Characterization of the Gut Microbiota in Patients with Psoriasis: A Systematic Review
Abstract
1. Introduction
2. Methods and Materials
2.1. Inclusion/Exclusion Criteria
2.2. Study Selection
2.3. Data Extraction
3. Results
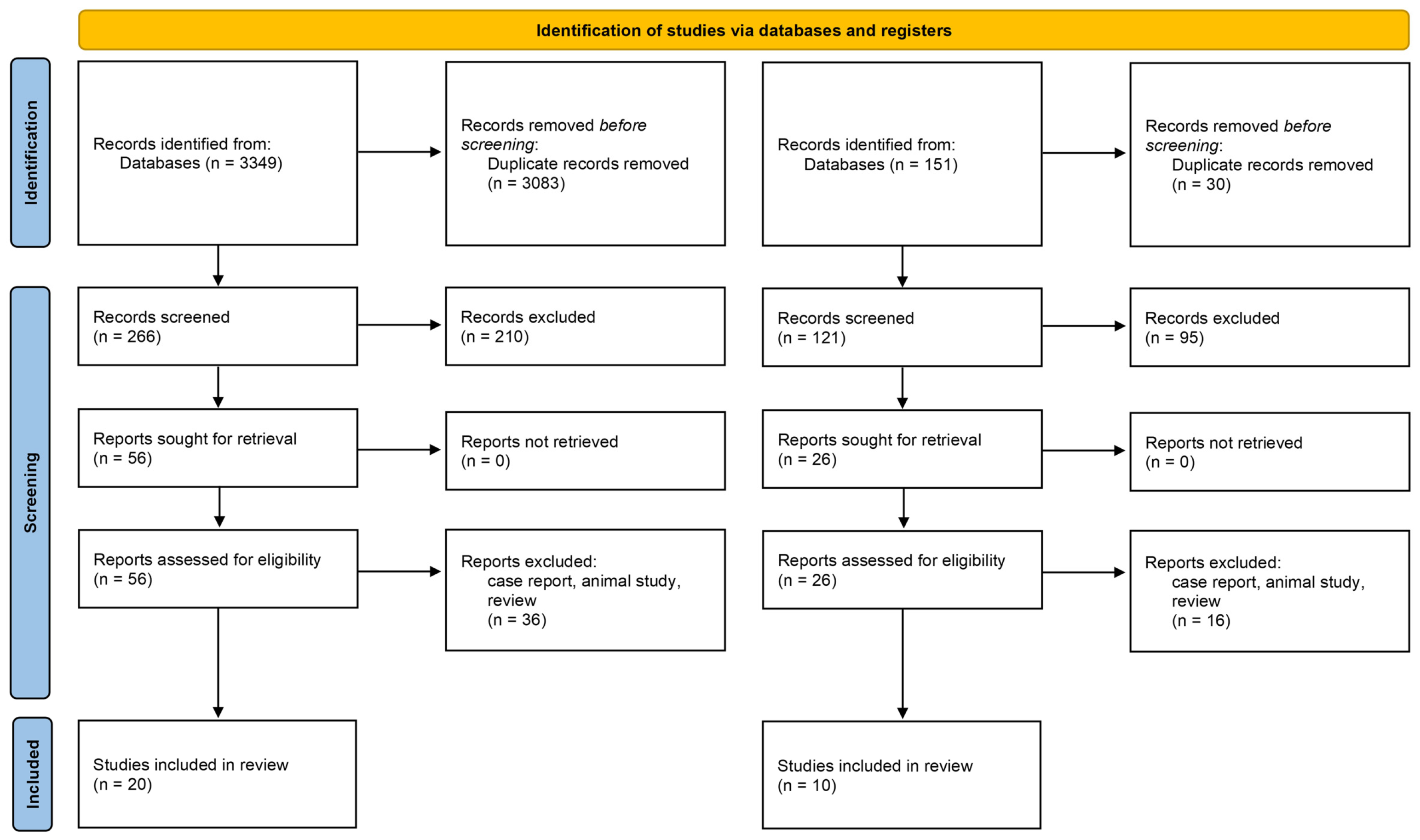
3.1. Study Selection and Population Characteristics
3.2. Analysis of α-Diversity in Psoriasis Studies
3.3. Analysis of β-Diversity in Psoriasis Studies
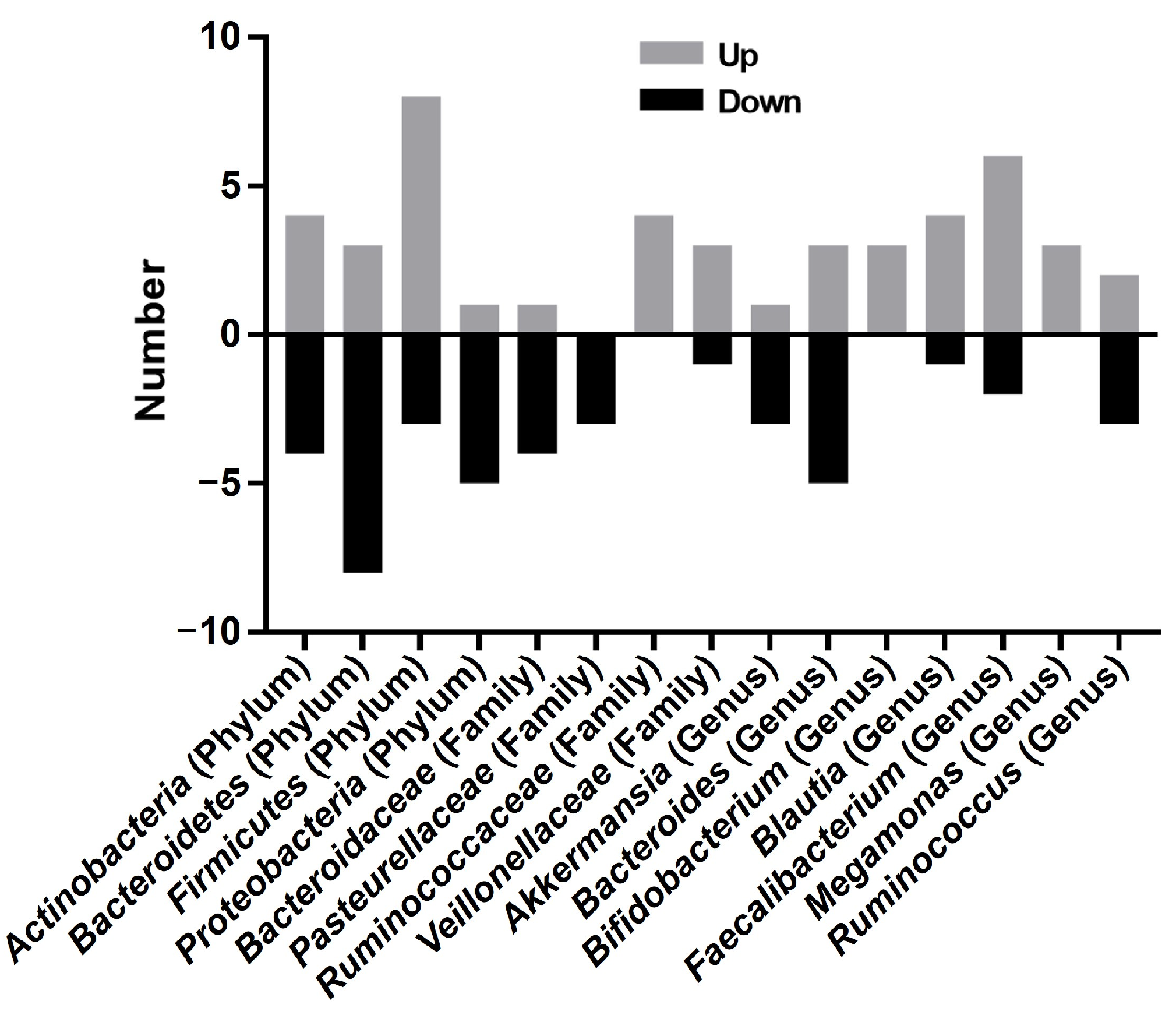
3.4. Taxonomic Alterations in Psoriasis Studies
3.5. Probiotics/FMT Intervention
| No. | Author/Year/Ref | Method | Cases/Age/Female | α-Diversity (Ps vs. C) | β-Diversity (Ps vs. C) | F/B | Phylum (Ps vs. C) | Class (Ps vs. C) | Order (Ps vs. C) | Family (Ps vs. C) | Genus (Ps vs. C) | Species (Ps vs. C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Scher et al., 2015 [8] | 16s rRNA gene sequencing (V1-V2) | P (n = 15) 39.4 PsA (n = 16) C (n = 17) 42.2 | Shannon index, Faith’s phylogenetic diversity index Lower diversity in psoriasis | Unweighted UniFrac analysis SD | NE | Actinobacteria ↓ Firmicutes ↑ Bacteroidetes ↓ | Actinobacteria ↓ | Erysipelotrichales ↓ | Erysipelotrichaceae ↓ Porphyromonadaceae ↓ | Parabacteroides ↓ UC_Clostridia ↓ Coprobacillus ↓ Ruminococcus ↓ Akkermansia ↓ (PSA) Ruminococcus ↓ (PSA) | Coprococcus species ↓ |
| 2 | Eppinga et al., 2016 [9] | 16s rRNA gene sequencing | P (n = 29) 46.0 ± 14.0 F (17) C (n = 33) 41 ± 14.9 F (23) | NE | NE | NE | Escherichia coli ↑ F. prausnitzii ↓ | |||||
| 3 | Doaa et al., 2016 [30] | 16s rRNA gene sequencing | P (n = 45) 42.3 ± 10.0 C (n = 45) 44.2 ± 7.1 | NE | NE | ↑ | Actinobacteria ↓ Firmicute ↑ Bacteroidetes ↓ | |||||
| 4 | Codoñer et al., 2018 [10] | 16s rRNA gene sequencing (V3-V4) | P (n = 52) C (n = 300) (from HMP) | Shannon Greater diversity in psoriasis | SD | NE | Bacteroides ↓ Faecalibacterium ↑ Akkermansia ↑ | |||||
| 5 | Chen et al., 2018 [11] | 16s rRNA gene sequencing (V3-V4) | P (n = 32) 42.8 ± 12.6 C (n = 64) 44.2 ± 10.8 | Shannon index, Simpson index, Chao1 index NSD | UniFrac analysis (weighted and unweighted analyses), Bray–Curtis index SD (psoriasis patients with BMI < 25) | ↑ | Bacteroidetes ↓ Firmicutes ↑ | Bacteroidaceae ↓ Prevotellaceae ↓ Ruminococcaceae ↑ Lachnospiraceae ↑ | ||||
| 6 | Tan et al., 2018 [12] | 16s rRNA gene sequencing (V4) | P (n = 14) 47.5 ± 4.7 C (n = 14) 40.4 ± 2.5 | Shannon, Simpson, ACE, Chao1 NSD | PCA, UPGMA, SD | NE | Verrucomicrobia ↓ Tenericutes ↓ | Verrucomicrobiae ↓ Mollicutes ↓ | Verrucomicrobiales ↓ | S24-7 ↓ Verrucomicrobiaceae ↓ Bacteroidaceae ↑ Enterococcaceae ↑ Veillonellaceae ↑ | Akkermansia ↓ Bacteroides ↑ Enterococcus ↑ | Akkermiansia muciniphila ↓ Clostridium citroniae ↑ |
| 7 | Shapiro et al., 2019 [13] | 16s rRNA gene sequencing (V4) | P (n = 24) 52.7 ± 11.6 C (n = 22) 43.9 ± 12.7 | Shannon, Chao1, Faith’s phylogenetic diversity index Lower diversity in psoriasis | UniFrac analysis (weighted and unweighted analyses) SD | ↑ | Bacteroidetes ↓ Proteobacteria ↓ Firmicutes ↑ Actinobacteria ↑ | Prevotella ↓ Lachnospira ↓ Faecalibacterium ↑ Ruminococcus ↑ Blautia ↑ Coprococcus ↑ Actinomyces ↑ Bifidobacterium ↑ Collinsella ↑ Dorea ↑ | Ruminococcus gnavus ↑ Dorea formicigenerans ↑ Collinsella aerofaciens ↑ Prevotella copri ↓ | |||
| 8 | Huang et al., 2019 [14] | 16s rRNA gene sequencing (V4–V5) | P (n = 16) 52.1 ± 3.0 C (n = 27) 52.9 ± 1.5 | Shannon index, Simpson index—no difference ACE index and Chao1 index decreased in psoriasis | PCA based on the Bray–Curtis dissimilarity distance, SD | ↓ | Firmicutes ↓ Proteobacteria ↓ Actinobacteria ↓ Bacteroidetes ↑ | Carnobacterium ↓ Granulicatella ↓ Rothia ↓ Streptococcus ↓ Bacteroides ↑ Parabacteroides ↑ Lachnospira ↑ Lachnospiraceae_UCG004 ↑ Lactococcus ↑ Bacillus ↑ | ||||
| 9 | Hidalgo-Cantabrana et al., 2019 [15] | 16s rRNA gene sequencing (V2–V3) | P (n = 19) 49 ± 11 C (n = 20) 43 ± 11 | Shannon index, Chao1 index, Faith’s phylogenetic diversity index Lower diversity in psoriasis | Unweighted Unifrac analysis, SD | ↑ | Bacteroidetes ↓ Proteobacteria ↓ Firmicutes ↑ Actinobacteria ↑ | Bacteroidaceae ↓ Prevotellaceae ↓ Burkholderiaceae ↓ Lactobacillaceae ↓ Streptococcaceae ↓ Veillonellaceae ↓ Ruminococcaceae ↑ Lachnospiraceae ↑ Clostridiales Family XIII ↑ Bifidobacteriaceae ↑ Coriobacteriaceae ↑ | Bacteroides ↓ Paraprevotella ↓ Barnesiella ↓ Parabacteroides ↓ Faecalibacterium ↓ Ruminococcus ↑ Blautia ↑ Bifidobacterium ↑ | |||
| 10 | Dei-Cas et al., 2020 [16] | 16s rRNA gene sequencing (V3–V4) | P (n = 55) 44.8 F (27) C (n = 27) 48.7 F (13) | Chao1 index NSD | SD | ↑ | Bacteroidetes ↓ Firmicutes ↑ Proteobacteria ↑ Fusobacteria ↑ Verrucomicrobia ↓ | Bacteroides ↓ Paraprevotella ↓ Faecalibacterium ↑ Blautia ↑ | ||||
| 11 | Yegorov et al., 2020 [17] | 16s rRNA gene sequencing (V3–V4) | P (n = 14) 34.5 F (10) C (n = 7) 33.0 F (10) | ↑ | Firmicutes ↓ | Lachnospiraceae ↓ Ruminococcaceae ↑ | Oscillibacter ↓ Roseburia ↓ Faecalibacterium ↑ | |||||
| 12 | Schade et al., 2021 [18] | 16s rRNA gene sequencing (V3–V4) | P (n = 21) 50.1 ± 11.73 F (14) C (n = 24) 49.4 ± 10.06 F (15) | NE | NE | NE | Ruminococcus ↓ Lachnospira ↓ Blautia ↓ Akkermansia muciniphila ↓ Dialister ↑ | Akkermansia muciniphila ↓ Prevotella copri ↑ | ||||
| 13 | Zhao et al., 2021 [29] | 16s rRNA gene sequencing (V4) | P (n = 13) C (n = 13) | Observed species and Chao index of BT and N showed a significant difference. The ACE index of AT and N had a significant difference. | NE | Bacteroides ↓ Clostridium ↓ Prevotella ↑ Lachnospira ↑ | ||||||
| 14 | Zhang et al., 2021 [19] | 16s rRNA gene sequencing (V3–V4) | P (n = 24) 43.13 ± 13.79 F (10) C (n = 30) 43.7 ± 13.21 F (10) | Sobs, Chao, ACE, Shannon, Simpson, Coverage NSD | SD | NE | Bacteroidetes ↓ Firmicutes ↑ Actinobacteria ↓ | Clostridia ↑ Fusobacteriia ↓ Actinobacteria ↓ | Bacteriodales ↓ Bifidobacteriales ↑ Burkholderiales ↓ Aeromonadales ↓ Fusobacteriales ↓ | Lachnospiraceae ↓ Veillonellaceae ↑ Ruminococcaceae ↑ Prevotellaceae ↑ Bacteroidaceae ↓ Enterobacteriaceae ↑ Fusobacteriaceae ↓ | Faecalibacterium ↑ Megamonas ↑ Prevotella ↑ Bacteroides ↓ | Prevotella_copri ↑ Faecalibacteriu, _prausnitzii ↑ Escherichia_coli ↑ Roseburia_faecis ↓ Bacteroides_uniformis ↑ |
| 15 | Xiao et al., 2021 [20] | Metagenomic sequencing | P (n = 30) 34, F 8 C (n = 15) 32, F 4 | Shannon index was high | PCoA SD | ↑ | Bacteroidetes ↓ Proteobacteria ↓ Euryarchaeota ↓ Actinobacteria ↑ Firmicutes ↑ Verrucomicrobia ↑ | Oxalobacteraceae ↓ Porphyromonadaceae ↓ Pasteurellaceae ↓ Rikenellaceae ↓ Sphingobacteriaceae ↓ Comamonadaceae ↓ | Prevotella ↓ Alistipes ↓ Eubacterium ↓ Butyricimonas ↓ Oxalobacter ↓ Actinobacillus ↓ Pseudoflavonifractor ↓ Faecalibacterium ↑ Bacteroides ↑ Bifidobacterium ↑ Megamonas ↑ Roseburia ↑ | Faecalibacterium prausnitzii ↑ | ||
| 16 | Wang et al., 2021 [21] | 16s rRNA gene sequencing (V4) | P (n = 20) C (n = 20) | Shannon, Simpson, Chao1, ACE | PCoA SD | NE | Negativicutes ↑ Bacilli ↑ | Lactobacillales ↑ Selenomonadales ↑ | Veillonellaceae ↑ | Romboutsia ↓ Megamonas ↑ | ||
| 17 | Chang et al., 2022 [22] | Metagenomic sequencing | P (n = 33) 43.2 ±14.6 F (17) C (n = 15) 45.8 ±13.9 F (12) | Chao indices NSD Greater diversity in psoriasis (Shannon and Simpson indices) | NE | Phascolarctobacterium succinatutens ↓ Bacteroides vulgatus ↑ Parasutterella excrementihominis ↑ | ||||||
| 18 | Wang et al., 2022 [23] | 16s rRNA gene sequencing (V4) | P (n = 28) 44.5 F (9) C (n = 21) 46.1 F (6) | Shannon, Simpson, Chao1, ACE NSD | SD | NE | Proteobacteria ↓ Bacteroidetes ↑ | Clostridia ↑ Bacteroidia ↓ | Clostridiales; ↓ Bacteroidales ↑ Enterobacteriales ↓ | Enterobacteriaceae ↓ Peptostreptococcaceae ↓ Lactobacillaceae ↑ Muribaculaceae ↑ | unidentified_Enterobacteriaceae ↓ unidentified_Lachnospiraceae ↓ Dorea ↓ Lactobacillus ↑ Dialister ↑ | Escherichia_coli ↓ bacterium_LF-3 ↓ Parabacteroides_distasonis ↑ Bacteroides_thetaiotaomicron ↑ Lactobacillus_reuteri ↑ |
| 19 | Todberg et al., 2022 [25] | Metagenomic sequencing | P (n = 53) 48.0 F (24) C (n = 52) 49.0 F (23) Cohabitant partners (n = 21) | Shannon index NSD lower MGS richness in PP | SD | NE | Actinobacteria ↑ Euryarchaeota ↑ | Methanobacteriaceae ↑ | Blautia ↑ Faecalibacerium ↓ | Faecalibacterium sp. Ruminococcus torques ↑ Ruminococcus gnavus ↑ F04-11AC ↓ | ||
| 20 | Wen et al., 2023 [28] | Metagenomic sequencing | P (n = 32) C (n = 32) | Richness Shannon NSD | PCoA showed a minor separation | ↓ | Firmicutes ↓ Bacteroidetes ↑ | Roseburia ↓ Eubacterium ↓ | Roseburia hominis ↓ Bacteroides eggerthii ↓ Bacteroides uniformis ↑ Escherichia spp. ↑ Alistipes finegoldii ↑ |
| No. | Author/Year/Ref | Study Subjects (n) | Intervention Group (n)/ Age/Female (n) | Control (n) | Intervention in the Study Group | Antipsoriasitic | Duration of Intervention | Study Type | Outcome Measurements | Microbiome and Biomarker | Conclusion (Supports the Hypothesis that Gut Microbiome Modulation via Ingestion Produces Clinical Improvement) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Groeger et al., 2013 [32] | Psoriasis patients (26) | n = 2 | Placebo (14) | Bifidobacteria infantis 35,624 | 6–8 weeks | NE | Yes | |||
| 2 | Navarro-López et al., 2019 [33] | Psoriasis patients (90) | n = 46 41.57 ± 13.23, F (27) | Placebo (44) 43.09 ± 10.32 F (27) | Bifidobacterium longum CECT 7347, B. lactis CECT 8145, and Lacticaseibacillus rhamnosus CECT 8361 | Topical corticosteroid betamethasone in combination with calcipotriol | 12 weeks | randomized, double-blind, placebo-controlled trial | PASI, PGA recurrence ↓ | genera Micromonospora and Rhodococcus disappearance Collinsella ↑ and Lactobacillus ↑ | Yes |
| 3 | Haidmayer et al., 2020 [26] | PsA (58) | n = 58 F (7) | No control | Nine bacterial strains of Lactobacillus and Bifidobacterium | Anti-TNF, Anti-IL-17, Methotrexate, NSAID | 12 weeks | pilot open-label study | mPASDAS ↓ | fecal zonulin ↓, antitrypsin ↓, calprotectin ↓ | Yes |
| 4 | Moludi et al., 2021 [34] | Psoriasis patients (50) | n = 25 42.70 ± 9.10 F (15) | Placebo (25) 43.10 ± 7.80, F (17) | Multistrain probiotics including Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium lactis, and Bifidobacterium longum | 8 weeks | single-center, randomized, placebo-controlled double-blind trial | BDI, PSS, PASI, and DLQI ↓TAC ↑, MDA ↓hs-CRP ↓, IL-6 ↓ | Yes | ||
| 5 | Akbarzadeh et al., 2021 [35] | Psoriasis patients (36) | n = 22 F (9) | Placebo (14), F (7) | Lactocare® | 12 weeks | double-blind, randomized, placebo-controlled study | serum levels of Fe, Zn, P, Mg, Ca, and Na are increased | Yes | ||
| 6 | Moludi et al., 2022 [37] | Psoriasis patients (46) | n = 23 42.04 ± 8.10, F (13) | Placebo (23) 43.76 ± 8.86, F (15) | Probiotic capsules (Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium lactis, and Bifidobacterium longum) | Routine drug while taking any antioxidants was forbidden | 8 weeks | randomized double-blind placebo-controlled clinical trial | QOL ↑hs-CRP ↓ IL1-β ↓, and LPS ↓ | Yes | |
| 7 | Suriano et al., 2023 [39] | Psoriasis patients (103) | n = 50 50 F (23) | Placebo (53) 52 F (27) | Lacticaseibacillus rhamnosus | Standard-of-care | 6 M | a randomized, parallel, placebo-controlled, double-blind study | PASI, DLQI | No | |
| 8 | Siu et al., 2024 [38] | Psoriasis patients (45) | n = 45 44.57 ± 11.5 F (17) | No control | Bifidobacterium and Lactobacilli | Usual medication or topical maintenance therapy | 8 weeks | a single-arm, pre–post-interventional trial | PASI, BSFS ↓, DLQI ↑ | a significant difference in the gut microbiome composition between the responders and non-responders | Yes |
| 9 | Zangrilli et al., 2022 [40] | Psoriasis vulgaris (198) | n = 100 | Control (98) | Streptococcus salivarius K12 | Topical treatments such as emollient and vitamin D derivatives | 24 weeks | Prospective randomized controlled trial | PASI DLQI | Yes | |
| 10 | Kragsnaes et al., 2021 [36] | PsA (31) | n = 15 42.04 ± 16.1 F (8) | Sham (16) 52.4 ± 11.0 F (12) | One gastroscopic-guided FMT or sham transplantation in combination with methotrexate | Intra-articular or systemic glucocorticoids and non-methotrexate conventional synthetic and biologic disease modifying antirheumatic drugs; a washout period of 12 weeks (26 weeks for biologic agents) was required | 26 weeks | HAQ-DI, ACR20 | FMT appeared to be inferior to sham in treating active peripheral PsA | No |
4. Discussion
4.1. The Diversity of the Intestinal Microbiota in Psoriasis Patients Exhibited Marked Heterogeneity
4.2. Firmicutes/Bacteroidetes Ratio and Metabolic Implications in Psoriasis
4.3. Reduced Actinobacterial Phylum Abundance in Psoriasis and Anti-Inflammatory Effects of Bifidobacterium Supplementation
4.4. Elevated Ruminococcaceae Family Abundance in Psoriasis and Its Functional Implications
4.5. The Abundance of Prevotella and Bacteroides Genera Was Decreased, and Megamonas, Ruminococcus, and Faecalibacterium Were Increased in Psoriasis
4.6. Limited Evidence for Fecal Microbiota Transplantation in Psoriasis, Requiring Further Validation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Springate, D.A.; Parisi, R.; Kontopantelis, E.; Reeves, D.; Griffiths, C.E.; Ashcroft, D.M. Incidence, prevalence and mortality of patients with psoriasis: A U.K. population-based cohort study. Br. J. Dermatol. 2016, 176, 650–658. [Google Scholar] [CrossRef]
- Alinaghi, F.; Tekin, H.G.; Burisch, J.; Wu, J.J.; Thyssen, J.P.; Egeberg, A. Global Prevalence and Bidirectional Association Between Psoriasis and Inflammatory Bowel Disease—A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2019, 14, 351–360. [Google Scholar] [CrossRef]
- Fu, Y.; Lee, C.-H.; Chi, C.-C. Association of Psoriasis with Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018, 154, 1417–1423. [Google Scholar] [CrossRef]
- Zakostelska, Z.; Málková, J.; Klimešová, K.; Rossmann, P.; Hornová, M.; Novosádová, I.; Stehlíková, Z.; Kostovcikova, M.; Hudcovic, T.; Štepánková, R.; et al. Intestinal Microbiota Promotes Psoriasis-Like Skin Inflammation by Enhancing Th17 Response. PLoS ONE 2016, 11, e0159539. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wu, C.-S.; Chao, Y.-H.; Lin, C.-C.; Tsai, H.-Y.; Li, Y.-R.; Chen, Y.Z.; Tsai, W.-H.; Chen, Y.-K. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J. Food Drug Anal. 2017, 25, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Stehlikova, Z.; Kostovcikova, K.; Kverka, M.; Rossmann, P.; Dvorak, J.; Novosadova, I.; Kostovcik, M.; Coufal, S.; Srutkova, D.; Prochazkova, P.; et al. Crucial Role of Microbiota in Experimental Psoriasis Revealed by a Gnotobiotic Mouse Model. Front. Microbiol. 2019, 10, 236. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased Bacterial Diversity Characterizes the Altered Gut Microbiota in Patients with Psoriatic Arthritis, Resembling Dysbiosis in Inflammatory Bowel Disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef]
- Eppinga, H.; Sperna Weiland, C.J.; Thio, H.B.; van der Woude, C.J.; Nijsten, T.E.C.; Peppelenbosch, M.P.; Konstantinov, S.R. Similar Depletion of Protective Faecalibacterium prausnitzii in Psoriasis and Inflammatory Bowel Disease, but not in Hidradenitis Suppurativa. J. Crohn’s Colitis 2016, 10, 1067–1075. [Google Scholar] [CrossRef]
- Codoñer, F.M.; Ramírez-Bosca, A.; Climent, E.; Carrión-Gutierrez, M.; Guerrero, M.; Pérez-Orquín, J.M.; Horga de la Parte, J.; Genovés, S.; Ramón, D.; Navarro-López, V.; et al. Gut microbial composition in patients with psoriasis. Sci. Rep. 2018, 8, 3812. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ho, H.J.; Tseng, C.H.; Lai, Z.L.; Shieh, J.J.; Wu, C.Y. Intestinal microbiota profiling and predicted metabolic dysregulation in psoriasis patients. Exp. Dermatol. 2018, 27, 1336–1343. [Google Scholar] [CrossRef]
- Tan, L.; Zhao, S.; Zhu, W.; Wu, L.; Li, J.; Shen, M.; Lei, L.; Chen, X.; Peng, C. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp. Dermatol. 2017, 27, 144–149. [Google Scholar] [CrossRef]
- Shapiro, J.; Cohen, N.A.; Shalev, V.; Uzan, A.; Koren, O.; Maharshak, N. Psoriatic patients have a distinct structural and functional fecal microbiota compared with controls. J. Dermatol. 2019, 46, 595–603. [Google Scholar] [CrossRef]
- Huang, L.; Gao, R.; Yu, N.; Zhu, Y.; Ding, Y.; Qin, H. Dysbiosis of gut microbiota was closely associated with psoriasis. Sci. China Life Sci. 2018, 62, 807–815. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Gómez, J.; Delgado, S.; Requena-López, S.; Queiro-Silva, R.; Margolles, A.; Coto, E.; Sánchez, B.; Coto-Segura, P. Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br. J. Dermatol. 2019, 181, 1287–1295. [Google Scholar] [CrossRef]
- Dei-Cas, I.; Giliberto, F.; Luce, L.; Dopazo, H.; Penas-Steinhardt, A. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: Development of a new Psoriasis-Microbiome Index. Sci. Rep. 2020, 10, 12754. [Google Scholar] [CrossRef]
- Yegorov, S.; Babenko, D.; Kozhakhmetov, S.; Akhmaltdinova, L.; Kadyrova, I.; Nurgozhina, A.; Nurgaziyev, M.; Good, S.V.; Hortelano, G.H.; Yermekbayeva, B.; et al. Psoriasis Is Associated with Elevated Gut IL-1α and Intestinal Microbiome Alterations. Front. Immunol. 2020, 11, 571319. [Google Scholar] [CrossRef]
- Schade, L.; Mesa, D.; Faria, A.R.; Santamaria, J.R.; Xavier, C.A.; Ribeiro, D.; Hajar, F.N.; Azevedo, V.F. The gut microbiota profile in psoriasis: A Brazilian case-control study. Lett. Appl. Microbiol. 2021, 74, 498–504. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, L.; Sun, T.; Guo, K.; Geng, S. Dysbiosis of gut microbiota and its correlation with dysregulation of cytokines in psoriasis patients. BMC Microbiol. 2021, 21, 78. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, G.; Jiang, C.; Liu, X.; Wang, X.; Li, Y.; Cheng, M.; Lv, H.; Xian, F.; Guo, X.; et al. Deciphering Gut Microbiota Dysbiosis and Corresponding Genetic and Metabolic Dysregulation in Psoriasis Patients Using Metagenomics Sequencing. Front. Cell. Infect. Microbiol. 2021, 11, 605825. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, W.; Ma, J.; Xu, S.; Liu, M.; Zhang, X.; Yang, S. Substantial alterations of the intestinal microbiota in psoriasis patients of China. Exp. Dermatol. 2021, 30, 1840–1841. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-W.; Yan, D.; Singh, R.; Bui, A.; Lee, K.; Truong, A.; Milush, J.M.; Somsouk, M.; Liao, W. Multiomic Analysis of the Gut Microbiome in Psoriasis Reveals Distinct Host—Microbe Associations. JID Innov. 2022, 2, 100115. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Qiao, S.; Zhu, Q.; Zuo, Z.; Guo, B. Analysis of Alterations of the Gut Microbiota in Moderate to Severe Psoriasis Patients Using 16S rRNA Gene Sequencing. Indian J. Dermatol. 2022, 67, 495–503. [Google Scholar] [CrossRef]
- Zhao, H.; Shang, L.; Zhang, Y.; Liang, Z.; Wang, N.; Zhang, Q.; Gao, C.; Luo, J. IL-17A inhibitors alleviate Psoriasis with concomitant restoration of intestinal/skin microbiota homeostasis and altered microbiota function. Front. Immunol. 2024, 15, 1344963. [Google Scholar] [CrossRef]
- Todberg, T.; Egeberg, A.; Zachariae, C.; Sørensen, N.; Pedersen, O.; Skov, L. Patients with psoriasis have a dysbiotic taxonomic and functional gut microbiota. Br. J. Dermatol. 2022, 187, 89–98. [Google Scholar] [CrossRef]
- Haidmayer, A.; Bosch, P.; Lackner, A.; D’orazio, M.; Fessler, J.; Stradner, M.H. Effects of Probiotic Strains on Disease Activity and Enteric Permeability in Psoriatic Arthritis–A Pilot Open-Label Study. Nutrients 2020, 12, 2337. [Google Scholar] [CrossRef] [PubMed]
- Yeh, N.-L.; Hsu, C.-Y.; Tsai, T.-F.; Chiu, H.-Y. Gut Microbiome in Psoriasis is Perturbed Differently During Secukinumab and Ustekinumab Therapy and Associated with Response to Treatment. Clin. Drug Investig. 2019, 39, 1195–1203. [Google Scholar] [CrossRef]
- Wen, C.; Pan, Y.; Gao, M.; Wang, J.; Huang, K.; Tu, P. Altered gut microbiome composition in nontreated plaque psoriasis patients. Microb. Pathog. 2023, 175, 105970. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Zhu, L.; Geng, S.; Guo, K. Effectiveness and safety of Adalimumab in psoriasis and its influence on gut microbiome. Microb. Pathog. 2022, 162, 105308. [Google Scholar] [CrossRef]
- Doaa, M.; Dalia, M.; Ahmed, F.S. Gut bacterial microbiota in psoriasis: A case control study. Afr. J. Microbiol. Res. 2016, 10, 1337–1343. [Google Scholar] [CrossRef]
- Eppinga, H.; Thio, H.B.; Schreurs, M.W.J.; Blakaj, B.; Tahitu, R.I.; Konstantinov, S.R.; Peppelenbosch, M.P.; Fuhler, G.M. Depletion of Saccharomyces cerevisiae in psoriasis patients, restored by Dimethylfumarate therapy (DMF). PLoS ONE 2017, 12, e0176955. [Google Scholar] [CrossRef]
- Groeger, D.; O’mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef]
- Navarro-López, V.; Martínez-Andrés, A.; Ramírez-Boscá, A.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Carrión-Gutiérrez, M.A.; Prieto-Merino, D.; Codoñer-Cortés, F.; Ramón-Vidal, D.; Genovés-Martínez, S.; et al. Efficacy and Safety of Oral Administration of a Mixture of Probiotic Strains in Patients with Psoriasis: A Randomized Controlled Clinical Trial. Acta Dermato-Venereol. 2019, 99, 1078–1084. [Google Scholar] [CrossRef]
- Moludi, J.; Khedmatgozar, H.; Saiedi, S.; Razmi, H.; Alizadeh, M.; Ebrahimi, B. Probiotic supplementation improves clinical outcomes and quality of life indicators in patients with plaque psoriasis: A randomized double-blind clinical trial. Clin. Nutr. ESPEN 2021, 46, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Taheri, M.; Ebrahimi, B.; Alirezaei, P.; Doosti-Irani, A.; Soleimani, M.; Nouri, F. Evaluation of Lactocare® Synbiotic Administration on the Serum Electrolytes and Trace Elements Levels in Psoriasis Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial Study. Biol. Trace Element Res. 2021, 200, 4230–4237. [Google Scholar] [CrossRef]
- Kragsnaes, M.S.; Kjeldsen, J.; Horn, H.C.; Munk, H.L.; Pedersen, J.K.; Just, S.A.; Ahlquist, P.; Pedersen, F.M.; de Wit, M.; Möller, S.; et al. Safety and efficacy of faecal microbiota transplantation for active peripheral psoriatic arthritis: An exploratory randomised placebo-controlled trial. Ann. Rheum. Dis. 2021, 80, 1158–1167. [Google Scholar] [CrossRef]
- Moludi, J.; Fathollahi, P.; Khedmatgozar, H.; Pourteymour Fard Tabrizi, F.; Ghareaghaj Zare, A.; Razmi, H.; Amirpour, M. Probiotics Supplementation Improves Quality of Life, Clinical Symptoms, and Inflammatory Status in Patients with Psoriasis. J. Drugs Dermatol. 2022, 21, 637–644. [Google Scholar] [CrossRef]
- Siu, P.L.K.; Choy, C.T.; Chan, H.H.Y.; Leung, R.K.K.; Chan, U.K.; Zhou, J.; Wong, C.H.; Lee, Y.W.; Chan, H.W.; Lo, C.J.Y.; et al. A Novel Multi-Strain E3 Probiotic Formula Improved the Gastrointestinal Symptoms and Quality of Life in Chinese Psoriasis Patients. Microorganisms 2024, 12, 208. [Google Scholar] [CrossRef]
- Suriano, E.S.; Souza, M.D.M.; Kobata, C.M.; Santos, F.H.Y.; Mimica, M.J. Efficacy of an adjuvant Lactobacillus rhamnosus formula in improving skin lesions as assessed by PASI in patients with plaque psoriasis from a university-affiliated, tertiary-referral hospital in São Paulo (Brazil): A parallel, double-blind, randomized clinical trial. Arch. Dermatol. Res. 2023, 315, 1621–1629. [Google Scholar] [CrossRef]
- Zangrilli, A.; Diluvio, L.; Di Stadio, A.; Di Girolamo, S. Improvement of Psoriasis Using Oral Probiotic Streptococcus salivarius K-12: A Case–Control 24-Month Longitudinal Study. Probiotics Antimicrob. Proteins 2022, 14, 573–578. [Google Scholar] [CrossRef]
- Ducret, A.; Grangeasse, C. Recent progress in our understanding of peptidoglycan assembly in Firmicutes. Curr. Opin. Microbiol. 2021, 60, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, L.; Li, Y.; Wang, S.; Lu, G.; Wang, H. Advances in the mechanism of action of short-chain fatty acids in psoriasis. Int. Immunopharmacol. 2024, 141, 112928. [Google Scholar] [CrossRef]
- Polak, K.; Bergler-Czop, B.; Szczepanek, M.; Wojciechowska, K.; Frątczak, A.; Kiss, N. Psoriasis and Gut Microbiome—Current State of Art. Int. J. Mol. Sci. 2021, 22, 4529. [Google Scholar] [CrossRef] [PubMed]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Elena, R.-I.M.; Gabriela, G.-D.; Arnulfod, G.-C.; Enrique, C.A. Studying the Gut Microbiome of Latin America and Hispanic/Latino Populations. Insight into Obesity and Diabetes: Systematic Review. Curr. Diabetes Rev. 2019, 15, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Maya-Lucas, O.; Murugesan, S.; Nirmalkar, K.; Alcaraz, L.D.; Hoyo-Vadillo, C.; Pizano-Zárate, M.L.; García-Mena, J. The gut microbiome of Mexican children affected by obesity. Anaerobe 2019, 55, 11–23. [Google Scholar] [CrossRef]
- Martín, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermúdez-Humarán, L.G.; Sokol, H.; Chatel, J.-M.; Langella, P. Faecalibacterium: A bacterial genus with promising human health applications. FEMS Microbiol. Rev. 2023, 47, fuad039. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Martinez-Medina, M.; Busquets, D.; Sabat-Mir, M.; Duncan, S.H.; Flint, H.J.; Aldeguer, X.; Garcia-Gil, L.J. Mucosa-associated Faecalibacterium prausnitzii and Escherichia coli co-abundance can distinguish Irritable Bowel Syndrome and Inflammatory Bowel Disease phenotypes. Int. J. Med. Microbiol. 2014, 304, 464–475. [Google Scholar] [CrossRef]
| Gut microbiota–psoriasis investigation | Keywords | “gastrointestinal microbiome”, “gut microbiota”, “intestinal microbiome”, “intestinal microbiota” “bacteria”, “dysbiosis”, “gut”, “gastrointestinal”, “intestine”, “stool”, “fecal”, and “psoriasis” |
| Inclusion criteria |
| |
| Exclusion criteria | Review papers, conference abstracts, case reports, expert opinions, editorials, and studies using animal models | |
| Probiotics or FMT in treating psoriasis | Keywords | “Probiotics” or “Fecal microbiota transplantation” and “psoriasis”. |
| Inclusion criteria | Human case–control studies investigating the efficacy of probiotics or fecal microbiota transplantation in psoriasis | |
| Exclusion criteria | 1. Review papers, conference abstracts, case reports, expert opinions, editorials, and studies using animal models; 2. Patients were ineligible if they had comorbidities—inflammatory bowel diseases (Crohn’s disease, ulcerative colitis), rheumatoid arthritis, ankylosing spondylitis, onset of a severe organ dysfunction, terminal illness, human immunodeficiency virus (HIV) infection, or cancer—throughout the study duration; patients treated with antibiotics during the last 8 weeks. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Lou, Y.; Hui, Y.; Chen, H.; Sang, H.; Liu, F. Characterization of the Gut Microbiota in Patients with Psoriasis: A Systematic Review. Pathogens 2025, 14, 358. https://doi.org/10.3390/pathogens14040358
Gao Y, Lou Y, Hui Y, Chen H, Sang H, Liu F. Characterization of the Gut Microbiota in Patients with Psoriasis: A Systematic Review. Pathogens. 2025; 14(4):358. https://doi.org/10.3390/pathogens14040358
Chicago/Turabian StyleGao, Yingjun, Yanfeng Lou, Yun Hui, Huan Chen, Hong Sang, and Fang Liu. 2025. "Characterization of the Gut Microbiota in Patients with Psoriasis: A Systematic Review" Pathogens 14, no. 4: 358. https://doi.org/10.3390/pathogens14040358
APA StyleGao, Y., Lou, Y., Hui, Y., Chen, H., Sang, H., & Liu, F. (2025). Characterization of the Gut Microbiota in Patients with Psoriasis: A Systematic Review. Pathogens, 14(4), 358. https://doi.org/10.3390/pathogens14040358






