Detection of Campylobacter jejuni in Lizard Faeces from Central Australia Using Quantitative PCR
Abstract
:1. Introduction
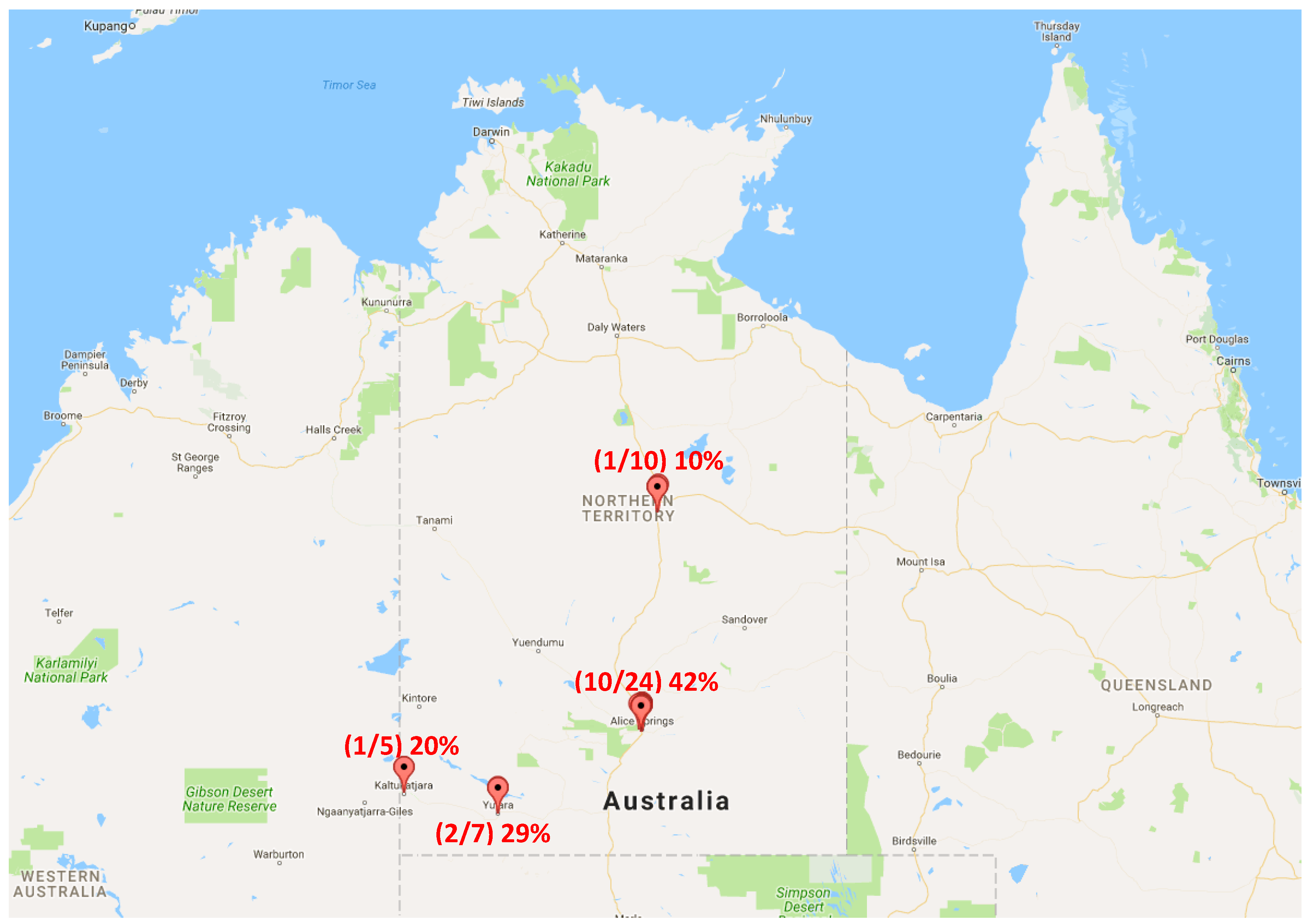
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. qPCR Detection of Campylobacter jejuni
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Pike, B.L.; Guerry, P.; Poly, F. Global distribution of Campylobacter jejuni penner serotypes: A systematic review. PLoS ONE 2013, 8, e67375. [Google Scholar] [CrossRef] [PubMed]
- Whiley, H.; van den Akker, B.; Giglio, S.; Bentham, R. The role of environmental reservoirs in human campylobacteriosis. Int. J. Environ. Res. Public Health 2013, 10, 5886–5907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitkänen, T. Review of Campylobacter spp. in drinking and environmental waters. J. Microbiol. Methods 2013, 95, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bronowski, C.; James, C.E.; Winstanley, C. Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol. Lett. 2014, 356, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Ivarsson, S.; Lofdahl, M.; Nauta, M.J. Estimating the true incidence of campylobacteriosis and salmonellosis in the European Union, 2009. Epidemiol. Infect. 2013, 141, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.; Angulo, F. Foodborne Illness Acquired in the United States—Major Pathogens; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2011.
- Department of Health, Australia’s National Notifiable Diseases Surveillance System (NNDSS). 2016. Available online: http://www9.health.gov.au/cda/source/cda-index.cfm.
- Kirk, M.; Ford, L.; Glass, K.; Hall, G. Foodborne illness, Australia, circa 2000 and circa 2010. Emerg. Infect. Dis. 2014, 20, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.E. Campylobacter jejuni: A review of its characteristics, pathogenicity, ecology, distribution, subspecies characterization and molecular methods of detection. Food Biotechnol. 2007, 21, 271–347. [Google Scholar] [CrossRef]
- Hiett, K.; Stern, N.; Fedorka-Cray, P.; Cox, N.; Musgrove, M.; Ladely, S. Molecular subtype analyses of Campylobacter spp. from Arkansas and California poultry operations. Appl. Environ. Microbiol. 2002, 68, 6220–6236. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Gardner, T.; McLaughlin, J.; Xavier, C. Campylobacteriosis outbreak due to consumption of raw peas-Alaska, 2008. In Proceedings of the IDSA 47th Annual Meeting: Bacterial Infections and Treatments, Philadelphia, PA, USA, 29 October 2009; Infectious Disease Society America.
- Angulo, F.J.; Steinmuller, N.; Demma, L.; Bender, J.B.; Eidson, M. Outbreaks of enteric disease associated with animal contact: Not just a foodborne problem anymore. Clin. Infect. Dis. 2006, 43, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Mermin, J.; Hutwagner, L.; Vugia, D.; Shallow, S.; Daily, P.; Bender, J.; Koehler, J.; Marcus, R.; Angulo, F.J.; Emerging Infections Program FoodNet Working Group. Reptiles, amphibians, and human Salmonella infection: A population-based, case-control study. Clin. Infect. Dis. 2004, 38, S253–S261. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, L.; Vercammen, F.; Bertrand, S.; Collard, J.M.; De Ceuster, S. Isolation of Salmonella from environmental samples collected in the reptile department of Antwerp Zoo using different selective methods. J. Appl. Microbiol. 2006, 101, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Callaway, Z.; Thomas, A.; Melrose, W.; Buttner, P.; Speare, R. Salmonella virchow and Salmonella weltevreden in a random survey of the Asian house gecko, Hemidactylus frenatus, in houses in northern Australia. Vector Borne Zoonotic Dis. 2011, 11, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Corrente, M.; Madio, A.; Friedrich, K.G.; Greco, G.; Desario, C.; Tagliabue, S.; D’Incau, M.; Campolo, M.; Buonavoglia, C. Isolation of Salmonella strains from reptile faeces and comparison of different culture media. J. Appl. Microbiol. 2004, 96, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Cyriac, J.; Wozniak, E. Infantile Salmonella meningitis associated with gecko-keeping. Commun. Dis. Public Health 2000, 3, 66–67. [Google Scholar] [PubMed]
- Ebani, V.V.; Cerri, D.; Fratini, F.; Meille, N.; Valentini, P.; Andreani, E. Salmonella enterica isolates from faeces of domestic reptiles and a study of their antimicrobial in vitro sensitivity. Res. Vet. Sci. 2005, 78, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Gugnani, H.C.; Oguike, J.U.; Sakazaki, R. Salmonellae and other enteropathogenic bacteria in the intestines of wall geckos in Nigeria. Antonie van Leeuwenhoek 1986, 52, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, K.; Moreno Switt, A.; Wiedmann, M. Animal contact as a source of human non-typhoidal salmonellosis. Vet. Res. 2011, 42, 34. [Google Scholar] [CrossRef] [PubMed]
- Keen, J.E.; Durso, L.M.; Meehan, T.P. Isolation of Salmonella enterica and shiga-toxigenic Escherichia coli o157 from feces of animals in public contact areas of United States zoological parks. Appl. Environ. Microbiol. 2007, 73, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Lowther, S.A.; Medus, C.; Scheftel, J.; Leano, F.; Jawahir, S.; Smith, K. Foodborne outbreak of Salmonella subspecies IV infections associated with contamination from bearded dragons. Zoonoses Public Health 2011, 58, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.J.; Myers, A.A. A review of Salmonella infections in reptiles with particular reference to Gekkonidae. Amphib. Reptil. 1993, 14, 357–371. [Google Scholar] [CrossRef]
- Ward, L. Salmonella perils of pet reptiles. Commun. Dis. Public Health 2000, 3, 2–3. [Google Scholar] [PubMed]
- Wobeser, G.A. Disease in Wild Animals, 2nd ed.; Plenum Press: New York, NY, USA, 2007; p. 393. [Google Scholar]
- Gilbert, M.J.; Kik, M.; Timmerman, A.J.; Severs, T.T.; Kusters, J.G.; Duim, B.; Wagenaar, J.A. Occurrence, diversity, and host association of intestinal Campylobacter, Arcobacter, and Helicobacter in reptiles. PLoS ONE 2014, 9, e101599. [Google Scholar] [CrossRef] [PubMed]
- Patrick, M.E.; Gilbert, M.J.; Blaser, M.J.; Tauxe, R.V.; Wagenaar, J.A.; Fitzgerald, C. Human infections with new subspecies of Campylobacter fetus. Emerg. Infect. Dis. 2013, 19, 1678–1680. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-M.; Shia, W.-Y.; Jhou, Y.-J.; Shyu, C.-L. Occurrence and molecular characterization of reptilian Campylobacter fetus strains isolated in Taiwan. Vet. Microbiol. 2013, 164, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Cogger, H.G. General description and definition of the Squamata. In Fauna of Australia Volume 2A; Glasby, C.G., Ross, G.J.B., Beesley, P.L., Eds.; Australian Government Publishing Service (AGPS): Canberra, Australia, 1993. [Google Scholar]
- Pianka, E.R. Habitat specificity, speciation, and species density in Australian desert lizards. Ecology 1969, 50, 498–502. [Google Scholar] [CrossRef]
- Ackley, J.W.; Wu, J.; Angilletta, M.J.; Myint, S.W.; Sullivan, B. Rich lizards: How affluence and land cover influence the diversity and abundance of desert reptiles persisting in an urban landscape. Biol. Conserv. 2015, 182, 87–92. [Google Scholar] [CrossRef]
- Google Maps 2016, Northern Territory. Available online: http://www.google.com.au/maps/place/northern+territory/@-18.4000078,129.0076453,6z/data=!3m1!4b1!4m5!3m4!1s0x2b5172c2e6f3190f:0x2775!8m2!3d-19.4914108!4d132.5509603 (accessed on 5 June 2016).
- Flekna, G.; Štefanič, P.; Wagner, M.; Smulders, F.J.M.; Možina, S.S.; Hein, I. Insufficient differentiation of live and dead Campylobacter jejuni and Listeria monocytogenes cells by ethidium monoazide (EMA) compromises EMA/real-time PCR. Res. Microbiol. 2007, 158, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Fratini, F. Bacterial zoonoses among domestic reptiles. Annali della Facoltà di Medicina Veterinaria 2005, 58, 85–91. [Google Scholar]
- Hemsworth, S.; Pizer, B. Pet ownership in immunocompromised children—A review of the literature and survey of existing guidelines. Eur. J. Oncol. Nurs. 2006, 10, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Levine, M.M.; Clements, M.L.; Hughes, T.P.; Blaser, M.J. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 1988, 157, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, F.A.; Groves, S.J.; Chambers, B.J. Pathogen survival during livestock manure storage and following land application. Bioresour. Technol. 2005, 96, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sinton, L.W.; Braithwaite, R.R.; Hall, C.H.; Mackenzie, M.L. Survival of indicator and pathogenic bacteria in bovine feces on pasture. Appl. Environ. Microbiol. 2007, 73, 7917–7925. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.L. Fly transmission of Campylobacter. Emerg. Infect. Dis. 2005, 11, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Templeton, J.M.; De Jong, A.J.; Blackall, P.; Miflin, J.K. Survival of Campylobacter spp. in darkling beetles (Alphitobius diaperinus) and their larvae in Australia. Appl. Environ. Microbiol. 2006, 72, 7909–7911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, N.; Fedorka-Cray, P.; Bailey, J.; Cox, N.; Craven, S.; Hiett, K.; Musgrove, M.; Ladely, S.; Cosby, D.; Mead, G. Distribution of Campylobacter spp. In selected US poultry production and processing operations. J. Food Prot. 2001, 64, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.E.; Hume, I.D. Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol. Rev. 1998, 78, 393–427. [Google Scholar] [PubMed]
- Zug, G.R.; Vitt, L.; Caldwell, J.P. Herpetology: An Introductory Biology of Amphibians and Reptiles; Academic Press: Waltham, MA, USA, 2001. [Google Scholar]
- Wasser, S.K.; Houston, C.S.; Koehler, G.M.; Cadd, G.G.; Fain, S.R. Techniques for application of faecal DNA methods to field studies of ursids. Mol. Ecol. 1997, 6, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Ariefdjohan, M.W.; Savaiano, D.A.; Nakatsu, C.H. Comparison of DNA extraction kits for PCR-DGGEanalysis of human intestinal microbial communities from fecal specimens. Nutr. J. 2010, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Best, E.L.; Powell, E.J.; Swift, C.; Grant, K.A.; Frost, J.A. Applicability of a rapid duplex real-time PCR assay for speciation of Campylobacter jejuni and Campylobacter coli directly from culture plates. FEMS Microbiol. Lett. 2003, 229, 237–241. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whiley, H.; McLean, R.; Ross, K. Detection of Campylobacter jejuni in Lizard Faeces from Central Australia Using Quantitative PCR. Pathogens 2017, 6, 1. https://doi.org/10.3390/pathogens6010001
Whiley H, McLean R, Ross K. Detection of Campylobacter jejuni in Lizard Faeces from Central Australia Using Quantitative PCR. Pathogens. 2017; 6(1):1. https://doi.org/10.3390/pathogens6010001
Chicago/Turabian StyleWhiley, Harriet, Ryan McLean, and Kirstin Ross. 2017. "Detection of Campylobacter jejuni in Lizard Faeces from Central Australia Using Quantitative PCR" Pathogens 6, no. 1: 1. https://doi.org/10.3390/pathogens6010001
APA StyleWhiley, H., McLean, R., & Ross, K. (2017). Detection of Campylobacter jejuni in Lizard Faeces from Central Australia Using Quantitative PCR. Pathogens, 6(1), 1. https://doi.org/10.3390/pathogens6010001





