicity and increased oral efficacy of artemether polymeric nanocapsules in Plasmodium berghei-infected mice. Parasitology 2018, 145, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Mosqueira, V.C.F.; Loiseau, P.M.; Bories, C.; Legrand, P.; Devissaguet, J.-P.; Barratt, G. Efficacy and Pharmacokinetics of Intravenous Nanocapsule Formulations of Halofantrine in Plasmodium berghei-Infected Mice. Antimicrob. Agents Chemother. 2004, 48, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Mosqueira, V.C.F.; Legrand, P.; Barratt, G. Surface-Modified and Conventional Nanocapsules as Novel Formulations for Parenteral Delivery of Halofantrine. J. Nanosci. Nanotechnol. 2006, 6, 3193–3202. [Google Scholar] [CrossRef] [PubMed]
- Tarlenton, R.L. Parasite persistence in the aetiology of Chagas disease. Int. J. Parasitol. 2001, 31, 550–554. [Google Scholar] [CrossRef]


| Formulation | ABZ mg/mL | Hydrodynamic Diameter ± SD 2 (nm) | PDI 3 | AFM 4 Mean Size ± DP 1 (nm) | Zeta Potential ± SD (mV) * | pH ± SD 2 |
|---|---|---|---|---|---|---|
| Blank NCs | 0 | 171.4 ± 0.8 | 0.127 ± 0.022 | 336 ± 144 | −50.1 ± 1.2 | 6.69 ± 0.01 |
| 0.5 NCs | 0.5 * | 201.5 ± 0.4 | 0.096 ± 0.019 | 195 ± 54 | −49.2 ± 3.4 | 6.44 ± 0.08 |
| 1 NCs | 1.0 * | 225.9 ± 2.1 | 0.193 ± 0.022 | - | −59.5 ± 5.1 | 7.02 ± 0.04 |
| 5 NCs | 5.0 5 | 155.9 ± 0.3 | 0.130 ± 0.004 | 250 ± 57 | −50.5 ± 3.0 | 7.10 ± 0.20 |
| ABZ Formulation | ABZ mg/mL (Feed) | ABZ in NCs (mg/mL ± SD 2) (Real) | EE 1 | % Drug Loading * | Payload # (µg/mg) |
|---|---|---|---|---|---|
| 0.5 NCs | 0.5 | 0.36 ± 0.18 | 71.4 ± 4.1 | 99.79± 0.38 | 7.83 |
| 1 NCs | 1.0 | 1.17± 0.03 | 117 ± 3.6 | 94.27± 0.15 | 25.43 |
| 5 NCs | 5.0 | 1.39 ± 0.32 | 27.9± 1.9 * | 94.18± 0.32 | 30.22 * |
| Evaluated Parameters | |||||
|---|---|---|---|---|---|
| Dose | Ataxia | Respiratory Changes | Convulsion | Survival * | |
| Free ABZ (intravenous solution) 1 | 25 mg/kg | - | - | - | 10/10 |
| 30 mg/kg | +++ | +++ | ++ | 5/8 | |
| 35 mg/kg | - | - | ++ | 2/10 | |
| 40 mg/kg | - | - | +++ | 0/9 | |
| ABZ NCs 2 | 80 mg/kg | - | - | - | 10/10 |
| 120 mg/kg | ++ | + | - | 10/10 | |
| 200 mg/kg | ++ | + | - | 8/8 | |
| 500 mg/kg | +++ | +++ | - | 5/5 | |
| Dose (mg/kg/Day) | Via | Survival |
MST 1 (Days) | Patent Period (Days ± SD 2) |
Maximal Parasitemia Level (×1000) ± SD 2 |
Negative Parasitological Tests 3 | |||
|---|---|---|---|---|---|---|---|---|---|
| During ttm. | After ttm. | d 90 | d 120 | ||||||
| Control (untreated) | SC | 0/10 | 11.4 ± 1.3 | 8.3 ± 1.5 | 1418 ± 969 | - | - | - | |
| Blank-NCs | SC | 0/10 | 10.2 ± 7.6 | 7.2 ± 7.6 | 1427 ± 3082 | - | - | - | |
| DMA/PEG 300 solution | SC | 0/10 | 8.4 ± 4.9 | 5.4 ± 4.9 | 1976 ± 420 | - | - | - | |
| Benznidazole (100 mg/kg/day) | PO | 10/10 | >60 | 0 | 0.5 ± 0 * | nd | 10/10 | 10/10 | |
| Free ABZ | 20 | PO | 10/10 | >60 * | 30 ± 4.8 # | 27 ± 29 # * | 7.5 ± 4.3 | - | - |
| 20 | SC | 10/10 | >60 * | 9.8 ± 12.4 # | 0.5 ± 1.6 * | 0.5 ± 1.7 | - | - | |
| 80 | SC | 7/10 | >60 4 * | 1.4 ± 0.8 # | 0 * | 0 | 3/7 # | 0/7 # | |
| 120 | SC | 0/10 | 4.7 ± 1.6 # | 1 ± 0.0 | 0 * | nd | - | - | |
| ABZ-NCs | 20 | IM | 9/10 | >60 5 * | 27.1 ± 3.1 # | 318.5 ± 558.5 # | 9.0 ± 11.5 | - | - |
| 20 | SC | 8/10 | >60 6 * | 30.1 ± 6.1 # | 103.0 ± 104.5 # | 12.7 ± 16.9 ** | - | - | |
| 40 (2 × 20) | SC | 9/10 | >60 7 * | 16.5 ± 14.6 # | 3.3 ± 5.6 * | 3.9 ± 8.6 | - | - | |
| 40 | SC | 10/10 | >60 * | 22.6 ± 11.9 # | 0.5 ± 1.6 * | 2.5 ± 3.5 | - | - | |
| 80 | SC | 10/10 | >60 * | 22.4 ± 12.8 # | 4.0 ± 12.6 # * | 2.0 ± 2.6 | 2/10 # | 2/10 # | |
| 120 | SC | 10/10 | >60 ** | 1.1 ± 0.3 | 0 * | 0 ** | 6/10 # ** | 6/10 # ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Barros, C.M.; Veloso, V.M.; Andrade, M.S.; Vilela, J.M.C.; de Oliveira, M.A.; de Lana, M.; Bahia, M.T.; Mosqueira, V.C.F. Albaconazole Polymeric Nanocapsules for Treating Trypanosoma cruzi Infections. Pathogens 2025, 14, 319. https://doi.org/10.3390/pathogens14040319
de Barros CM, Veloso VM, Andrade MS, Vilela JMC, de Oliveira MA, de Lana M, Bahia MT, Mosqueira VCF. Albaconazole Polymeric Nanocapsules for Treating Trypanosoma cruzi Infections. Pathogens. 2025; 14(4):319. https://doi.org/10.3390/pathogens14040319
Chicago/Turabian Stylede Barros, Cristina Maria, Vanja Maria Veloso, Margareth Spangler Andrade, José Mário Carneiro Vilela, Maria Alice de Oliveira, Marta de Lana, Maria Terezinha Bahia, and Vanessa Carla Furtado Mosqueira. 2025. "Albaconazole Polymeric Nanocapsules for Treating Trypanosoma cruzi Infections" Pathogens 14, no. 4: 319. https://doi.org/10.3390/pathogens14040319
APA Stylede Barros, C. M., Veloso, V. M., Andrade, M. S., Vilela, J. M. C., de Oliveira, M. A., de Lana, M., Bahia, M. T., & Mosqueira, V. C. F. (2025). Albaconazole Polymeric Nanocapsules for Treating Trypanosoma cruzi Infections. Pathogens, 14(4), 319. https://doi.org/10.3390/pathogens14040319









