FlpS, the FNR-Like Protein of Streptococcus suis Is an Essential, Oxygen-Sensing Activator of the Arginine Deiminase System
Abstract
:1. Introduction
2. Results
2.1. FlpS is Involved in the Regulation of the arcABC Operon and Metabolic Genes
2.2. Metabolic Characterization of the FlpS Knock-Out Strain 10ΔflpS
2.3. Loss of FlpS, but not CcpA, Is Dispensable for Virulence Properties of S. suis in Mice Infection Models
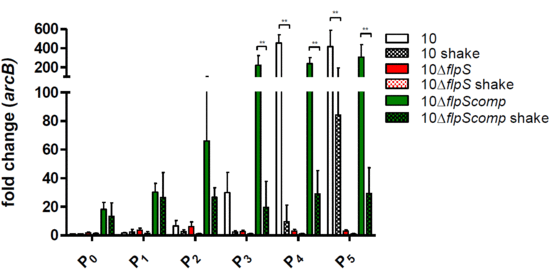
2.4. FlpS is an Oxygen-Sensing Regulator and Essential for arcABC Operon Expression In Vitro
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Mutagenesis of S. suis
4.3. Episomal Complementation of S. suis 10∆flpS
4.4. Microarray Analysis
4.5. Real-Time Quantitative RT-PCR (qRT-PCR)
4.6. Western Blot Analysis
4.7. GFP Reporter Studies
4.8. Isotopologue Profiling
4.9. Intracellular Survival in HEp-2 Cells
4.10. Experimental Infection of Mice
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADS | arginine deiminase system |
| CcpA | catabolite control protein A |
| exp | exponential |
| FlpS | FNR-like protein of S. suis |
| stat | stationary |
References
- Varela, N.P.; Gadbois, P.; Thibault, C.; Gottschalk, M.; Dick, P.; Wilson, J. Antimicrobial resistance and prudent drug use for Streptococcus suis. Anim. Health Res. Rev. 2013, 14, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M.; Xu, J.G.; Calzas, C.; Segura, M. Streptococcus suis: A new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010, 5, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Huong, V.T.L.; Ha, N.; Huy, N.T.; Horby, P.; Nghia, H.D.T.; Thiem, V.D.; Zhu, X.T.; Hoa, N.T.; Hien, T.T.; Zamora, J.; et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg. Infect. Dis. 2014, 20, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Mai, N.T.; Hoa, N.T.; Nga, T.V.; le Linh, D.; Chau, T.T.; Sinh, D.X.; Phu, N.H.; Chuong, L.V.; Diep, T.S.; Campbell, J.; et al. Streptococcus suis meningitis in adults in vietnam. Clin. Infect. Dis. 2008, 46, 659–667. [Google Scholar] [PubMed]
- Tang, J.Q.; Wang, C.J.; Feng, Y.J.; Yang, W.Z.; Song, H.D.; Chen, Z.H.; Yu, H.J.; Pan, X.Z.; Zhou, X.J.; Wang, H.R.; et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med. 2006, 3, e151. [Google Scholar]
- Fittipaldi, N.; Segura, M.; Grenier, D.; Gottschalk, M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012, 7, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Scott, C.; Guest, J.R. Functional versatility in the CRP-FNR superfamily of transcription factors: FNR and FLP. Adv. Microb. Physiol. 2001, 44, 1–34. [Google Scholar] [PubMed]
- Spiro, S.; Guest, J.R. FNR and its role in oxygen-regulated gene-expression in Escherichia coli. FEMS Microbiol. Lett. 1990, 75, 399–428. [Google Scholar] [CrossRef]
- Stulke, J.; Hillen, W. Carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 1999, 2, 195–201. [Google Scholar] [CrossRef]
- Green, J.; Bennett, B.; Jordan, P.; Ralph, E.T.; Thomson, A.J.; Guest, J.R. Reconstitution of the [4Fe-4S] cluster in FNR and demonstration of the aerobic-anaerobic transcription switch in vitro. Biochem. J. 1996, 316, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Irvine, A.S.; Meng, W.; Guest, J.R. FNR DNA interactions at natural and semi-synthetic promoters. Mol. Microbiol. 1996, 19, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Lazazzera, B.A.; Beinert, H.; Khoroshilova, N.; Kennedy, M.C.; Kiley, P.J. DNA binding and dimerization of the Fe-S-containing FNR protein from Escherichia coli are regulated by oxygen. J. Biol. Chem. 1996, 271, 2762–2768. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Q.; Chen, Y.Y.M.; Burne, R.A. Control of expression of the arginine deiminase operon of Streptococcus gordonii by CcpA and Flp. J. Bacteriol. 2004, 186, 2511–2514. [Google Scholar] [CrossRef] [PubMed]
- Gostick, D.O.; Green, J.; Irvine, A.S.; Gasson, M.J.; Guest, J.R. A novel regulatory switch mediated by the FNR-like protein of Lactobacillus casei. Microbiology 1998, 144 Pt 3, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.; Guest, J.R.; Green, J. Characterization of the Lactococcus lactis transcription factor FlpA and demonstration of an in vitro switch. Mol. Microbiol. 2000, 35, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Dong, Y.Q.; Chen, Y.Y.M.; Burne, R.A. Environmental and growth phase regulation of the Streptococcus gordonii arginine deiminase genes. Appl. Environ. Microb. 2008, 74, 5023–5030. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Dong, Y.Q.; Burne, R.A. Characterization of cis-acting sites controlling arginine deiminase gene expression in Streptococcus gordonii. J. Bacteriol. 2006, 188, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Q.; Chen, Y.Y.M.; Snyder, J.A.; Burne, R.A. Isolation and molecular analysis of the gene cluster for the arginine deiminase system from Streptococcus gordonii DL1. Appl. Environ. Microb. 2002, 68, 5549–5553. [Google Scholar] [CrossRef]
- Seitz, M.; Beineke, A.; Singpiel, A.; Willenborg, J.; Dutow, P.; Goethe, R.; Valentin-Weigand, P.; Klos, A.; Baums, C.G. Role of capsule and suilysin in mucosal infection of complement-deficient mice with Streptococcus suis. Infect. Immun. 2014, 82, 2460–2471. [Google Scholar] [CrossRef] [PubMed]
- Maghnouj, A.; Abu-Bakr, A.A.W.; Baumberg, S.; Stalon, V.; Vander Wauven, C. Regulation of anaerobic arginine catabolism in Bacillus licheniformis by a protein of the CRP/FNR family. FEMS Microbiol. Lett. 2000, 191, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Gamper, M.; Zimmermann, A.; Haas, D. Anaerobic regulation of transcription initiation in the arcDABC operon of Pseudomonas aeruginosa. J. Bacteriol. 1991, 173, 4742–4750. [Google Scholar] [PubMed]
- Barcelona-Andres, B.; Marina, A.; Rubio, V. Gene structure, organization, expression, and potential regulatory mechanisms of arginine catabolism in Enterococcus faecalis. J. Bacteriol. 2002, 184, 6289–6300. [Google Scholar] [CrossRef] [PubMed]
- Gruening, P.; Fulde, M.; Valentin-Weigand, P.; Goethe, R. Structure, regulation, and putative function of the arginine deiminase system of Streptococcus suis. J. Bacteriol. 2006, 188, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Galimand, M.; Gamper, M.; Zimmermann, A.; Haas, D. Positive FNR-like control of anaerobic arginine degradation and nitrate respiration in Pseudomonas aeruginosa. J. Bacteriol. 1991, 173, 1598–1606. [Google Scholar] [PubMed]
- Lu, C.D.; Winteler, H.; Abdelal, A.; Haas, D. The ArgR regulatory protein, a helper to the anaerobic regulator ANR during transcriptional activation of the arcD promoter in Pseudomonas aeruginosa. J. Bacteriol. 1999, 181, 2459–2464. [Google Scholar] [PubMed]
- Makhlin, J.; Kofman, T.; Borovok, I.; Kohler, C.; Engelmann, S.; Cohen, G.; Aharonowitz, Y. Staphylococcus aureus ArcR controls expression of the arginine deiminase operon. J. Bacteriol. 2007, 189, 5976–5986. [Google Scholar] [CrossRef] [PubMed]
- Schofield, P.J.; Costello, M.; Edwards, M.R.; Osullivan, W.J. The arginine dihydrolase pathway is present in Giardia intestinalis. Int. J. Parasitol. 1990, 20, 697–699. [Google Scholar] [CrossRef]
- Zuniga, M.; Perez, G.; Gonzalez-Candelas, F. Evolution of arginine deiminase (adi) pathway genes. Mol. Phylogenet. Evol. 2002, 25, 429–444. [Google Scholar] [CrossRef]
- Cunin, R.; Glansdorff, N.; Pierard, A.; Stalon, V. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 1986, 50, 314–352. [Google Scholar] [PubMed]
- Marquis, R.E.; Bender, G.R.; Murray, D.R.; Wong, A. Arginine deiminase system and bacterial adaptation to acid environments. Appl. Environ. Microb. 1987, 53, 198–200. [Google Scholar]
- Burne, R.A.; Marquis, R.E. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol. Lett. 2000, 193, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Hill, C. Surviving the acid test: Responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef] [PubMed]
- Fulde, M.; Willenborg, J.; de Greeff, A.; Benga, L.; Smith, H.E.; Valentin-Weigand, P.; Goethe, R. ArgR is an essential local transcriptional regulator of the arcABC operon in Streptococcus suis and is crucial for biological fitness in an acidic environment. Microbiology 2011, 157, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Willenborg, J.; de Greeff, A.; Jarek, M.; Valentin-Weigand, P.; Goethe, R. The CcpA regulon of Streptococcus suis reveals novel insights into the regulation of the streptococcal central carbon metabolism by binding of Ccpa to two distinct binding motifs. Mol. Microbiol. 2014, 92, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Chaussee, M.S.; Somerville, G.A.; Reitzer, L.; Musser, J.M. Rgg coordinates virulence factor synthesis and metabolism in Streptococcus pyogenes. J. Bacteriol. 2003, 185, 6016–6024. [Google Scholar] [CrossRef] [PubMed]
- Willenborg, J.; Huber, C.; Koczula, A.; Lange, B.; Eisenreich, W.; Valentin-Weigand, P.; Goethe, R. Characterization of the pivotal carbon metabolism of Streptococcus suis serotype 2 under ex vivo and chemically defined in vitro conditions by isotopologue profiling. J. Biol. Chem. 2015, 290, 5840–5854. [Google Scholar] [CrossRef] [PubMed]
- Seitz, M.; Beineke, A.; Seele, J.; Fulde, M.; Valentin-Weigand, P.; Baums, C.G. A novel intranasal mouse model for mucosal colonization by Streptococcus suis serotype 2. J. Med. Microbiol. 2012, 61, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Willenborg, J.; Fulde, M.; de Greeff, A.; Rohde, M.; Smith, H.E.; Valentin-Weigand, P.; Goethe, R. Role of glucose and CcpA in capsule expression and virulence of Streptococcus suis. Microbiology 2011, 157, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Goerke, B.; Stulke, J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Gostick, D.O.; Griffin, H.G.; Shearman, C.A.; Scott, C.; Green, J.; Gasson, M.J.; Guest, J.R. Two operons that encode FNR-like proteins in Lactococcus lactis. Mol. Microbiol. 1999, 31, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Maghnouj, A.; Cabral, T.F.D.; Stalon, V.; Vander Wauven, C. The arcABDC gene cluster, encoding the arginine deiminase pathway of Bacillus licheniformis, and its activation by the arginine repressor ArgR. J. Bacteriol. 1998, 180, 6468–6475. [Google Scholar] [PubMed]
- Korner, H.; Sofia, H.J.; Zumft, W.G. Phylogeny of the bacterial superfamily of CRP-FNR transcription regulators: Exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol. Rev. 2003, 27, 559–592. [Google Scholar] [CrossRef]
- Kiley, P.J.; Beinert, H. Oxygen sensing by the global regulator, FNR: The role of the iron-sulfur cluster. FEMS Microbiol. Rev. 1998, 22, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Spiro, S. The FNR family of transcriptional regulators. Anton. Leeuw. 1994, 66, 23–36. [Google Scholar] [CrossRef]
- Fei, F.; Mendonca, M.L.; McCarry, B.E.; Bowdish, D.M.E.; Surette, M.G. Metabolic and transcriptomic profiling of Streptococcus intermedius during aerobic and anaerobic growth. Metabolomics 2016, 12, 1–13. [Google Scholar] [CrossRef]
- Ahn, S.J.; Wen, Z.T.; Burne, R.A. Effects of oxygen on virulence traits of Streptococcus mutans. J. Bacteriol. 2007, 189, 8519–8527. [Google Scholar] [CrossRef] [PubMed]
- Fulde, M.; Willenborg, J.; Huber, C.; Hitzmann, A.; Willms, D.; Seitz, M.; Eisenreich, W.; Valentin-Weigand, P.; Goethe, R. The arginine-ornithine antiporter ArcD contributes to biological fitness of Streptococcus suis. Front. Cell. Infect. Microbiol. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.E.; Damman, M.; van der Velde, J.; Wagenaar, F.; Wisselink, H.J.; Stockhofe-Zurwieden, N.; Smits, M.A. Identification and characterization of the cps locus of Streptococcus suis serotype 2: The capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 1999, 67, 1750–1756. [Google Scholar] [PubMed]
- Baums, C.G.; Kaim, U.; Fulde, M.; Ramachandran, G.; Goethe, R.; Valentin-Weigand, P. Identification of a novel virulence determinant with serum opacification activity in Streptococcus suis. Infect. Immun. 2006, 74, 6154–6162. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.A.; Haefliger, J.A.; Francioli, P.; Moreillon, P. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect. Immun. 2000, 68, 3516–3522. [Google Scholar] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willenborg, J.; Koczula, A.; Fulde, M.; De Greeff, A.; Beineke, A.; Eisenreich, W.; Huber, C.; Seitz, M.; Valentin-Weigand, P.; Goethe, R. FlpS, the FNR-Like Protein of Streptococcus suis Is an Essential, Oxygen-Sensing Activator of the Arginine Deiminase System. Pathogens 2016, 5, 51. https://doi.org/10.3390/pathogens5030051
Willenborg J, Koczula A, Fulde M, De Greeff A, Beineke A, Eisenreich W, Huber C, Seitz M, Valentin-Weigand P, Goethe R. FlpS, the FNR-Like Protein of Streptococcus suis Is an Essential, Oxygen-Sensing Activator of the Arginine Deiminase System. Pathogens. 2016; 5(3):51. https://doi.org/10.3390/pathogens5030051
Chicago/Turabian StyleWillenborg, Jörg, Anna Koczula, Marcus Fulde, Astrid De Greeff, Andreas Beineke, Wolfgang Eisenreich, Claudia Huber, Maren Seitz, Peter Valentin-Weigand, and Ralph Goethe. 2016. "FlpS, the FNR-Like Protein of Streptococcus suis Is an Essential, Oxygen-Sensing Activator of the Arginine Deiminase System" Pathogens 5, no. 3: 51. https://doi.org/10.3390/pathogens5030051
APA StyleWillenborg, J., Koczula, A., Fulde, M., De Greeff, A., Beineke, A., Eisenreich, W., Huber, C., Seitz, M., Valentin-Weigand, P., & Goethe, R. (2016). FlpS, the FNR-Like Protein of Streptococcus suis Is an Essential, Oxygen-Sensing Activator of the Arginine Deiminase System. Pathogens, 5(3), 51. https://doi.org/10.3390/pathogens5030051