Abstract
Helicobacter pylori (H. pylori) represents an independent risk factor for Gastric Cancer (GC). First Degree Relatives (FDR) of GC subjects and Autoimmune Gastritis (AG) patients are both at increased risk for GC. H. pylori genetic heterogeneity within the gastric niche of FDR and AG individuals has been little explored. To understand whether they exploit an increased H. pylori stability and virulence, 14 AG, 25 FDR, 39 GC and 13 dyspeptic patients (D) were investigated by a cultural PCR-based approach characterizing single colonies-forming-units. We chose three loci within the Cytotoxin-associated gene-A Pathogenicity Island (CagPAI) (cagA,cagE,virB11), vacA, homA and homB as markers of virulence with reported association to GC. Inflammatory/precancerous lesions were staged according to Sydney System. When compared to D, FDR, similarly to GC patients, were associated to higher atrophy (OR = 6.29; 95% CI:1.23–31.96 in FDR; OR = 7.50; 95% CI:1.67–33.72 in GC) and a lower frequency of mixed infections (OR = 0.16; 95% CI:0.03–0.81 in FDR; OR = 0.10; 95% CI:0.02–0.48 in GC). FDR presented also an increased neutrophil infiltration (OR = 7.19; 95% CI:1.16–44.65). Both FDR and GC carried a higher proportion of CagPAI+vacAs1i1mx+homB+ profiles (OR = 2.71; 95% CI: 1.66–4.41 and OR = 3.43; 95% CI: 2.16–5.44, respectively). Conversely, AG patients presented a lower frequency of subtypes carrying a stable CagPAI and vacAs1i1mx. These results underline different H. pylori plasticity in FDR and AG individuals, and thus, a different host-bacterium interaction capacity that should be considered in the context of eradication therapies.
1. Introduction
H. pylori has presumably co-evolved with humans for at least 50,000 years to be transmitted from person to person and become a commensal of the stomach [1,2]. An equilibrium between H. pylori and host responses allows microbial persistence resulting in an increased risk of gastric neoplasia. H. pylori has been classified as a class I human carcinogen by the International Agency for Research on Cancer working group for its association, in particular, with non-cardia gastric cancer (GC) and mucosa-associated lymphoid tissue (MALT) lymphoma [3]. Since then, H. pylori infection has been shown to be the primary cause of gastric neoplasms [4], although its effects are multi-factorial. The mechanisms by which H. pylori may express its pathogenetic potential is related to bacterial structure and induced chronic inflammation, that triggers chronic active gastritis and development of GC lesions according the currently accepted model of precancerous Correa’s cascade (in order, non-atrophic gastritis, multifocal atrophic gastritis, intestinal metaplasia, dysplasia and, finally, cancer) [5].
Histopathological changes in the gastric mucosa can be associated with H. pylori fitness adaptation through multiple and subtle genetic events which allow its persistence in the microenvironment [6]. As a consequence, each host is colonized by a multitude of genetically closely related microorganisms, similar to quasispecies, which interfere with signaling pathways influencing host cell growth and death [7,8,9]. Several studies suggested a functional relation of particular combinations of genes and proteins, determining certain traits of H. pylori and specific pre-cancerous or pathological conditions [10,11,12,13,14,15]. In particular, the composition of the Cytotoxin-associated gene A Pathogenicity Island (CagPAI) modulates bacterial motility, survival, production of proinflammatory cytokines and antimicrobial susceptibility [16,17,18,19,20]. It has been highlighted that a single H. pylori strain may include variable proportions of subtypes with different CagPAI genotypes [21,22]. Deletions of CagPAI genes were more frequently detected among individuals with metaplasia and atrophic gastritis than non-atrophic gastritis or duodenal ulcers [23,24]. Another H. pylori virulence factor is the vacuolating toxin A (VacA) [25,26]. Different VacA isoforms are generated through the combination of three polymorphic regions, namely the signal (s), the intermediate (i) and the middle (m) regions, which affect the anion-selective channels formation, the vacuolating activity and the binding to different cell surface receptors, respectively [27,28,29,30]. The outer membrane protein (OMP) family includes surface molecules that are involved in H. pylori adherence and in the induction of a robust inflammatory response. Among OMP genes, homA and homB are poorly studied [31]. HomB had been associated with GC in USA, Colombia and Iran and a lower frequency of homA had been evidenced in patients with GC compared to those with chronic gastritis [32,33]. However, very few studies have examined the association between hom genes and GC in European countries [34].
Several studies have shown that long term colonization by specific H. pylori strains and the outcome of the infection are strictly dependent on interactions between H. pylori and the host genetic factors [25,35,36,37,38]. Subjects with a family history of GC or affected by autoimmune gastritis (AG) displayed a 1.5–3.0 fold higher risk to develop GC when compared to the general population [39,40,41] and in these individuals H. pylori is a recognized causative agent of gastritis. However, the importance of H. pylori virulence factors, along with conditions such as being First Degree Relatives (FDR) or having AG, which could increase the risk of GC development, has been little explored in non-endemic areas [42,43,44,45,46,47].
The aim of the present study is to understand whether an increased GC risk in populations, such as FDR and AG, could be associated with an intrinsic H. pylori high virulence. The results will increase the knowledge on H. pylori pathogenesis and have implications in guiding the choice of eradication in these patients. For this purpose, we dissected H. pylori strain heterogeneity in the gastric niche of FDR and AG individuals by using a focused genetic analysis on the most representative inflammation-related H. pylori virulence factors: virB11, cagE and cagA, located in the CagPAI, homA and homB genes, and vacA s, m and i regions. We applied virulent gene profiling on single bacterial isolates from primary plates obtained from the biopsies of each single patient (at least 10 colony-forming-units (CFU) per patient). Results could highlight possible virulence factors associated with predisposition to GC development in specific populations at risk for GC.
2. Results
2.1. Patients Characteristics
Patients were selected from a larger population performing endoscopy at the Oncological Gastroenterology Division, Centro di Riferimento Oncologico (CRO), Aviano (Italy) and submitted to H. pylori infection diagnostic workup. Inclusion criteria and strategies for variable clustering were defined in the Materials and Methods section. Table 1 shows the demographic characteristics of the subjects and the Sydney classification of gastric lesions related to the site of H. pylori isolation. When compared to dyspeptic patients (D), no statistically significant difference in the distribution of the patients by age and sex was observed. FDR were associated with a significantly higher gastric neutrophil activity (OR = 7.19; 95% CI: 1.16–44.65, p = 0.03) and glandular atrophy (OR = 6.29; 95% CI: 1.23–31.96, p = 0.03). This last characteristic was shared with GC patients (OR = 7.50; 95% CI: 1.67–33.72, p = 0.009), while the association of AG with atrophy was nearly significant (OR = 5.14; 95% CI: 0.81–32.77, p = 0.08). No statistically significant difference in intestinal metaplasia was evidenced by all the groups, while the degree of chronic inflammation and H. pylori density were similar in FDR and slightly lower in GC and AG when compared to D group.

Table 1.
Demographic characteristics and Sydney System classification of the studied subjects.
2.2. H. pylori Putative Virulent Gene Load in the Gastric Niche
The H. pylori gastric niche was globally analyzed in each subject pooling the subtypes positive for the studied virulent factors. The concomitant presence of cagA, cagE and virB11 was considered as an indicator of CagPAI stability. VacA haplotypes were clustered considering s1i1mx with a highest vacuolization property than sxi2m2 haplotype. The presence of homB haplotype, which putatively confers to H. pylori higher level pathogenicity than homA, was also evaluated.
GC patients showed a higher risk than D to carry a stable CagPAI (OR = 19.20; 95% CI: 3.79–97.36, p = 0.0004), and a nearly significant association to the most virulent forms of the vacA gene (OR = 3.44; 95% CI: 0.83-14.17, p = 0.09) (Table 2). No difference in homB gene distribution was observed. A lower presence of cagA gene was registered in the H. pylori niche from AG subjects in comparison to D (OR = 0.17; 95% CI: 0.03–0.90, p = 0.04). Overall, AG patients showed a tendency to have a lower association with all the analyzed highly virulent factors. VirB11 was the most conserved CagPAI gene in all the tested populations. We then evaluated the prevalence of H. pylori mixed infections defined as the presence in a patient of at least one subtype different from the others for at least one virulence factor. We showed that mixed infections were less frequent in GC (OR = 0.10; 95% CI: 0.02–0.48, p = 0.004) and FDR groups (OR = 0.16; 95% CI: 0.03–0.81, p = 0.03) compared to D, whereas no statistically significant difference was observed for AG.

Table 2.
H. pylori putative virulent gene load in the gastric niche of the studied subjects.
2.3. Association Between H. pylori Virulence Factors within H. pylori Subtypes
CagPAI-positive H. pylori strains are most likely to carry the highly toxic forms of vacA [48]. Thus, we next analyzed whether there was any association among vacA haplotypes and a stable CagPAI in the subtypes, and we compared their frequencies within groups. An association between vacA s1i1mx forms and a stable CagPAI was shown in all the groups (OR = 15.18; 95% CI: 6.82-33.78, p < 0.0001 in GC; OR = 34.47; 95% CI: 9.78-121.42, p < 0.001 in D; OR = 108.65; 95% CI: 25.66-459.99, p < 0.0001 in FDR; OR = 3.16; 95% CI: 1.50-6.69, p = 0.003 in AG) (Table 3). The frequency of the subtypes with the coexistence of these virulence factors was particularly high in GC (81.6% of the subtypes) (Figure 1a). In this group, the less virulent vacA sxi2m2 subtypes were also mainly associated to the presence of a stable CagPAI, but this genotype was present only in a minority of cases (10.2% of the subtypes) (Figure 1a). Subtypes carrying vacA s1i1mx and stable CagPAI were the less represented within the AG group (18.8% of the subtypes) (Figure 1a), and in comparison to D (OR = 0.33; 95% CI: 0.17–0.66, p = 0.0016).

Table 3.
Association between CagPAI status and vacA polymorphisms or hom haplotypes, and between hom haplotypes and vacA polymorphisms within H. pylori CFU by group.
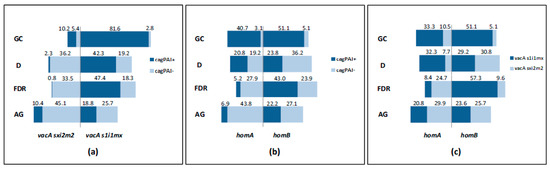
Figure 1.
Schematic representation of the distribution of H. pylori subtypes by study group. (a) Frequency of H. pylori subtypes carrying a stable/unstable CagPAI associated to vacA variants; (b) frequency of H. pylori subtypes carrying a stable/unstable CagPAI associated to hom haplotypes; (c) frequency of H. pylori subtypes harboring the simultaneous presence of hom and vacA haplotypes. CagPAI+, subtypes with stable CagPAI (positive for virB11, cagE and cagA); CagPAI-, subtypes with at least one deletion for virB11, cagE or cagA.
HomB was found to coexist with the most virulent genotypes [31]; hence, we further assessed whether hom haplotypes were associated to the presence of CagPAI or vacA haplotypes. The frequency of homB haplotype was slightly higher in the GC group if compared to homA (56.2% versus 43.8% of the subtypes) (Figure 1b,c). However, while the subtypes with a stable CagPAI were equally associated with homB and A genes (51.1% and 40.7% of the subtypes) (Figure 1b), the virulent form of vacA showed a significant high risk of being linked with homB (OR = 3.14; 95% CI: 1.76–5.60, p = 0.0001) (Table 3). In the D group subjects, the distribution of the subtypes carrying a stable CagPAI was similar independently of hom status (23.8% in homB, 20.8% in homA) (Figure 1b); a low association between homB and vacA s1i1mx was found (OR = 0.23; 95% CI: 0.10–0.51, p = 0.0004) (Table 3).
FDR showed a high proportion of subtypes displaying homB associated to the presence of a stable CagPAI (43.0% of the subtypes) (Figure 1b) (OR = 9.70; 95% CI: 5.00–18.96, p < 0.0001) (Table 3) or vacA s1i1mx (57.3% of the subtypes) (Figure 1c) (OR = 17.71; CI: 9.18–34.17, p < 0.0001) (Table 3). These associations were statistically significant also in comparison to D (homB and stable CagPAI compresence: OR = 2.73; 95% CI: 1.57-4.74, p = 0.0004; homB and vacA s1i1mx compresence: OR = 7.58; 95% CI: 4.02–14.29, p < 0.0001). Thus, subtypes owning all the three virulence factors (stable CagPAI, vacAs1i1mx and homB) were significantly associated with FDR (OR = 2.71, 95% CI: 1.66–4.41, p = 0.0001) as demonstrated by GC (OR = 3.43, 95% CI: 2.16-5.54, p < 0.0001) (Table 4). Although with a low statistical significance, the highly virulent profile appeared to be more prevalent in FDR and GC patients with intestinal metaplasia than in those without (OR = 9.33; 95% CI: 0.85–101.96, p = 0.07 in FDR; 3.18; 95% CI: 0.86–11.79, p = 0.08 in GC).

Table 4.
Subtypes carrying highly virulent profile (concomitant presence of stable CagPAI, vacA s1i1mx and homB) by group.
Concerning AG, we found a similar proportion of subtypes harboring homA and homB genes within this group (50.7% and 49.3%, respectively) (Figure 1b,c). However, a high frequency of subtypes carrying homA associated with an unstable CagPAI was present within this group (43.8% of the subtypes) (Figure 1b) (OR = 5.17; 95% CI: 2.29–11.67, p = 0.0001) (Table 3). Although not significant, H. pylori subtypes from AG exploited a reduced risk to carry all the three virulence factors compared to D (OR = 0.84, 95% CI: 0.47–1.52, p = 0.57) (Table 4).
3. Discussion
It has been proposed that H. pylori infection and host factors play an important role in determining the clinical outcome of the infection [25,49]. Differences in H. pylori strains concerning specific virulent genes could be involved in the progression of gastric precancerous lesions to GC [10,11]. However, H. pylori strains exhibit a high degree of heterogeneity that helps its adaptation to and persistence in an evolving gastric environment [9,50]. To evaluate if populations with an increased GC risk could be associated with an intrinsic heightened virulence of the bacterium, molecular analyses of H. pylori isolates assessing the heterogeneity of the virulent gene profile were performed in AG and FDR subjects, and compared to those obtained from H. pylori subtypes isolated from D, and from GC as a positive control.
Consistent with data reported in the literature [49,51,52,53], GC patients included in this study showed an association with H. pylori strains harboring the CagPAI, which was accompanied by the presence of the highly virulent forms of the vacA gene, in accordance with previously reported data [54,55]. However, although some authors evidenced an association between GC and H. pylori strains positive for homB gene [32,33], our population-based analysis did not confirm this relation. This discrepancy could be explained by the different geographical origin of the patients from whom the strains had been isolated, and by the use of different preanalytical approaches [32,33,34,56]. Indeed, when we considered the H. pylori heterogeneity by the characterization of several subtypes shaping the gastric niche, we found that GC patients appear to host a high proportion of homB associated with the virulent form of vacA, and have a significant risk to carry subtypes containing all the three virulence factors.
In this study, H. pylori isolates from FDR mainly carried the highest virulent vacA s1i1mx genotype combined with a stable CagPAI. A similar vacA haplotype distribution was also evidenced in the D group, consistent with previous results obtained in studies conducted on similar cohorts [42,43]. For the first time, we assessed the presence of hom genes in FDR and AG populations. We showed a high frequency of subtypes carrying the homB haplotype associated both to a stable CagPAI and to vacA s1i1mx, specifically in FDR. In the latter group, similarly to GC patients, a high frequency of the genotype including all the three virulence factors was evidenced. It has been reported that homB was more frequent in H. pylori strains carrying cagA gene and the most virulent forms of vacA [31,32]; moreover, in the presence of the CagPAI, homB was found to promote a considerable proinflammatory response in vitro [31]. These observations suggest that homB might have a role in the development of a more severe clinical outcome of the infection in subjects such as FDR, who present a higher risk for GC than the general population. In addition, we interestingly found a lower prevalence of mixed infection in FDR than in D. It is well known that mixed infections determine the availability of exogenous DNA, which allows bacterial genome diversification and adaptation to an unfavorable environment [57]. Indeed, a higher frequency of recombination events during chronic infections have been reported in genes that influence bacterial adherence to epithelial cells and immune response [9,58]. It could be hypothesized that the reduced frequency of mixed infections in FDR could be the result of a selective pressure from the host, which promotes the emergence of H. pylori subtypes able to exacerbate the recruitment of immune cells at the site of infection in an attempt to reduce the bacterial survival, but actually reducing the possibilities of virulence attenuation. In accordance with this hypothesis, histological analyses in the present and other studies revealed a significant higher frequency of neutrophil infiltration (activity) and atrophy in the gastric mucosa of FDR when compared to that of D [44,59,60]. Moreover, IL-8 up-regulation and a more severe inflammatory reaction during H. pylori infection have been documented in FDR [61,62]. Our data, showing the predominance of more virulent H. pylori strains in FDR, reinforce the model involving a contribution of H. pylori in the progression of precancerous lesions towards GC in this population. The fact that any significant difference in H. pylori density was evidenced in FDR compared to D supports the importance of specific host and bacterial features rather than the quantity of H. pylori in determining the type of the response to the infection.
Then, we analyzed patients with antiparietal cell autoantibodies. It is reported that this condition precedes the onset of severe forms of autoimmune gastritis that expose the patients to the risk of non-cardia gastric adenocarcinoma [63,64]. Consistent with data previously reported, in our AG patients, vital H. pylori was isolated from a minority of cases (21%) [65,66,67,68]. The low recovery of H. pylori from AG subjects could suggest the presence of low amounts of H. pylori strains in their gastric niche. Indeed, we found a lower H. pylori density (Sydney 2-3) in AG (7%) than D (31%, Table 1). Low H. pylori amounts could be related to low fitness, which in turn could be dependent on changes in gastric relative abundance of other bacterial species that could compete for the vital space within the niche [69,70], participating in the gastric pathogenesis and in the exchange of resistance determinants [71,72]. It is worth noting that, in a subset of AG patients, we found a high proportion of individuals carrying a heterogeneous bacterial flora [73]. Herein, a high prevalence of Streptococcaceae was evidenced by a semiquantitative cultural method (data not shown), in agreement with recent studies conducted on AG series [69]. H. pylori isolated from AG showed a significant reduced risk to carry the cagA gene and a reduced frequency of all the evaluated virulence factors; as the chance to develop GC is higher when virulence bacterial factors are present, our findings are in agreement with the observation that only a small percentage of AG patients with active H. pylori infection is at higher risk to develop GC [40]. In these AG patients, we found that subtypes carrying unstable CagPAI associated with homA or vacA sxi2m2 haplotypes were the most represented. These results confirm that homA is linked with strains lacking some important H. pylori virulence genes [31,32], which, on the other hand, suggest an adaptation of the bacterium to the particular gastric environment produced during the chronic autoimmune disorder [2,74,75]. The residence of less virulent subtypes probably owning a higher fitness in the gastric niche and a different interaction capacity with the host is supported by a previous proteomic study revealing that H. pylori strains isolated from AG subjects have a tendency to express proteins involved in survival and bacterial protection from the gastric environment, rather than molecules involved in biosynthetic pathways and in invasion of the gastric mucosa [14]. Immune response over the long course of H. pylori colonization might play a role in selecting H. pylori subtypes which display target bacterial genes [37]. A role of chronic H. pylori infection in the development of AG has been previously proposed on the basis of a positive correlation between parietal auto-antibodies and antibodies specific for H. pylori antigens in the majority of AG patients, a phenomenon explained by molecular mimicry [76,77]. Homologies between self and H. pylori proteins, such as CagA, have been documented [78,79,80,81,82]. It is conceivable that, in inflammatory chronic diseases with autoimmune components and H. pylori infection, the equilibrium between H. pylori and host immune mechanisms might contribute to the removal of the H. pylori more virulent strains carrying CagA, as was found in our AG series [83,84].
The possible association of H. pylori with AG, related to the antigenicity of specific virulence traits involved in the initiation of the disease and in the modulation of H. pylori pathogenicity, does not exclude that the bacterium might contribute to support the functional loss of the stomach through the persistence of its virulence factors. By dissecting individual histological features and associated bacterial characteristics, we found a predominance of subtypes with stable CagPAI in 4 out of 14 AG patients, who had a higher median age than those hosting subtypes with CagPAI deletions. Interestingly, three of these four patients had a moderate corpus atrophy (Sydney 2). Whole genome sequencing studies evidenced a temporal and spatial evolution of H. pylori genome, with gain and loss of multiple virulence and resistance genes [85,86,87]. However, CagPAI appears to be relatively stable during disease progression to gastric carcinoma [86]. This highlights the possibility that AG patients could also be associated with a persistently virulent H. pylori. Hence, eradication therapy may be indicated in a subset of these patients, especially in the presence of signs of atrophy or old age. However, it must be kept in mind that the intactness or deletions of specific virulence factors, such as CagPAI and cagA, or the presence of less virulent forms of vacA, could be associated with phenotypic resistance to antibiotics [19,88,89,90,91] and high risk of eradication failure [92]. These data imply that, in the presence of diversity within single H. pylori strains, antibiotics therapies could select resistant subtypes and, in addition, they stimulate research on feasible methods to assess the presence of resistance within heterogeneous populations.
The present study has some limitations and merits. First, the number of the patients could be considered low, and made possible only univariate analyses. However, for the first time the presence of some H. pylori virulence genes has been assessed in non-endemic populations at risk to develop GC, such as FDR and AG, thoroughly analyzing 10–12 single colonies isolated from biopsies of single patients. This could not be representative of the entire heterogeneity of the H. pylori gastric niche, although this number was higher in comparison to that investigated in other studies with similar culture-based approaches [93,94]. An implementation of the study could derive from investigations on biopsies collected on a greater number of paired antrum-corpus samples, given that our study was basically conducted on biopsies isolated from the preferential niche of H. pylori, the antrum region. Second, if compared to more advanced molecular techniques which explore the entire H. pylori genome [58], the method we used allowed to examine a limited set of H. pylori virulence factors. However, the sensitivity of PCR-based virulent profiling of multiple H. pylori subtypes is higher if compared with other methods, such as RFLP and RAPD PCR [21,95]. In the future, the complete genome sequence data could deepen this point, providing information about still unknown bacterial genes [86]. Finally, our study produced a collection of well-characterized bacterial isolates useful to dissect specific pathogenetic mechanisms of H. pylori.
Multicenter perspective studies enrolling a high number of patients at early phases of gastric diseases and performed with high throughput molecular techniques avoiding time-consuming bacterial culture [96] could improve the knowledge of H. pylori patho-physiology and the management of individuals at risk of GC.
4. Materials and Methods
4.1. Study Population
A total of 340 subjects who underwent gastrointestinal endoscopy at the Oncological Gastroenterology Division, Centro di Riferimento Oncologico (CRO), Aviano (Italy) were submitted to H. pylori infection diagnostic workup. All the patients were subjected to an accurate clinical interview to ascertain the presence of already diagnosed diseases and current or recent pharmacological or surgical treatments. Subjects were included in the study according to the following criteria: positive culture for H. pylori, absence of previous H. pylori eradication therapy, of continuous or occasional proton pump inhibitor or antibiotic cures in the last month, and of chronic nonsteroidal anti-inflammatory drug treatments. Patients affected by gastritis and having auto-antibodies against parietal cells were defined as AG. Antiparietal cell antibody levels were estimated by means of indirect immunofluorescence with a cutoff of ≥1/80 (Euroimmun, Lubeck, Germany). Subjects with one or more cases of GC among their first-degree relatives were defined as FDR. Dyspeptic subjects without familiarity or autoimmunity (D) were included in the study as controls. In addition, confirmed GC cases were evaluated for the known association between H. pylori virulence factors and the pathology. Positive H. pylori cultures were obtained from 14 out of 67 AG (20.9%), 26 out of 105 FDR (24.8%), 42 out of 126 GC (33.3%) and 16 out of 42 D (38.1%). Seven subjects were subsequently excluded due to the loss of viability or bacteria contamination during culture steps. 39 high GC risk (14 AG, 25 FDR), 39 GC and 13 D were finally involved in the analysis. The study was approved by the Internal Review Board of CRO, Aviano (Italy) (IRB 14-2013). All study participants, or their legal guardian, provided informed written consent before enrollment. Ethical guidelines for research involving human subjects were respected.
4.2. Endoscopy and H. pylori Culture and Identification
Multiple biopsy specimens were taken for histological examination: two biopsies from the antrum, two from the corpus and two from the fundus. One antrum biopsy from all the patients, and one additional corpus biopsy from four FDR, four AG, eight D and 10 GC subjects were received in the laboratory and cultured for H. pylori. The specimens were obtained by using sterilized biopsy forceps, which were cleansed with a detergent, rinsed with sterile water after each examination. Biopsies were collected in a vial containing 1 mL of 0.9% NaCl solution and delivered to the laboratory within 2 hours. After subtle fragmentation, they were cultured in Pylori Selective Medium (Bio-Mérieux, Florence, Italy) following standard cultivation conditions for H. pylori [97]. Briefly, cultures were incubated at 37°C under sachet-generating microaerophilic athmosphere (Oxoid, Basingstoke, UK) for 3–5 days. If growth was not observable, incubation was prolonged for further 9–11 days and culture plates were controlled for growth twice/thrice weekly before considering them definitively negative. Concerning patients involved in this study, negative cultures were obtained from the antrum of two AG patients and from the corpus of two GC patients. The cultured bacteria were identified as H. pylori based on Gram-negative staining, curved or spiral shape, and positivity for oxidase, catalase and urease production.
In order to represent the possible genetic heterogeneity of H. pylori populations that colonize the gastric niche, 10 to 12 CFU were isolated from each single H. pylori positive selective primary plate, as previously described [21,24]. In brief, each CFU was spotted on Columbia Sheep Blood Agar (CA) (Kima, Padua, Italy), and then expanded on three CA plates. Each incubation was carried out under microaerophilic atmosphere at 37°C for a maximum of 2–3 days and four passages, to limit the appearance of H. pylori coccoid form. After confluent bacterial growth, two plates were used for bacterial DNA extraction and one for cryo-conservation (Microbank, Pro-Lab Diagnostics, Richmond Hill, Canada). A total of 915 H. pylori single colonies defining possibly subtypes were isolated from 91 patients.
4.3. Histological Study
For histological examination, biopsy specimens were fixed in buffered formalin 10%, embedded in paraffin and stained with hematoxylin and eosin by means of the modified Giemsa method for H. pylori. Available slides were retrieved retrospectively: 13 antrum and 11 corpus for D, 14 antrum and 14 corpus for AG, 25 antrum and 21 corpus for FDR and 39 antrum and 19 corpus for GC patients. A pathologist, who was unaware of endoscopic, microbiological or serological data, evaluated the inflammatory and precancerous parameters in accordance with the Sydney System classification [98], assigning a score from 0 to 3 (absent, mild, moderate, severe) to each of the following structural variables: activity (amount of neutrophilic infiltration), inflammation (amount of mononuclear-cell infiltration), atrophy (loss of glandular tissue), intestinal metaplasia and H. pylori density. The Sydney System’s parameters related to the profiled gastric regions were included in the analyses of patients’ characteristics.
4.4. Genomic DNA Extraction
Grown bacteria were collected from the CA plates and resuspended in 1 mL of 0.9% NaCl. Bacterial pellets were then obtained by centrifugation at 6797g for 6 minutes. Genomic DNA was extracted by QIAmp DNA Mini Kit (QIAgen, Hilden, Germany) following manufacturer’s instructions. NanoDrop 1000 Spectrophotometer (Thermo Scientific) was used for the calculation of DNA concentration. A fixed amount of DNA corresponding to 4 ng was used in each PCR reaction.
4.5. PCR-Based Virulent Gene Profiling
Each PCR reaction was performed in a final volume of 50 μL containing 10mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.5 μM of each primer, 0.020 U/μL of AmpliTaq® DNA Polymerase (Applied Biosystems, Foster City, CA, USA). After validation of the results in double reactions with known samples, for the evaluation of vacA polymorphisms and hom genes, 0.015 U/μL per reaction of GoTaq® G2 DNA Polymerase was used (Promega, Madison, WI, USA).
In order to speed-up the genotyping procedure, a different thermal profile was applied to a multiplex PCR including cagA and vacA s and m regions. The test was reliable and provided results overlapping those obtained through the previous single PCR assays. The multiplex PCR reaction mix contained 1.5 mM MgCl2, 0.25 mM dNTPs, 18 pmol of each vacA primer, 28 pmol of cagA primers, 0.8 U/μL of GoTaq® G2 DNA Polymerase (Promega, Madison, WI, USA) in a final volume of 25 μL. Primer sequences, thermal PCR conditions and length of the amplified fragments are reported in Table 5.

Table 5.
Primers and PCR conditions for the virulent gene profiling.
CagPAI and hom PCR products were analyzed by 8% polyacrylamide gel electrophoresis, while vacA s, m and i PCR products were loaded in 2% agarose gel electrophoresis. After ethidium bromide staining, all the gels were visualized under a ultraviolet transilluminator (Gel Doc 2000, Biorad).
Specificity of the amplification was confirmed by Sanger sequencing after PCR product purification with Centricon-100 concentrator columns (Amicon, Beverly, Mass). Sequencing reactions were run on ABI Prism 3130XL automated DNA Sequencer (Applied Biosystems).
4.6. Definitions and Statistical Analysis
Parameters under study were dichotomized on the basis of their frequencies in the studied groups. The presence of activity, atrophy and intestinal metaplasia was defined when the Sydney score was equal or greater than 1. A stratification of the patients by the grade of inflammation and H. pylori density 0–1 versus 2–3 was made. Age was dichotomized considering the median age of the D controlgroup.
CagA, cagE and virB11 within a subtype were studied as single genes and as a whole: the concomitant presence of virB11 (located in the left half of the CagPAI), cagA and cagE (both located in the right half of the CagPAI) in a subtype was considered as an indicator of CagPAI stability (herein named stable CagPAI); conversely, an unstable CagPAI was defined by the deletion of at least one of the aforementioned CagPAI genes. VacA haplotypes were clustered based on the presence of s, m, i allelic heterogeneity, considering s1i1mx with a highest vacuolization property than sxi2m2 haplotype, and where x indicated the alternative allele 1 or 2 [25,28]. 23 out of 915 total subtypes without vacA (2.51%) were grouped with sxi2m2 positive isolates. 44 out of 915 total subtypes carrying both homB and homA (4.81%) were assembled within the strains keeping the homB haplotype, which putatively confers higher level pathogenicity than homA [31]. The very few subtypes without hom (10/915, 1.09%) were grouped with strains carrying homA.
Since antrum is the preferential niche of H. pylori [101], when identical bacterial profiles from paired antrum corpus biopsy samples were obtained, those related to only the antrum were included in the statistical analyses (three in FDR, two in AG, six in D, eight in GC). In three cases (one in FDR and two in D) a difference in genotypes by topography was evidenced, nonetheless the profile related to antrum region was considered. Since no significant differences were shown in the distribution of the Sydney parameters between matched antrum and corpus samples (Supplementary Table S1), corpus-related H. pylori profiles of the two AG cases with negative biopsy culture from the antrum were included.
In order to evaluate the H. pylori putative virulent gene load in the gastric niche of the studied subjects, based on the frequency of the subtypes carrying virulent genetic traits in each group, an individual was a priori considered owner of a highly virulent H. pylori load in the niche when a number of subtypes ≥9 was positive for the virulent factors analyzed. A mixed infection in a patient was defined based on the presence of at least one subtype different from the others for at least one virulence factor.
Univariate Odds ratios (OR) and their corresponding 95% confidence intervals (CI) were computed to estimate the differences in the distribution of the patients by demographic, histological and virulent profiles and to assess virulence factor associations in the subtypes within and among the groups under study. Two-tailed p-value <0.05 was considered to indicate statistical significance. Analyses were performed by the SAS System software, version 9.40 (SAS Institute Inc., Cary, NC, USA, 1999–2001).
5. Conclusions
Less virulent H. pylori subtypes were mainly isolated from AG patients, whereas highly virulent H. pylori profiles predominated in FDR, suggesting a selection exerted by the host in the gastric niche. These results underline that differences in H. pylori genome might play differential roles in gastric pathogenesis. Our observations strengthen the guidelines statements that recommend eradication of the H. pylori infection in FDR subjects [102]. Eradication therapy in H. pylori positive subjects at high risk of GC should be considered in relation to the complexity of the factors involved in the gastric tumor development, not least, to the alterations of the gastric microbiota of which H. pylori is part and within which it can compete for survival [103,104].
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/8/2/65/s1, Table S1: Sydney classification of histological parameters in AG patients by gastric topography.
Author Contributions
Conceptualization, S.Z., E.B.; Methodology, M.C., S.Z.; Acquisition of data: M.C., S.Z., G.B., V.C., R.C., S.M., R.M.; Analysis and interpretation of data: M.C., S.Z.; Statistical analysis: S.Z., E.B., M.C.; Original draft preparation: M.C., S.Z., C.P.; Review and editing the manuscript: All the authors; Resources, G.B., A.S.; Supervision, S.Z.
Funding
This research work was partially supported by Ministry of Health, “Ricerca Corrente 2019 – Linea 5”, CUP J36C19000010001, Italy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Blaser, M.J.; Atherton, J.C. Helicobacter pylori persistence: Biology and disease. J. Clin. Investig. 2004, 113, 321–333. [Google Scholar] [CrossRef]
- Atherton, J.C.; Blaser, M.J. Coadaptation of Helicobacter pylori and humans: Ancient history, modern implications. J. Clin. Investig. 2009, 119, 2475–2487. [Google Scholar] [CrossRef] [PubMed]
- IARC. Infection with Helicobacter pylori. Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 177–240. [Google Scholar]
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef]
- Correa, P.; Piazuelo, M.B. The gastric precancerous cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.L.; Blaser, M.J. Helicobacter pylori in health and disease. Gastroenterology 2009, 136, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Saberi, S.; Douraghi, M.; Azadmanesh, K.; Shokrgozar, M.A.; Zeraati, H.; Hosseini, M.E.; Mohagheghi, M.A.; Parsaeian, M.; Mohammadi, M. A potential association between Helicobacter pylori CagA EPIYA and multimerization motifs with cytokeratin 18 cleavage rate during early apoptosis. Helicobacter 2012, 17, 350–357. [Google Scholar] [CrossRef]
- Greenfield, L.K.; Jones, N.L. Modulation of autophagy by Helicobacter pylori and its role in gastric carcinogenesis. Trends Microbiol. 2013, 21, 602–612. [Google Scholar] [CrossRef]
- Suerbaum, S.; Josenhans, C. Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat. Rev. Microbiol. 2007, 5, 441–452. [Google Scholar] [CrossRef]
- Plummer, M.; van Doorn, L.J.; Franceschi, S.; Kleter, B.; Canzian, F.; Vivas, J.; Lopez, G.; Colin, D.; Muñoz, N.; Kato, I. Helicobacter pylori cytotoxin-associated genotype and gastric precancerous lesions. J. Natl. Cancer Inst. 2007, 99, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Figueiredo, C.; Lic, C.B.; Ferreira, R.M.; Pardo, M.L.; Ruiz Liso, J.M.; Alonso, P.; Sala, N.; Capella, G.; Sanz-Anquela, J.M. Helicobacter pylori cagA and vacA genotypes as predictors of progression of gastric preneoplastic lesions: A long-term follow-up in a high-risk area in Spain. Am. J. Gastroenterol. 2011, 106, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Figura, N.; Marano, L.; Moretti, E.; Ponzetto, A. Helicobacter pylori infection and gastric carcinoma: Not all the strains and patients are alike. World J. Gastrointest. Oncol. 2016, 8, 40–54. [Google Scholar] [CrossRef]
- Figura, N.; Valassina, M.; Moretti, E.; Vindigni, C.; Collodel, G.; Iacoponi, F.; Giordano, N.; Roviello, F.; Marrelli, D. Histological variety of gastric carcinoma and Helicobacter pylori cagA and vacA polymorphism. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Repetto, O.; Zanussi, S.; Casarotto, M.; Canzonieri, V.; De Paoli, P.; Cannizzaro, R.; De Re, V. Differential proteomics of Helicobacter pylori associated with autoimmune atrophic gastritis. Mol. Med. 2014, 20, 57–71. [Google Scholar] [CrossRef]
- Bernardini, G.; Figura, N.; Ponzetto, A.; Marzocchi, B.; Santucci, A. Application of proteomics to the study of Helicobacter pylori and implications for the clinic. Expert Rev. Proteom. 2017, 14, 477–490. [Google Scholar] [CrossRef]
- Karita, M.; Blaser, M.J. Acid-tolerance response in Helicobacter pylori and differences between cagA+ and cagA- strains. J. Infect. Dis. 1998, 178, 213–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suerbaum, S.; Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 2002, 347, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Figura, N.; Trabalzini, L.; Mini, R.; Bernardini, G.; Scaloni, A.; Talamo, F.; Lusini, P.; Ferro, E.; Martelli, P.; Santucci, A. Inactivation of Helicobacter pylori cagA gene affects motility. Helicobacter 2004, 9, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Basaglia, G.; Sperandio, P.; Tomasini, M.L.; Calzavara, S.S.; Giordari, F.; De Paoli, P. Analysis of antimicrobial susceptibility and virulence factors in Helicobacter pylori clinical isolates. J. Chemother. 2004, 16, 504–506. [Google Scholar] [CrossRef]
- De Paoli, P.; Tomasini, M.L.; Basaglia, G. The predictive value of Helicobacter pylori in-vitro metronidazole resistance. Clin. Microbiol. Infect. 2004, 10, 1105–1106. [Google Scholar] [CrossRef]
- Tomasini, M.L.; Zanussi, S.; Sozzi, M.; Tedeschi, R.; Basaglia, G.; De Paoli, P. Heterogeneity of cag genotypes in Helicobacter pylori isolates from human biopsy specimens. J. Clin. Microbiol. 2003, 41, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, M.; Crosatti, M.; Kim, S.K.; Romero, J.; Blaser, M.J. Heterogeneity of Helicobacter pylori cag genotypes in experimentally infected mice. FEMS Microbiol. Lett. 2001, 203, 109–114. [Google Scholar] [CrossRef][Green Version]
- Sozzi, M.; Valentini, M.; Figura, N.; De Paoli, P.; Tedeschi, R.M.; Gloghini, A.; Serraino, D.; Poletti, M.; Carbone, A. Atrophic gastritis and intestinal metaplasia in Helicobacter pylori infection: The role of CagA status. Am. J. Gastroenterol. 1998, 93, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, M.; Tomasini, M.L.; Vindigni, C.; Zanussi, S.; Tedeschi, R.; Basaglia, G.; Figura, N.; De Paoli, P. Heterogeneity of cag genotypes and clinical outcome of Helicobacter pylori infection. J. Lab. Clin. Med. 2005, 146, 26–70. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, L.E.; Peek, R.M., Jr.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Jones, K.R.; Olsen, C.H.; Joo, Y.M.; Yoo, Y.J.; Chung, I.S.; Cha, J.H.; Merrell, D.S. Epidemiological link between gastric disease and polymorphisms in VacA and CagA. J. Clin. Microbiol. 2010, 48, 559–567. [Google Scholar] [CrossRef]
- Cover, T.L.; Tummuru, M.K.; Cao, P.; Thompson, S.A.; Blaser, M.J. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J. Biol. Chem. 1994, 269, 10566–10573. [Google Scholar]
- Rhead, J.L.; Letley, D.P.; Mohammadi, M.; Hussein, N.; Mohagheghi, M.A.; Eshagh Hosseini, M.; Atherton, J.C. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology 2007, 133, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.; Zambon, C.F.; Letley, D.P.; Stranges, A.; Marchet, A.; Rhead, J.L.; Schiavon, S.; Guariso, G.; Ceroti, M.; Nitti, D.; et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology 2008, 135, 91–99. [Google Scholar] [CrossRef]
- Fahimi, F.; Tohidkia, M.R.; Fouladi, M.; Aghabeygi, R.; Samadi, N.; Omidi, Y. Pleiotropic cytotoxicity of VacA toxin in host cells and its impact on immunotherapy. Bioimpacts 2017, 7, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Oleastro, M.; Cordeiro, R.; Ferrand, J.; Nunes, B.; Lehours, P.; Carvalho-Oliveira, I.; Mendes, A.I.; Penque, D.; Monteiro, L.; Mégraud, F.; et al. Evaluation of the clinical significance of homB, a novel candidate marker of Helicobacter pylori strains associated with peptic ulcer disease. J. Infect. Dis. 2008, 198, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Sugimoto, M.; Graham, D.Y.; Yamaoka, Y. homB status of Helicobacter pylori as a novel marker to distinguish gastric cancer from duodenal ulcer. J. Clin. Microbiol. 2009, 47, 3241–3245. [Google Scholar] [CrossRef]
- Talebi Bezmin Abadi, A.; Rafiei, A.; Ajami, A.; Hosseini, V.; Taghvaei, T.; Jones, K.R.; Merrell, D.S. Helicobacter pylori homB, but not cagA, is associated with gastric cancer in Iran. J. Clin. Microbiol. 2011, 49, 3191–3197. [Google Scholar] [CrossRef]
- Oleastro, M.; Monteiro, L.; Lehours, P.; Mégraud, F.; Ménard, A. Identification of markers for Helicobacter pylori strains isolated from children with peptic ulcer disease by suppressive subtractive hybridization. Infect. Immun. 2006, 74, 4064–4074. [Google Scholar] [CrossRef]
- Atherton, J.C. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu. Rev. Pathol. 2006, 1, 63–96. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, M.C.; Yu, J.; Peppelenbosch, M.P.; Fuhler, G.M. Genetic host factors in Helicobacter pylori-induced carcinogenesis: Emerging new paradigms. Biochim. Biophys. Acta 2018, 1869, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fero, J.B.; Mendez, M.; Carpenter, B.M.; Servetas, S.L.; Rahman, A.; Goldman, M.D.; Boren, T.; Salama, N.R.; Merrell, D.S.; et al. Analysis of a single Helicobacter pylori strain over a 10-year period in a primate model. Int. J. Med. Microbiol. 2015, 305, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.J.; Danon, S.J.; Wilson, J.E.; O’Rourke, J.L.; Salama, N.R.; Falkow, S.; Mitchell, H.; Lee, A. Chronic Helicobacter pylori infection with Sydney strain 1 and a newly identified mouse-adapted strain (Sydney strain 2000) in C57BL/6 and BALB/c mice. Infect. Immun. 2004, 72, 4668–4679. [Google Scholar] [CrossRef]
- Brenner, H.; Arndt, V.; Stürmer, T.; Stegmaier, C.; Ziegler, H.; Dhom, G. Individual and joint contribution of family history and Helicobacter pylori infection to the risk of gastric carcinoma. Cancer 2000, 88, 274–279. [Google Scholar] [CrossRef]
- Toh, B.H. Diagnosis and classification of autoimmune gastritis. Autoimmun. Rev. 2014, 13, 459–462. [Google Scholar] [CrossRef]
- Yaghoobi, M.; Bijarchi, R.; Narod, S.A. Family history and the risk of gastric cancer. Br. J. Cancer 2010, 102, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Stec-Michalska, K.; Peczek, L.; Michalski, B.; Wisniewska-Jarosinska, M.; Krakowiak, A.; Nawrot, B. Helicobacter pylori infection and family history of gastric cancer decrease expression of FHIT tumor suppressor gene in gastric mucosa of dyspeptic patients. Helicobacter 2009, 14, 126–134. [Google Scholar] [CrossRef]
- Siavoshi, F.; Asgharzadeh, A.; Ghadiri, H.; Massarrat, S.; Latifi-Navid, S.; Zamani, M. Helicobacter pylori genotypes and types of gastritis in first-degree relatives of gastric cancer patients. Int. J. Med. Microbiol. 2011, 301, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, D.M.; Silva, C.I.; Goncalves, M.H.; Braga-Neto, M.B.; Fialho, A.B.; Fialho, A.M.; Rocha, G.A.; Rocha, A.M.; Batista, S.A.; Guerrant, R.L.; et al. Higher frequency of cagA EPIYA-C phosphorylation sites in H. pylori strains from first-degree relatives of gastric cancer patients. BMC Gastroenterol. 2012, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Pinto, R.; Dinis-Ribeiro, M.; Carneiro, F.; Wen, X.; Lopes, C.; Figueiredo, C.; Machado, J.C.; Ferreira, R.M.; Reis, C.A.; Canedo, P.; et al. First-degree relatives of early-onset gastric cancer patients show a high risk for gastric cancer: Phenotype and genotype profile. Virchows Arch. 2013, 463, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Venerito, M.; Radünz, M.; Reschke, K.; Reinhold, D.; Frauenschläger, K.; Jechorek, D.; Di Mario, F.; Malfertheiner, P. Autoimmune gastritis in autoimmune thyroid disease. Aliment. Pharmacol. Ther. 2015, 41, 686–693. [Google Scholar] [CrossRef]
- Zhang, Y.; Weck, M.N.; Schöttker, B.; Rothenbacher, D.; Brenner, H. Gastric parietal cell antibodies, Helicobacter pylori infection, and chronic atrophic gastritis: Evidence from a large population-based study in Germany. Cancer Epidemiol. Biomark. Prev. 2013, 22, 821–826. [Google Scholar] [CrossRef]
- Yadegar, A.; Alebouyeh, M.; Zali, M.R. Analysis of the intactness of Helicobacter pylori cag pathogenicity island in Iranian strains by a new PCR-based strategy and its relationship with virulence genotypes and EPIYA motifs. Infect. Genet. Evol. 2015, 35, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, J.; Prasad, K.N.; Prakash Rai, R.; Ghoshal, U.C.; Krishnani, N. Association of heterogenicity of Helicobacter pylori cag pathogenicity island with peptic ulcer diseases and gastric cancer. Br. J. Biomed. Sci. 2017, 74, 121–126. [Google Scholar] [CrossRef]
- Achtman, M.; Azuma, T.; Berg, D.E.; Ito, Y.; Morelli, G.; Pan, Z.J.; Suerbaum, S.; Thompson, S.A.; van der Ende, A.; van Doorn, L.J. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol. Microbiol. 1999, 32, 459–470. [Google Scholar] [CrossRef]
- Parsonnet, J.; Friedman, G.D.; Orentreich, N.; Vogelman, H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 1997, 40, 297–301. [Google Scholar] [CrossRef]
- Queiroz, D.M.; Mendes, E.N.; Rocha, G.A.; Oliveira, A.M.; Oliveira, C.A.; Magalhães, P.P.; Moura, S.B.; Cabral, M.M.; Nogueira, A.M. cagA-positive Helicobacter pylori and risk for developing gastric carcinoma in Brazil. Int. J. Cancer 1998, 78, 135–139. [Google Scholar] [CrossRef]
- Torres, J.; Pérez-Pérez, G.I.; Leal-Herrera, Y.; Muñoz, O. Infection with CagA+ Helicobacter pylori strains as a possible predictor of risk in the development of gastric adenocarcinoma in Mexico. Int. J. Cancer 1998, 78, 298–300. [Google Scholar] [CrossRef]
- Miehlke, S.; Kirsch, C.; Agha-Amiri, K.; Günther, T.; Lehn, N.; Malfertheiner, P.; Stolte, M.; Ehninger, G.; Bayerdörffer, E. The Helicobacter pylori vacA s1, m1 genotype and cagA is associated with gastric carcinoma in Germany. Int. J. Cancer 2000, 87, 322–327. [Google Scholar] [CrossRef]
- Sheikh, A.F.; Yadyad, M.J.; Goodarzi, H.; Hashemi, S.J.; Aslani, S.; Assarzadegan, M.A.; Ranjbar, R. CagA and vacA allelic combination of Helicobacter pylori in gastroduodenal disorders. Microb. Pathog. 2018, 122, 144–150. [Google Scholar] [CrossRef]
- Kang, J.; Jones, K.R.; Jang, S.; Olsen, C.H.; Yoo, Y.J.; Merrell, D.S.; Cha, J.H. The geographic origin of Helicobacter pylori influences the association of the homB gene with gastric cancer. J. Clin. Microbiol. 2012, 50, 1082–1085. [Google Scholar] [CrossRef]
- Kauser, F.; Khan, A.A.; Hussain, M.A.; Carroll, I.M.; Ahmad, N.; Tiwari, S.; Shouche, Y.; Das, B.; Alam, M.; Ali, S.M.; et al. The cag pathogenicity island of Helicobacter pylori is disrupted in the majority of patient isolates from different human populations. J. Clin. Microbiol. 2004, 42, 5302–5308. [Google Scholar] [CrossRef] [PubMed]
- Kennemann, L.; Didelot, X.; Aebischer, T.; Kuhn, S.; Drescher, B.; Droege, M.; Reinhardt, R.; Correa, P.; Meyer, T.F.; Josenhans, C.; et al. Helicobacter pylori genome evolution during human infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5033–5038. [Google Scholar] [CrossRef] [PubMed]
- Motta, C.R.; Cunha, M.P.; Queiroz, D.M.; Cruz, F.W.; Guerra, E.J.; Mota, R.M.; Braga, L.L. Gastric precancerous lesions and Helicobacter pylori infection in relatives of gastric cancer patients from Northeastern Brazil. Digestion 2008, 78, 3–8. [Google Scholar] [CrossRef]
- Rokkas, T.; Sechopoulos, P.; Pistiolas, D.; Margantinis, G.; Koukoulis, G. Helicobacter pylori infection and gastric histology in first-degree relatives of gastric cancer patients: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1128–1133. [Google Scholar] [CrossRef]
- Liao, J.; Wen, S.; Cao, L.; Zhou, Y.; Feng, Z. Effect of eradication of Helicobacter pylori on expression levels of FHIT, IL-8 and P73 in gastric mucosa of first-degree relatives of gastric cancer patients. PLoS ONE 2015, 10, e0124576. [Google Scholar] [CrossRef]
- Vilkin, A.; Levi, Z.; Morgenstern, S.; Shmuely, H.; Gal, E.; Hadad, B.; Hardi, B.; Niv, Y. Higher gastric mucin secretion and lower gastric acid output in first-degree relatives of gastric cancer patients. J. Clin. Gastroenterol. 2008, 42, 36–41. [Google Scholar] [CrossRef]
- Murphy, G.; Dawsey, S.M.; Engels, E.A.; Ricker, W.; Parsons, R.; Etemadi, A.; Lin, S.W.; Abnet, C.C.; Freedman, N.D. Cancer risk after pernicious anemia in the US elderly population. Clin. Gastroenterol. Hepatol. 2015, 13, 2282–2289.e4. [Google Scholar] [CrossRef]
- Mahmud, N.; Stashek, K.; Katona, B.W.; Tondon, R.; Shroff, S.G.; Roses, R.; Furth, E.E.; Metz, D.C. The incidence of neoplasia in patients with autoimmune metaplastic atrophic gastritis: A renewed call for surveillance. Ann. Gastroenterol. 2019, 32, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Annibale, B.; Lahner, E.; Negrini, R.; Baccini, F.; Bordi, C.; Monarca, B.; Delle Fave, G. Lack of specific association between gastric autoimmunity hallmarks and clinical presentations of atrophic body gastritis. World J. Gastroenterol. 2005, 11, 5351–5357. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Fassan, M.; Pizzi, M.; Zorzetto, V.; Maddalo, G.; Realdon, S.; De Bernard, M.; Betterle, C.; Cappellesso, R.; Pennelli, G.; et al. Autoimmune gastritis: Histology phenotype and OLGA staging. Aliment. Pharmacol. Ther. 2012, 35, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Presotto, F.; Sabini, B.; Cecchetto, A.; Plebani, M.; De Lazzari, F.; Pedini, B.; Betterle, C. Helicobacter pylori infection and gastric autoimmune diseases: Is there a link? Helicobacter 2003, 8, 578–584. [Google Scholar] [CrossRef]
- Minalyan, A.; Benhammou, J.N.; Artashesyan, A.; Lewis, M.S.; Pisegna, J.R. Autoimmune atrophic gastritis: Current perspectives. Clin. Exp. Gastroenterol. 2017, 10, 19–27. [Google Scholar] [CrossRef]
- Parsons, B.N.; Ijaz, U.Z.; D’Amore, R.; Burkitt, M.D.; Eccles, R.; Lenzi, L.; Duckworth, C.A.; Moore, A.R.; Tiszlavicz, L.; Varro, A.; et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 2017, 13, e1006653. [Google Scholar] [CrossRef] [PubMed]
- Klymiuk, I.; Bilgilier, C.; Stadlmann, A.; Thannesberger, J.; Kastner, M.T.; Högenauer, C.; Püspök, A.; Biowski-Frotz, S.; Schrutka-Kölbl, C.; Thallinger, G.G.; et al. The human gastric microbiome is predicated upon infection with Helicobacter pylori. Front. Microbiol. 2017, 8, 2508. [Google Scholar] [CrossRef]
- Adamsson, I.; Edlund, C.; Nord, C.E. Impact of treatment of Helicobacter pylori on the normal gastrointestinal microflora. Clin. Microbiol. Infect. 2000, 6, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.; Wreiber, K.; Fall, K.; Fjelstad, B.; Nyrén, O.; Engstrand, L. Macrolide resistance in the normal microbiota after Helicobacter pylori treatment. Scand. J. Infect. Dis. 2007, 39, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Zanussi, S.; Casarotto, M.; Basaglia, G.; Tedeschi, R.; Giacomini, S.; Canzonieri, V.; De Re, V.; Maiero, S.; Cannizzaro, R.; De Paoli, P. Prevalence of Helicobacter pylori infection and its genetic heterogeneity in autoimmune atrophic chronic gastritis patients. Helicobacter 2013, 18 (Suppl. 1), 112–113. [Google Scholar]
- Kraft, C.; Stack, A.; Josenhans, C.; Niehus, E.; Dietrich, G.; Correa, P.; Fox, J.G.; Falush, D.; Suerbaum, S. Genomic changes during chronic Helicobacter pylori infection. J. Bacteriol. 2006, 188, 249–254. [Google Scholar] [CrossRef]
- Lan, R.; Reeves, P.R. Intraspecies variation in bacterial genomes: The need for a species genome concept. Trends Microbiol. 2000, 8, 396–401. [Google Scholar] [CrossRef]
- D’Elios, M.M.; Appelmelk, B.J.; Amedei, A.; Bergman, M.P.; Del Prete, G. Gastric autoimmunity: The role of Helicobacter pylori and molecular mimicry. Trends Mol. Med. 2004, 10, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Amedei, A.; Bergman, M.P.; Appelmelk, B.J.; Azzurri, A.; Benagiano, M.; Tamburini, C.; van der Zee, R.; Telford, J.L.; Vandenbroucke-Grauls, C.M.; D’Elios, M.M.; et al. Molecular mimicry between Helicobacter pylori antigens and H+, K+ --adenosine triphosphatase in human gastric autoimmunity. J. Exp. Med. 2003, 198, 1147–1156. [Google Scholar] [CrossRef]
- Roujeinikova, A. Phospholipid binding residues of eukaryotic membrane-remodelling F-BAR domain proteins are conserved in Helicobacter pylori CagA. BMC Res. Notes 2014, 7, 525. [Google Scholar] [CrossRef] [PubMed]
- Kalim, K.W.; Yang, J.Q.; Li, Y.; Meng, Y.; Zheng, Y.; Guo, F. Reciprocal regulation of glycolysis-driven Th17 pathogenicity and regulatory T cell stability by Cdc42. J. Immunol. 2018, 200, 2313–2326. [Google Scholar] [CrossRef]
- Chmiela, M.; Gonciarz, W. Molecular mimicry in Helicobacter pylori infections. World J. Gastroenterol. 2017, 23, 3964–3977. [Google Scholar] [CrossRef]
- Roy, A.; Ganesh, G.; Sippola, H.; Bolin, S.; Sawesi, O.; Dagälv, A.; Schlenner, S.M.; Feyerabend, T.; Rodewald, H.R.; Kjellén, L.; et al. Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation. J. Biol. Chem. 2014, 289, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Lennon, E.M.; Borst, L.B.; Edwards, L.L.; Moeser, A.J. Mast cells exert anti-inflammatory effects in an IL10-/- model of spontaneous colitis. Mediators Inflamm. 2018, 2018, 7817360. [Google Scholar] [CrossRef] [PubMed]
- Zárate-Bladés, C.R.; Horai, R.; Caspi, R.R. Regulation of autoimmunity by the Microbiome. DNA Cell Biol. 2016, 35, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, D.; Majkowska-Skrobek, G.; Roszkowiak, J.; Dorotkiewicz-Jach, A. Defensive and offensive cross-reactive antibodies elicited by pathogens: The good, the bad and the ugly. Curr. Med. Chem. 2017, 24, 4002–4037. [Google Scholar] [CrossRef] [PubMed]
- Gressmann, H.; Linz, B.; Ghai, R.; Pleissner, K.P.; Schlapbach, R.; Yamaoka, Y.; Kraft, C.; Suerbaum, S.; Meyer, T.F.; Achtman, M. Gain and loss of multiple genes during the evolution of Helicobacter pylori. PLoS Genet. 2005, 1, e43. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.D.; Kling-Bäckhed, H.; Giannakis, M.; Xu, J.; Fulton, R.S.; Fulton, L.A.; Cordum, H.S.; Wang, C.; Elliott, G.; Edwards, J.; et al. The complete genome sequence of a chronic atrophic gastritis Helicobacter pylori strain: Evolution during disease progression. Proc. Natl. Acad. Sci. USA 2006, 103, 9999–10004. [Google Scholar] [CrossRef]
- Suzuki, R.; Shiota, S.; Yamaoka, Y. Molecular epidemiology, population genetics, and pathogenic role of Helicobacter pylori. Infect. Genet. Evol. 2012, 12, 203–213. [Google Scholar] [CrossRef]
- Vilaichone, R.K.; Mahacahai, V.; Tumwasorn, S.; Kachintorn, U. CagA genotype and metronidazole resistant strain of Helicobacter pylori in functional dyspepsia in Thailand. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. 3), 46–48. [Google Scholar] [CrossRef]
- Bachir, M.; Allem, R.; Tifrit, A.; Medjekane, M.; Drici, A.E.; Diaf, M.; Douidi, K.T. Primary antibiotic resistance and its relationship with cagA and vacA genes in Helicobacter pylori isolates from Algerian patients. Braz. J. Microbiol. 2018, 49, 544–551. [Google Scholar] [CrossRef]
- Fasciana, T.; Calà, C.; Bonura, C.; Di Carlo, E.; Matranga, D.; Scarpulla, G.; Manganaro, M.; Camilleri, S.; Giammanco, A. Resistance to clarithromycin and genotypes in Helicobacter pylori strains isolated in Sicily. J. Med. Microbiol. 2015, 64, 1408–1414. [Google Scholar] [CrossRef][Green Version]
- Brennan, D.E.; Dowd, C.; O’Morain, C.; McNamara, D.; Smith, S.M. Can bacterial virulence factors predict antibiotic resistant Helicobacter pylori infection? World J. Gastroenterol. 2018, 24, 971–981. [Google Scholar] [CrossRef]
- Sugimoto, M.; Yamaoka, Y. Virulence factor genotypes of Helicobacter pylori affect cure rates of eradication therapy. Arch. Immunol. Ther. Exp. (Warsz.) 2009, 57, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.C.; Wang, W.H.; Berg, D.E.; Fung, F.M.; Wong, K.W.; Wong, W.M.; Lai, K.C.; Cho, C.H.; Hui, W.M.; Lam, S.K. High prevalence of mixed infections by Helicobacter pylori in Hong Kong: Metronidazole sensitivity and overall genotype. Aliment. Pharmacol Ther. 2001, 15, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Ben Mansour, K.; Fendri, C.; Battikh, H.; Garnier, M.; Zribi, M.; Jlizi, A.; Burucoa, C. Multiple and mixed Helicobacter pylori infections: Comparison of two epidemiological situations in Tunisia and France. Infect. Genet. Evol. 2016, 37, 43–48. [Google Scholar] [CrossRef]
- Matteo, M.J.; Armitano, R.I.; Granados, G.; Wonaga, A.D.; Sánches, C.; Olmos, M.; Catalano, M. Helicobacter pylori oipA, vacA and dupA genetic diversity in individual hosts. J. Med. Microbiol. 2010, 59, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Hu, B.; Zhao, F.; Xiang, P.; Ji, D.; Chen, F.; Liu, X.; Yang, F.; Wu, Y.; et al. Direct detection of Helicobacter pylori in biopsy specimens using a high-throughput multiple genetic detection system. Future Microbiol. 2016, 11, 1521–1534. [Google Scholar] [CrossRef]
- Isenberg, H.D. Clinical Microbiology Procedure Handbook, 2nd ed.; ASM Press, American Society Press for Microbiology: Washington, DC, USA, 2004. [Google Scholar]
- Genta, R.M. Recognizing atrophy: Another step toward a classification of gastritis. Am. J. Surg. Pathol. 1996, 20, S23–S30. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.M.; Andres, S.; Nilsson, C.; Kovach, Z.; Kaakoush, N.O.; Engstrand, L.; Goh, K.L.; Fock, K.M.; Forman, D.; Mitchell, H. The cag PAI is intact and functional but HP0521 varies significantly in Helicobacter pylori isolates from Malaysia and Singapore. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Patra, R.; Ramamurthy, T.; Chowdhury, A.; Santra, A.; Dhali, G.K.; Bhattacharya, S.K.; Berg, D.E.; Nair, G.B.; Mukhopadhyay, A.K. Multiplex PCR assay for rapid detection and genotyping of Helicobacter pylori directly from biopsy specimens. J. Clin. Microbiol. 2004, 42, 2821–2824. [Google Scholar] [CrossRef]
- Lash, J.G.; Genta, R.M. Adherence to the Sydney System guidelines increases the detection of Helicobacter gastritis and intestinal metaplasia in 400738 sets of gastric biopsies. Aliment. Pharmacol. Ther. 2013, 38, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. European helicobacter and microbiota study group and consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Matsumoto, A.; Tanaka, H.; Matsumura, I. Gastric microbiota: An emerging player in Helicobacter pylori-induced gastric malignancies. Cancer Lett. 2018, 414, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human gut microbiota and gastrointestinal cancer. Genom. Proteom. Bioinform. 2018, 16, 33–49. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).