The Role of Staphylococcus aureus Virulence Factors in Skin Infection and Their Potential as Vaccine Antigens
Abstract
:1. Staphylococcus aureus Skin Infection
2. Requirements for an Effective Anti-S. aureus Vaccine
3. Skin
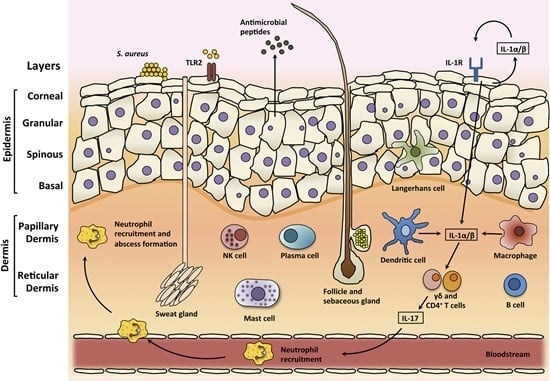
3.1. Skin Structure
3.2. Skin Infections
3.3. Skin Immune Response
4. Virulence Factors Involved in the Pathogenesis of S. aureus SSTIs
4.1. Membrane Damaging Toxins
4.2. Cell Wall-Anchored Proteins
4.2.1. Clumping Factor A
4.2.2. Clumping Factor B
4.2.3. Fibronectin-Binding Proteins
4.2.4. SasX
4.2.5. Protein A
4.2.6. Iron Regulated Surface Proteins
5. CWA Proteins as T Cell Antigens
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hersh, A.L.; Chambers, H.F.; Maselli, J.H.; Gonzales, R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch. Internal Med. 2008, 168, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- David, M.Z.; Daum, R.S. Community-associated methicillin-resistant Staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Basis of virulence in community-associated methicillin-resistant staphylococcus aureus. Annu. Rev. Microbiol. 2010, 64, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.A. Challenges for a universal Staphylococcus aureus vaccine. Clin. Infect. Dis. 2012, 54, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G., Jr.; Proctor, R.A. Where does a Staphylococcus aureus vaccine stand? Clin. Microbiol. Infect. 2014, 20 (Suppl. 5), 66–75. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Ross, T.; Gregson, D.B. Staphylococcus aureus bloodstream infections: Risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J. Infect. Dis. 2008, 198, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Ibrahim, A.S.; Yeaman, M.R.; Lin, L.; Fu, Y.; Avanesian, V.; Bayer, A.S.; Filler, S.G.; Lipke, P.; Otoo, H.; et al. The antifungal vaccine derived from the recombinant n terminus of als3p protects mice against the bacterium Staphylococcus aureus. Infect. Immunity 2008, 76, 4574–4580. [Google Scholar] [CrossRef] [PubMed]
- Daum, R.S.; Spellberg, B. Progress toward a Staphylococcus aureus vaccine. Clin. Infect. Dis. 2012, 54, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Misstear, K.; McNeela, E.A.; Murphy, A.G.; Geoghegan, J.A.; O’Keeffe, K.M.; Fox, J.; Chan, K.; Heuking, S.; Collin, N.; Foster, T.J.; et al. Targeted nasal vaccination provides antibody-independent protection against Staphylococcus aureus. J. Infect. Dis. 2014, 209, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Wiese, L.; Mejer, N.; Schonheyder, H.C.; Westh, H.; Jensen, A.G.; Larsen, A.R.; Skov, R.; Benfield, T. A nationwide study of comorbidity and risk of reinfection after Staphylococcus aureus bacteraemia. J. Infect. 2013, 67, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.M.; DeLeo, F.R.; Elloumi, H.Z.; Hsu, A.P.; Uzel, G.; Brodsky, N.; Freeman, A.F.; Demidowich, A.; Davis, J.; Turner, M.L.; et al. Stat3 mutations in the hyper-ige syndrome. N. Engl. J. Med. 2007, 357, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Litjens, N.H.; Huisman, M.; van den Dorpel, M.; Betjes, M.G. Impaired immune responses and antigen-specific memory cd4+ T cells in hemodialysis patients. J. Am. Soc. Nephrol. 2008, 19, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.D.; Brenchley, J.M.; Laurence, A.; Freeman, A.F.; Hill, B.J.; Elias, K.M.; Kanno, Y.; Spalding, C.; Elloumi, H.Z.; Paulson, M.L.; et al. Impaired t(h)17 cell differentiation in subjects with autosomal dominant hyper-ige syndrome. Nature 2008, 452, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Ishigame, H.; Kakuta, S.; Nagai, T.; Kadoki, M.; Nambu, A.; Komiyama, Y.; Fujikado, N.; Tanahashi, Y.; Akitsu, A.; Kotaki, H.; et al. Differential roles of interleukin-17a and -17f in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 2009, 30, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.F.; Murphy, A.G.; Lalor, S.J.; Leech, J.M.; O’Keeffe, K.M.; Mac Aogain, M.; O’Halloran, D.P.; Lacey, K.A.; Tavakol, M.; Hearnden, C.H.; et al. Memory th1 cells are protective in invasive Staphylococcus aureus infection. PLoS Pathog. 2015, 11, e1005226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pier, G.B. Will there ever be a universal Staphylococcus aureus vaccine? Hum. Vaccin. Immunother. 2013, 9, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; Cho, J.S. Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol. 2011, 11, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Miller, L.S. Host-pathogen interactions between the skin and Staphylococcus aureus. Curr. Opin. Microbiol. 2012, 15, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.J.; Abrahamian, F.M.; LoVecchio, F.; Talan, D.A. Acute bacterial skin infections: Developments since the 2005 infectious diseases society of america (idsa) guidelines. J. Emerg. Med. 2013, 44, e397–e412. [Google Scholar] [CrossRef] [PubMed]
- Ki, V.; Rotstein, C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184. [Google Scholar] [PubMed]
- Moran, G.J.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; McDougal, L.K.; Carey, R.B.; Talan, D.A. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 2006, 355, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.G.; Tonthat, G.T.; Stryjewski, M.E.; Rude, T.H.; Reilly, L.F.; Barriere, S.L.; Genter, F.C.; Corey, G.R.; Fowler, V.G., Jr. Presence of genes encoding the panton-valentine leukocidin exotoxin is not the primary determinant of outcome in patients with complicated skin and skin structure infections due to methicillin-resistant Staphylococcus aureus: Results of a multinational trial. J. Clin. Microbiol. 2009, 47, 3952–3957. [Google Scholar] [PubMed]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Pathogenesis of Staphylococcus aureus abscesses. Am. J. Pathol. 2015, 185, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Men, S.; Akhan, O.; Koroglu, M. Percutaneous drainage of abdominal abcess. Eur. J. Radiol. 2002, 43, 204–218. [Google Scholar] [CrossRef]
- Kupper, T.S.; Fuhlbrigge, R.C. Immune surveillance in the skin: Mechanisms and clinical consequences. Nat. Rev. Immunol. 2004, 4, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S. Toll-like receptors in skin. Adv. Dermatol. 2008, 24, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Von Bernuth, H.; Picard, C.; Jin, Z.; Pankla, R.; Xiao, H.; Ku, C.L.; Chrabieh, M.; Mustapha, I.B.; Ghandil, P.; Camcioglu, Y.; et al. Pyogenic bacterial infections in humans with myd88 deficiency. Science 2008, 321, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.; Puel, A.; Bonnet, M.; Ku, C.L.; Bustamante, J.; Yang, K.; Soudais, C.; Dupuis, S.; Feinberg, J.; Fieschi, C.; et al. Pyogenic bacterial infections in humans with irak-4 deficiency. Science 2003, 299, 2076–2079. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; O’Connell, R.M.; Gutierrez, M.A.; Pietras, E.M.; Shahangian, A.; Gross, C.E.; Thirumala, A.; Cheung, A.L.; Cheng, G.; Modlin, R.L. Myd88 mediates neutrophil recruitment initiated by il-1r but not tlr2 activation in immunity against Staphylococcus aureus. Immunity 2006, 24, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.L.; von Bernuth, H.; Picard, C.; Zhang, S.Y.; Chang, H.H.; Yang, K.; Chrabieh, M.; Issekutz, A.C.; Cunningham, C.K.; Gallin, J.; et al. Selective predisposition to bacterial infections in irak-4-deficient children: Irak-4-dependent tlrs are otherwise redundant in protective immunity. J. Exp. Med. 2007, 204, 2407–2422. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.E.; Lalor, S.J.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.C.; Mills, K.H. Interleukin-1 and il-23 induce innate il-17 production from gammadelta T cells, amplifying th17 responses and autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Cua, D.J.; Tato, C.M. Innate il-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. Il-17 and th17 cells. Nat. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Pietras, E.M.; Garcia, N.C.; Ramos, R.I.; Farzam, D.M.; Monroe, H.R.; Magorien, J.E.; Blauvelt, A.; Kolls, J.K.; Cheung, A.L.; et al. Il-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Investig. 2010, 120, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Tan, X.Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (il)-22 and il-17 are coexpressed by th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Filler, S.G.; Chaili, S.; Barr, K.; Wang, H.; Kupferwasser, D.; Hennessey, J.P., Jr.; Fu, Y.; Schmidt, C.S.; Edwards, J.E., Jr.; et al. Mechanisms of ndv-3 vaccine efficacy in mrsa skin versus invasive infection. Proc. Natl. Acad. Sci. USA 2014, 111, E5555–E5563. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.C.; Chaili, S.; Filler, S.G.; Barr, K.; Wang, H.; Kupferwasser, D.; Edwards, J.E., Jr.; Xiong, Y.Q.; Ibrahim, A.S.; Miller, L.S.; et al. Non-redundant roles of il-17a and il-22 in murine host defense against cutaneous and hematogenous infection due to methicillin-resistant Staphylococcus aureus. Infect. Immunity 2015. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Henry, T.; van Rooijen, W.J.; Perret, M.; Badiou, C.; Aerts, P.C.; Kemmink, J.; de Haas, C.J.; van Kessel, K.P.; Vandenesch, F.; et al. The staphylococcal toxin panton-valentine leukocidin targets human c5a receptors. Cell Host Microbe 2013, 13, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Loffler, B.; Hussain, M.; Grundmeier, M.; Bruck, M.; Holzinger, D.; Varga, G.; Roth, J.; Kahl, B.C.; Proctor, R.A.; Peters, G. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010, 6, e1000715. [Google Scholar] [CrossRef] [PubMed]
- Malachowa, N.; Kobayashi, S.D.; Braughton, K.R.; Whitney, A.R.; Parnell, M.J.; Gardner, D.J.; Deleo, F.R. Staphylococcus aureus leukotoxin gh promotes inflammation. J. Infect. Dis. 2012, 206, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, N.; Kaihou, Y.; Noda, M. Purification, cloning and characterization of variant luke-lukd with strong leukocidal activity of staphylococcal bi-component leukotoxin family. Microbiol. Immunol. 2003, 47, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Malachowa, N.; Whitney, A.R.; Braughton, K.R.; Gardner, D.J.; Long, D.; Bubeck Wardenburg, J.; Schneewind, O.; Otto, M.; Deleo, F.R. Comparative analysis of usa300 virulence determinants in a rabbit model of skin and soft tissue infection. J. Infect. Dis. 2011, 204, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Lipinska, U.; Hermans, K.; Meulemans, L.; Dumitrescu, O.; Badiou, C.; Duchateau, L.; Haesebrouck, F.; Etienne, J.; Lina, G. Panton-valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: A rabbit model. PLoS ONE 2011, 6, e22864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malachowa, N.; Kobayashi, S.D.; Sturdevant, D.E.; Scott, D.P.; DeLeo, F.R. Insights into the Staphylococcus aureus-host interface: Global changes in host and pathogen gene expression in a rabbit skin infection model. PLoS ONE 2015, 10, e0117713. [Google Scholar] [CrossRef] [PubMed]
- Yoong, P.; Torres, V.J. Counter inhibition between leukotoxins attenuates Staphylococcus aureus virulence. Nat. Commun. 2015, 6, 8125. [Google Scholar] [CrossRef] [PubMed]
- Ezepchuk, Y.V.; Leung, D.Y.; Middleton, M.H.; Bina, P.; Reiser, R.; Norris, D.A. Staphylococcal toxins and protein a differentially induce cytotoxicity and release of tumor necrosis factor-alpha from human keratinocytes. J. Investig. Dermatol. 1996, 107, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Hocke, A.C.; Temmesfeld-Wollbrueck, B.; Schmeck, B.; Berger, K.; Frisch, E.M.; Witzenrath, M.; Brell, B.; Suttorp, N.; Hippenstiel, S. Perturbation of endothelial junction proteins by Staphylococcus aureus alpha-toxin: Inhibition of endothelial gap formation by adrenomedullin. Histochem. Cell Biol. 2006, 126, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Thelestam, M.; Mollby, R.; Wadstrom, T. Effects of staphylococcal alpha-, beta-, delta-, and gamma-hemolysins on human diploid fibroblasts and hela cells: Evaluation of a new quantitative as say for measuring cell damage. Infect. Immunity 1973, 8, 938–946. [Google Scholar]
- Walev, I.; Martin, E.; Jonas, D.; Mohamadzadeh, M.; Muller-Klieser, W.; Kunz, L.; Bhakdi, S. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect. Immunity 1993, 61, 4972–4979. [Google Scholar]
- Wilke, G.A.; Bubeck Wardenburg, J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc. Natl. Acad. Sci. USA 2010, 107, 13473–13478. [Google Scholar] [CrossRef] [PubMed]
- Ebsen, H.; Schröder, A.; Kabelitz, D.; Janssen, O. Differential surface expression of adam10 and adam17 on human T lymphocytes and tumor cells. PLoS ONE 2013, 8, e76853. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.D.; Bubeck Wardenburg, J.; Gardner, D.J.; Long, D.; Whitney, A.R.; Braughton, K.R.; Schneewind, O.; DeLeo, F.R. Targeting of alpha-hemolysin by active or passive immunization decreases severity of usa300 skin infection in a mouse model. J. Infect. Dis. 2010, 202, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yeh, A.J.; Cheung, G.Y.; Villaruz, A.E.; Tan, V.Y.; Joo, H.S.; Chatterjee, S.S.; Yu, Y.; Otto, M. Basis of virulence in a panton-valentine leukocidin-negative community-associated methicillin-resistant Staphylococcus aureus strain. J. Infect. Dis. 2015, 211, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.Y.; Monk, I.R.; Lin, Y.H.; Seemann, T.; Tuck, K.L.; Porter, J.L.; Stepnell, J.; Coombs, G.W.; Davies, J.K.; Stinear, T.P.; et al. Hyperexpression of alpha-hemolysin explains enhanced virulence of sequence type 93 community-associated methicillin-resistant Staphylococcus aureus. BMC Microbiol. 2014, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, C.; Hua, L.; Varkey, R.; Shi, Y.; Dettinger, L.; Woods, R.; Barnes, A.; MacGill, R.S.; Wilson, S.; Chowdhury, P.; et al. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin. Vaccine Immunol. 2012, 19, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Phenol-soluble modulins. Int. J. Med. Microbiol. 2014, 304, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.-H.L.; Queck, S.Y.; Li, M.; Kennedy, A.D.; Dorward, D.W.; Klebanoff, S.J.; Peschel, A.; et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated mrsa. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.; Kretschmer, D.; Queck, S.Y.; Joo, H.S.; Wang, R.; Duong, A.C.; Nguyen, T.H.; Bach, T.H.; Porter, A.R.; DeLeo, F.R.; et al. Insight into structure-function relationship in phenol-soluble modulins using an alanine screen of the phenol-soluble modulin (psm) alpha3 peptide. FASEB J. 2014, 28, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Surewaard, B.G.; de Haas, C.J.; Vervoort, F.; Rigby, K.M.; DeLeo, F.R.; Otto, M.; van Strijp, J.A.; Nijland, R. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell. Microbiol. 2013, 15, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Geiger, T.; Francois, P.; Liebeke, M.; Fraunholz, M.; Goerke, C.; Krismer, B.; Schrenzel, J.; Lalk, M.; Wolz, C. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular psms expression. PLoS Pathog. 2012, 8, e1003016. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Hook, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, A.P.; Budzik, J.M.; Oh, S.Y.; Schneewind, O. Architects at the bacterial surface—Sortases and the assembly of pili with isopeptide bonds. Nat. Rev. Microbiol. 2011, 9, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, G.; Jensen, E.R.; Lenoy, E.; Schneewind, O. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. USA 2000, 97, 5510–5515. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.G.; Kim, H.K.; Burts, M.L.; Krausz, T.; Schneewind, O.; Missiakas, D.M. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009, 23, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.; Jin, T.; Josefsson, E. Surface proteins of Staphylococcus aureus play an important role in experimental skin infection. APMIS 2014, 122, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, D.; Nanavaty, T.; House-Pompeo, K.; Bell, E.; Turner, N.; McIntire, L.; Foster, T.; Hook, M. Characterization of the interaction between the Staphylococcus aureus clumping factor (clfa) and fibrinogen. Eur. J. Biochem. 1997, 247, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, E.; Hartford, O.; O’Brien, L.; Patti, J.M.; Foster, T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor a, a novel virulence determinant. J. Infect. Dis. 2001, 184, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- McAdow, M.; Kim, H.K.; Dedent, A.C.; Hendrickx, A.P.; Schneewind, O.; Missiakas, D.M. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 2011, 7, e1002307. [Google Scholar] [CrossRef] [PubMed]
- Moreillon, P.; Entenza, J.M.; Francioli, P.; McDevitt, D.; Foster, T.J.; Francois, P.; Vaudaux, P. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immunity 1995, 63, 4738–4743. [Google Scholar]
- Higgins, J.; Loughman, A.; van Kessel, K.P.; van Strijp, J.A.; Foster, T.J. Clumping factor a of Staphylococcus aureus inhibits phagocytosis by human polymorphonuclear leucocytes. FEMS Microbiol. Lett. 2006, 258, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Flick, M.J.; Du, X.; Prasad, J.M.; Raghu, H.; Palumbo, J.S.; Smeds, E.; Hook, M.; Degen, J.L. Genetic elimination of the binding motif on fibrinogen for the S. aureus virulence factor clfa improves host survival in septicemia. Blood 2013, 121, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Kolata, J.B.; Kuhbandner, I.; Link, C.; Normann, N.; Vu, C.H.; Steil, L.; Weidenmaier, C.; Broker, B.M. The fall of a dogma? Unexpected high T-cell memory response to Staphylococcus aureus in humans. J. Infect. Dis. 2015, 212, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, M.E.; Geoghegan, J.A.; Monk, I.R.; O’Keeffe, K.M.; Walsh, E.J.; Foster, T.J.; McLoughlin, R.M. Nasal colonisation by Staphylococcus aureus depends upon clumping factor b binding to the squamous epithelial cell envelope protein loricrin. PLoS Pathog. 2012, 8, e1003092. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, A.C.; Solinga, R.M.; Cocchiaro, J.; Portoles, M.; Kiser, K.B.; Risley, A.; Randall, S.M.; Valtulina, V.; Speziale, P.; Walsh, E.; et al. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect. Immunity 2006, 74, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.; Walsh, E.; Choudhurry, R.; Melles, D.C.; Boelens, H.A.; Miajlovic, H.; Verbrugh, H.A.; Foster, T.; van Belkum, A. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med. 2008, 5, e17. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.J.; Miajlovic, H.; Gorkun, O.V.; Foster, T.J. Identification of the Staphylococcus aureus mscramm clumping factor B (clfb) binding site in the alphac-domain of human fibrinogen. Microbiology 2008, 154, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.J.; O’Brien, L.M.; Liang, X.; Hook, M.; Foster, T.J. Clumping factor B, a fibrinogen-binding mscramm (microbial surface components recognizing adhesive matrix molecules) adhesin of Staphylococcus aureus, also binds to the tail region of type I cytokeratin 10. J. Biol. Chem. 2004, 279, 50691–50699. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.C.; Steinert, P.M. Protein composition of cornified cell envelopes of epidermal keratinocytes. J. Cell Sci. 1994, 107, 693–700. [Google Scholar] [PubMed]
- Palmqvist, N.; Foster, T.; Fitzgerald, J.R.; Josefsson, E.; Tarkowski, A. Fibronectin-binding proteins and fibrinogen-binding clumping factors play distinct roles in staphylococcal arthritis and systemic inflammation. J. Infect. Dis. 2005, 191, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Peacock, S.J.; Foster, T.J.; Cameron, B.J.; Berendt, A.R. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 1999, 145 Pt 12, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Francois, P.P.; Nusse, O.; Foti, M.; Hartford, O.M.; Vaudaux, P.; Foster, T.J.; Lew, D.P.; Herrmann, M.; Krause, K.H. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin alpha5beta1. Cell. Microbiol. 1999, 1, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Massey, R.C.; Kantzanou, M.N.; Fowler, T.; Day, N.P.; Schofield, K.; Wann, E.R.; Berendt, A.R.; Hook, M.; Peacock, S.J. Fibronectin-binding protein a of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell. Microbiol. 2001, 3, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.M.; Potts, J.R.; Josefsson, E.; Massey, R.C. Staphylococcus aureus host cell invasion and virulence in sepsis is facilitated by the multiple repeats within fnbpa. PLoS Pathog. 2010, 6, e1000964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinji, H.; Yosizawa, Y.; Tajima, A.; Iwase, T.; Sugimoto, S.; Seki, K.; Mizunoe, Y. Role of fibronectin-binding proteins a and B in in vitro cellular infections and in vivo septic infections by Staphylococcus aureus. Infect. Immunity 2011, 79, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.M.; Potter, U.; Meenan, N.A.; Potts, J.R.; Massey, R.C. Staphylococcus aureus keratinocyte invasion is dependent upon multiple high-affinity fibronectin-binding repeats within fnbpa. PLoS ONE 2011, 6, e18899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Du, X.; Villaruz, A.E.; Diep, B.A.; Wang, D.; Song, Y.; Tian, Y.; Hu, J.; Yu, F.; Lu, Y.; et al. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat. Med. 2012, 18, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Du, X.; Hong, X.; Li, T.; Zheng, B.; He, L.; Wang, Y.; Otto, M.; Li, M. Targeting surface protein sasx by active and passive vaccination to reduce Staphylococcus aureus colonization and infection. Infection Immunity 2015, 83, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Immune evasion by staphylococci. Nat. Rev. Microbiol. 2005, 3, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, R.; Thoren-Tolling, K.; Sjoquist, J. Binding of immunoglobulins to protein a and immunoglobulin levels in mammalian sera. J. Immunol. Methods 1983, 62, 1–13. [Google Scholar] [CrossRef]
- Goodyear, C.S.; Silverman, G.J. Death by a B cell superantigen: In vivo vh-targeted apoptotic supraclonal B cell deletion by a staphylococcal toxin. J. Exp. Med. 2003, 197, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.I.; Lee, A.; Reddy, B.; Muir, A.; Soong, G.; Pitt, A.; Cheung, A.; Prince, A. Staphylococcus aureus protein a induces airway epithelial inflammatory responses by activating tnfr1. Nat. Med. 2004, 10, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kim, H.Y.; Schneewind, O.; Missiakas, D. Identifying protective antigens of Staphylococcus aureus, a pathogen that suppresses host immune responses. FASEB J. 2011, 25, 3605–3612. [Google Scholar] [CrossRef] [PubMed]
- Classen, A.; Kalali, B.N.; Schnopp, C.; Andres, C.; Aguilar-Pimentel, J.A.; Ring, J.; Ollert, M.; Mempel, M. Tnf receptor I on human keratinocytes is a binding partner for staphylococcal protein a resulting in the activation of NF Kappa b, ap-1, and downstream gene transcription. Exp. Dermatol. 2011, 20, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Grigg, J.C.; Ukpabi, G.; Gaudin, C.F.; Murphy, M.E. Structural biology of heme binding in the Staphylococcus aureus isd system. J. Inorganic Biochem. 2010, 104, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.R.; Brummell, K.J.; Horsburgh, M.J.; McDowell, P.W.; Mohamad, S.A.; Stapleton, M.R.; Acevedo, J.; Read, R.C.; Day, N.P.; Peacock, S.J.; et al. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J. Infect. Dis. 2006, 193, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.R.; Andre, G.; Walsh, E.J.; Dufrene, Y.F.; Foster, T.J.; Foster, S.J. Iron-regulated surface determinant protein a mediates adhesion of Staphylococcus aureus to human corneocyte envelope proteins. Infect. Immunity 2009, 77, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.R.; Mohamed, R.; Bian, L.; Routh, A.F.; Kokai-Kun, J.F.; Mond, J.J.; Tarkowski, A.; Foster, S.J. The Staphylococcus aureus surface protein isda mediates resistance to innate defenses of human skin. Cell Host Microbe 2007, 1, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.R.; Foster, S.J. Isda protects Staphylococcus aureus against the bactericidal protease activity of apolactoferrin. Infect. Immunity 2008, 76, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Kuklin, N.A.; Clark, D.J.; Secore, S.; Cook, J.; Cope, L.D.; McNeely, T.; Noble, L.; Brown, M.J.; Zorman, J.K.; Wang, X.M.; et al. A novel Staphylococcus aureus vaccine: Iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect. Immunity 2006, 74, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.; Smith, S.; Pancari, G.; Clark, D.; Hampton, R.; Secore, S.; Towne, V.; Fan, H.; Wang, X.M.; Wu, X.; et al. A fully human monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (isdb) with functional activity in vitro and in vivo. Hum. Antib. 2010, 19, 113–128. [Google Scholar]
- Brown, M.; Kowalski, R.; Zorman, J.; Wang, X.M.; Towne, V.; Zhao, Q.; Secore, S.; Finnefrock, A.C.; Ebert, T.; Pancari, G.; et al. Selection and characterization of murine monoclonal antibodies to Staphylococcus aureus iron-regulated surface determinant B with functional activity in vitro and in vivo. Clin. Vaccine Immunol. 2009, 16, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; DeDent, A.; Cheng, A.G.; McAdow, M.; Bagnoli, F.; Missiakas, D.M.; Schneewind, O. Isda and isdb antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine 2010, 28, 6382–6392. [Google Scholar] [CrossRef] [PubMed]
- Stranger-Jones, Y.K.; Bae, T.; Schneewind, O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2006, 103, 16942–16947. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Hu, D.L.; Mori, F.; Wakabayashi, K.; Iwakura, Y.; Nakane, A. Role of interleukin-17a in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor a. Infect. Immunity 2010, 78, 4234–4242. [Google Scholar] [CrossRef] [PubMed]
- Nissen, M.; Marshall, H.; Richmond, P.; Shakib, S.; Jiang, Q.; Cooper, D.; Rill, D.; Baber, J.; Eiden, J.; Gruber, W.; et al. A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (sa3ag) in healthy adults. Vaccine 2015, 33, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.A. Recent developments for Staphylococcus aureus vaccines: Clinical and basic science challenges. Eur. Cells Mater. 2015, 30, 315–326. [Google Scholar]
- Schmidt, C.S.; White, C.J.; Ibrahim, A.S.; Filler, S.G.; Fu, Y.; Yeaman, M.R.; Edwards, J.E., Jr.; Hennessey, J.P., Jr. Ndv-3, a recombinant alum-adjuvanted vaccine for candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 2012, 30, 7594–7600. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacey, K.A.; Geoghegan, J.A.; McLoughlin, R.M. The Role of Staphylococcus aureus Virulence Factors in Skin Infection and Their Potential as Vaccine Antigens. Pathogens 2016, 5, 22. https://doi.org/10.3390/pathogens5010022
Lacey KA, Geoghegan JA, McLoughlin RM. The Role of Staphylococcus aureus Virulence Factors in Skin Infection and Their Potential as Vaccine Antigens. Pathogens. 2016; 5(1):22. https://doi.org/10.3390/pathogens5010022
Chicago/Turabian StyleLacey, Keenan A., Joan A. Geoghegan, and Rachel M. McLoughlin. 2016. "The Role of Staphylococcus aureus Virulence Factors in Skin Infection and Their Potential as Vaccine Antigens" Pathogens 5, no. 1: 22. https://doi.org/10.3390/pathogens5010022
APA StyleLacey, K. A., Geoghegan, J. A., & McLoughlin, R. M. (2016). The Role of Staphylococcus aureus Virulence Factors in Skin Infection and Their Potential as Vaccine Antigens. Pathogens, 5(1), 22. https://doi.org/10.3390/pathogens5010022