Characterization of Monoclonal Antibodies against σA Protein and Cross-Reactive Epitope Identification and Application for Detection of Duck and Chicken Reovirus Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Sera
2.2. Expression of σ A Protein
2.3. Monoclonal Antibodies Production and Coupling to Horseradish Peroxidase
2.4. Characterization of MAbs by Western Blot, Immunofluorescence, and Dot Blotting Assays
2.5. Antibody Binding Assay and Competitive Binding Assay (CBA)
2.6. Epitope Mapping
2.7. Sequence Analysis
2.8. Cross-Reactivity of the Epitopes
2.9. Competitive Inhibition Binding Assay
2.10. Epitope Peptides for Detection of DRV and ARV Infections
3. Results
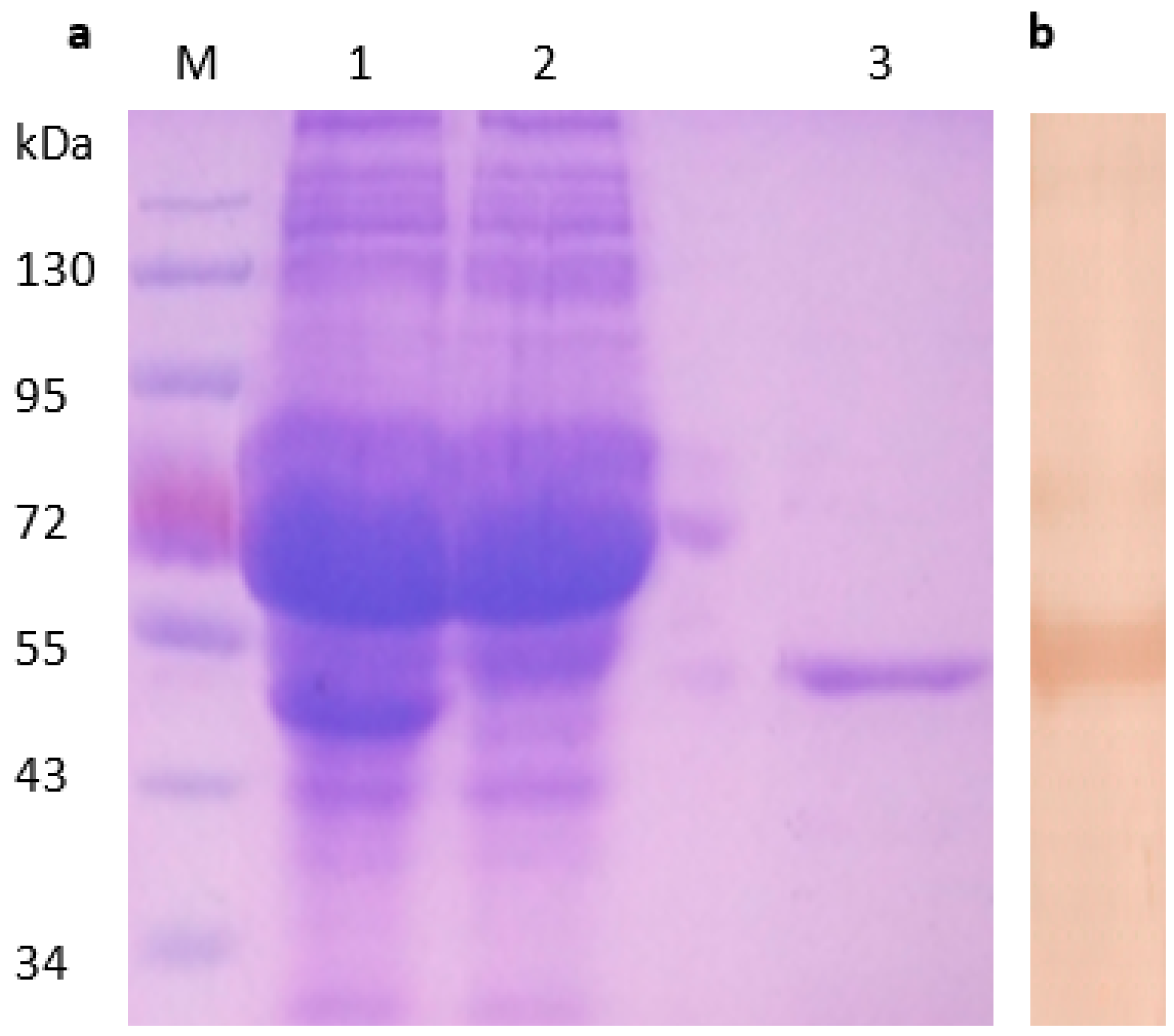
3.1. σA Protein Expression
3.2. Characterization of MAbs
3.3. Competitive Binding Assay
3.4. Epitope Mapping
3.5. Sequence Analysis of the Identified Epitopes Among DRV and ARV
3.6. Fine Mapping of the Epitopes by Western Blot
3.7. Epitopes Cross-Reactivity to DRV and ARV-Positive Sera
3.8. Competitive Inhibition of Recombinant Peptides Binding to MAbs 1A7 and 4E2
3.9. An Epitope-Based Peptide ELISA for Diagnosis of DRV and ARV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Heffels-Redmann, U.; Muller, H.; Kaleta, E.F. Structural and biological characteristics of reoviruses isolated from Muscovy ducks (Cairina moschata). Avian Pathol. 1992, 21, 481–491. [Google Scholar] [CrossRef]
- Kuntz-Simon, G.; Le Gall-Recule, G.; de Boisseson, C.; Jestin, V. Muscovy duck reovirus σC protein is a typically encoded by the smallest genome segment. J. Gen. Virol. 2002, 83, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Le Gall-Recule, G.; Cherbonnel, M.; Arnauld, C.; Blanchard, P.; Jestin, A.; Jestin, V. Molecular characterization and expression of the S3 gene of Muscovy duck reovirus strain 89026. J. Gen. Virol. 1999, 80, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, D.; Geng, H.; Liu, M.; Hu, L.; Wang, J.; Tong, G.; Kong, X.; Liu, C. Characterization of M-class genome segments of Muscovy duck reovirus S14. Virus Res. 2007, 125, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, D.C.; Liu, M.; Geng, H.; Hu, Q.L. Characterization of the σB-encoding genes of musocvy duck reovirus: σC-σB-ELISA for antibodies against duck reovirus in ducks. Vet. Microbiol. 2007, 121, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, M.; Hu, Q.L.; Ouyang, S.; Tong, G.Z. Characterization of the σC-encoding gene from Musocvy duck reovirus. Virus Genes 2006, 32, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, M.; Qu, L.; Xiang, W.; Guo, D.; Yuan, X.; Ge, M.; Zhang, C. Sequence and phylogenetic analysis of the S-class genome segments of a duck orthoreovirus. Acta Virol. 2007, 51, 239–247. [Google Scholar] [PubMed]
- Geng, H.; Zhang, Y.; Liu-Partanen, Y.; Guo, D.; Wang, Y.; Liu, M.; Tong, G. Apoptosis Induced by Duck Reovirus p10.8 Protein in Primary Duck Embryonated Fibroblast and Vero E6 Cells. Avian Dis. 2009, 53, 434–440. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, G.; Huang, Y.; Ren, G.; Chen, L.; Gao, J.; Zhang, D.; Han, B.; Su, W.; Zhao, J.; et al. Isolation and characterization of a reovirus causing spleen necrosis in Pekin ducklings. Vet. Microbiol. 2011, 148, 200–206. [Google Scholar] [CrossRef]
- Yun, T.; Yu, B.; Ni, Z.; Ye, W.C.; Chen, L.; Hua, J.G.; Zhang, C. Genomic characteristics of a novel reovirus from Muscovy duckling in China. Vet. Microbiol. 2014, 168, 261–271. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Zhu, Y.Q.; Li, C.F.; Liu, G.Q. Outbreak-associated novel duck reovirus, China, 2011. Emerg. Infect. Dis. 2012, 18, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Malkinson, M.; Perk, K.; Weisman, Y. Reovirus infection of young muscovy ducks (Cairina moschata). Avian Pathol. 1981, 10, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Palya, V.; Glávits, R.; Dobos-Kovács, M.; Ivanics, E.; Nagy, E.; Bányai, K.; Reuter, G.; Szücs, G.; Dán, A.; Benkö, M. Reovirus identified as cause of disease in young geese. Avian Pathol. 2003, 32, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, J.K.; Olson, N.O. Reovirus infections. In Diseases of Poultry; Canlnek, B.W., Barnes, H.J., Beard, C.W., Reid, W.M., Yoder, H.W., Jr., Eds.; Iowa State University Press: Ames, IA, USA, 1991; pp. 639–647. [Google Scholar]
- Rosenberger, J.K.; Sterner, F.J.; Botts, S.; Lee, K.P.; Margolin, A. In vitro and in vivo characterization of avian reoviruses I. Pathogenicity and antigenic relatedness of several avian reovirus isolates. Avian Dis. 1989, 33, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, L.E.; Gupta, A.; Fricke, J.; Ahmed, K.A.; Popowich, S.; Lockerbie, B.; Tikoo, S.K.; Ojkic, D.; Gomis, S. Phenotypic, genotypic and antigenic characterization of emerging avian reoviruses isolated from clinical cases of arthritis in broilers in Saskatchewan, Canada. Sci. Rep. 2017, 7, 3565. [Google Scholar] [CrossRef]
- Palomino-Tapia, V.; Mitevski, D.; Inglis, T.; van der Meer, F.; Abdul-Careem, M.F. Molecular characterization of emerging avian reovirus variants isolated from viral arthritis cases in Western Canada 2012–2017 based on partial sigma (σ)C gene. Virology 2018, 522, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y.; Lee, D.H.; Lim, T.H.; Lee, J.H.; Day, J.M.; Song, C.S. Isolation and genomic characterization of a novel avian orthoreovirus strain in Korea, 2014. Arch. Virol. 2018, 163, 1307–1316. [Google Scholar] [CrossRef]
- Kuntz-Simon, G.; Blanchard, P.; Cherbonnel, M.; Jestin, A.; Jestin, V. Baculovirus-expressed muscovy duck reovirus sC protein induces serum neutralizing antibodies and protection against challenge. Vaccine 2002, 20, 3113–3122. [Google Scholar] [CrossRef]
- Day, J.M. The diversity of the orthoreoviruses: Molecular taxonomy and phylogentic divides. Infect. Genet. Evol. 2009, 9, 390–400. [Google Scholar] [CrossRef]
- Dermody, T.S.; Schiff, L.A.; Nibert, M.L.; Coombs, K.M.; Fields, B.N. The S2 Gene Nucleotide Sequences of Prototype Strains of the Three Reovirus Serotypes: Characterization of Reovirus Core Protein σ2. J. Virol. 1991, 65, 5721–5731. [Google Scholar]
- Liu, H.J.; Huang, P.H. Sequence and phylogenetic analysis of the sigma A-encoding gene of avian reovirus. J. Virol. Methods 2001, 98, 99–107. [Google Scholar] [CrossRef]
- Benavente, J.; Martínez-Costas, J. Avian reovirus: Structure and biology. Virus Res. 2007, 123, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.S.; Shieh, H.K.; Lee, L.H. Synthesis in Escherichia coli of avian reovirus core protein σA and its dsRNA-binding activity. Virology 2000, 266, 33–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guardado-Calvo, P.; Vazquez-Iglesias, L.; Martinez-Costas, J.; Llamas-Saiz, A.L.; Schoehn, G.; Fox, G.C.; Hermo-Parrado, X.L.; Benavente, J.; van Raaij, M.J. Crystal structure of the avian reovirus inner capsid protein σA. J. Virol. 2008, 82, 11208–11216. [Google Scholar] [CrossRef] [PubMed]
- Mor, S.K.; Verma, H.; Sharafeldin, T.A.; Porter, R.E.; Jindal, N.; Ziegler, A.; Goyal, S.M. Characterization of S class gene segments of a newly isolated turkey arthritis reovirus. Virology 2014, 464–465, 33–44. [Google Scholar] [CrossRef]
- Dandar, E.; Farkas, S.L.; Marton, S.; Oldal, M.; Jakab, F.; Mato, T.; Palya, V.; Banyai, K. The complete genome sequence of a European goose reovirus strain. Arch. Virol. 2014, 159, 2165–2169. [Google Scholar] [CrossRef]
- Day, J.M.; Pantin-Jackwood, M.J.; Spackman, E. Sequence and phylogenetic analysis of the S1 genome segment of turkey-origin reoviruses. Virus Genes 2007, 35, 235–242. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Ouyan, S.D.; Hu, Q.L.; Guo, D.C.; Han, Z. Detection and identification of avian, duck, and goose reoviruses by RT-PCR: Goose and duck reoviruses aggregated the same specified genogroup in Orthoreovirus Genus II. Arch. Virol. 2006, 151, 1525–1538. [Google Scholar] [CrossRef]
- Liu, M.; Chen, X.; Wang, Y.; Zhang, Y.; Li, Y.; Wang, Y.; Shen, N.; Chen, H. Characterization of monoclonal antibodies against Muscovy duck reovirus σB protein. Virol. J. 2010, 7, 133. [Google Scholar] [CrossRef]
- Li, Y.; Yin, X.; Chen, X.; Li, X.; Li, J.; Liu, C.; Liu, M.; Zhang, Y. Antigenic analysis monoclonal antibodies against different epitopes of σB protein of Muscovy duck reovirus. Virus Res. 2012, 163, 546–551. [Google Scholar] [CrossRef]
- Hall, R.A.; Broom, A.K.; Hartnett, A.C.; Howard, M.J.; Mackenzie, J.S. Immunodominant epitopes on the NS1 protein of MVE and KUN viruses serve as targets for a blocking ELISA to detect virus-specific antibodies in sentinel animal serum. J. Virol. Methods 1995, 51, 201–210. [Google Scholar] [CrossRef]
- Li, C.; Bai, X.; Meng, R.; Shaozhou, W.; Zhang, Q.; Hua, R.; Liu, J.; Liu, M.; Zhang, Y. Identification of a New Broadly Cross-reactive Epitope within Domain III of the Duck Tembusu Virus E Protein. Sci. Rep. 2016, 6, 36288. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, J.; Shaozhou, W.; Bai, X.; Zhang, Q.; Hua, R.; Liu, J.; Liu, M.; Zhang, Y. Epitope Identification and Application for Diagnosis of Duck Tembusu Virus Infections in Ducks. Viruses 2016, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Wawegama, N.K.; Tarigan, S.; Indriani, R.; Selleck, P.; Abdul Adjid, R.M.; Syafriati, T.; Hardiman; Durr, P.A.; Ignjatovic, J. Evaluation of a homologous HA 274–288 epitope to detect antibodies to highly pathogenic avian influenza virus H5N1 in Indonesian commercial poultry. Avian Pathol. 2016, 45, 478–492. [Google Scholar] [CrossRef]
- Kouzmitcheva, G.A.; Petrenko, V.A.; Smith, G.P. Identifying Diagnostic Peptides for Lyme Disease through Epitope Discovery. Clin. Diagn. Lab. Immunol. 2001, 8, 150–160. [Google Scholar] [CrossRef]
- Wilson, M.B.; Nakane, P.K. Recent developments in the periodate method of conjugating horseradish peroxidase (HRPO) to antibodies. In Immunofluorescence and Related Staining Techniques; Elsevier/North-Holland: New York, NY, USA, 1978; pp. 215–224. [Google Scholar]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. Methods Mol. Biol. 2000, 132, 71–91. [Google Scholar]
- Huang, P.H.; Li, Y.J.; Su, Y.P.; Lee, L.H.; Liu, H.J. Epitope mapping and functional analysis of sigma A and sigma NS proteins of avian reovirus. Virology 2005, 332, 584–595. [Google Scholar] [CrossRef] [PubMed]







| Competitor | Clone Type | HRP-Labled Mab | ||||||
|---|---|---|---|---|---|---|---|---|
| 1A7 | 2B7 | 3F4 | 2D5 | 3C7 | 4E2 | |||
| Epitope I | 1A7 | IgG1 | ++ | ++ | ++ | −− | −− | −− |
| 2B7 | IgG2b | ++ | ++ | ++ | −− | −− | −− | |
| 3F4 | IgG1 | ++ | ++ | ++ | −− | −− | −− | |
| Epitope II | 2D5 | IgG1 | −− | −− | −− | ++ | −− | −− |
| Epitope III | 3C7 | IgG2b | −− | −− | −− | −− | ++ | ++ |
| 4E2 | IgG1 | −− | −− | −− | −− | ++ | ++ | |
| Phage Clones | Sequences | Phage Clones | Sequences | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | T | M | A | N | E | A | P | Y | P | G | T | Q | 1 | A | F | W | S | V | P | G | T | V | S | M | T | |||||
| 2 | H | W | D | P | P | Y | P | G | S | G | T | Q | 2 | N | W | V | V | G | G | S | V | A | A | V | T | |||||
| 4 | T | E | A | P | Y | P | G | S | S | V | T | N | 3 | Y | N | F | V | M | A | G | L | V | A | T | T | |||||
| 5 | S | A | L | I | D | A | A | Y | P | G | T | Q | 6 | I | W | V | M | A | G | A | I | S | L | S | M | |||||
| 8 | S | E | A | P | Y | P | G | A | G | V | Y | N | 8 | Y | V | M | A | G | L | I | L | S | I | P | N | |||||
| 9 | T | F | E | A | P | Y | P | G | T | M | L | V | 9 | C | F | T | M | A | S | L | I | T | I | T | A | |||||
| 10 | S | T | E | A | P | Y | P | G | I | A | V | Q | 11 | G | A | W | V | V | A | G | L | I | M | T | V | |||||
| Consensus | E | A | P | Y | P | G | 12 | Q | W | V | V | A | G | L | V | S | V | L | G | |||||||||||
| DRV | V | S | T | P | E | A | P | Y | P | G | S | S | L | Y | Q | Consensus | W | V | V/M | A | G | L | I/V | |||||||
| DRV | Q | Q | W | V | V | A | G | L | I | S | A | A | K | G | ||||||||||||||||
| Peptide | Primers | Sequences | Muted Epitope Peptide |
|---|---|---|---|
| S1 | pSF1 | 5-aattc gag gcg cct tac ccg ggc c-3 | GST-EAPYPG |
| pSR1 | 5-tcgag gcc cgg gta agg cgc ctc g-3 | ||
| S2 | pSF2 | 5-aattc gcg cct tac ccg ggc c-3 | GST-APYPG |
| pSR2 | 5-tcgag gcc cgg gta agg cgc g-3 | ||
| S3 | pSF3 | 5-aattc gag gcg cct tac ccg c-3 | GST-EAPYP |
| pSR3 | 5-tcgag cgg gta agg cgc ctc g-3 | ||
| S4 | pSF4 | 5-aattc gcg gcg cct tac ccg ggc c-3 | GST-AAPYPG |
| pSR4 | 5-tcgag gcc cgg gta agg cgc cgc g-3 | ||
| S5 | pSF5 | 5-aattc gag gcg cct tac ccg gcg c-3 | GST-EAPYPA |
| pSR5 | 5-tcgag cgc cgg gta agg cgc ctc g-3 | ||
| P1 | pF1 | 5-aattc tgg gtc gtg gct ggt ctg att c-3 | GST-WVVAGLI |
| pR1 | 5-tcgag aat cag acc agc cac gac cca g-3 | ||
| P2 | pF4 | 5-aattc gtc gtg gct ggt ctg att c-3 | GST-VVAGLI |
| pR4 | 5-tcgag aat cag acc agc cac gac g-3 | ||
| P3 | pF3 | 5-aattc tgg gtc atg gct ggt ctg att c-3 | GST-WVMAGLI |
| pR3 | 5-tcgag aat cag acc agc cat gac cca g-3 | ||
| P4 | pF3 | 5-aattc tgg gtc gtg gct ggt ctg gtg c-3 | GST-WVVAGLV |
| pR3 | 5-tcgag cac cag acc agc cac gac cca g-3 | ||
| P5 | pF5 | 5-aattc tgg gtc gtg gct ggt ctg c-3 | GST-WVVAGL |
| pR5 | 5-tcgag cag acc agc cac gac cca g-3 | ||
| P6 | pF6 | 5-aattc tgg gtc atg gct ggt ctg gtg c-3 | GST-WVMAGLV |
| pR6 | 5-tcgag cac cag acc agc cat gac cca g-3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, T.; Chen, X.; Li, C.; Lin, W.; Liu, H.; Song, S.; Bai, X.; Zhang, Y. Characterization of Monoclonal Antibodies against σA Protein and Cross-Reactive Epitope Identification and Application for Detection of Duck and Chicken Reovirus Infections. Pathogens 2019, 8, 140. https://doi.org/10.3390/pathogens8030140
Chen X, Li T, Chen X, Li C, Lin W, Liu H, Song S, Bai X, Zhang Y. Characterization of Monoclonal Antibodies against σA Protein and Cross-Reactive Epitope Identification and Application for Detection of Duck and Chicken Reovirus Infections. Pathogens. 2019; 8(3):140. https://doi.org/10.3390/pathogens8030140
Chicago/Turabian StyleChen, Xueming, Tongtong Li, Xiaodan Chen, Chenxi Li, Weiwei Lin, Hongyu Liu, Shuping Song, Xiaofei Bai, and Yun Zhang. 2019. "Characterization of Monoclonal Antibodies against σA Protein and Cross-Reactive Epitope Identification and Application for Detection of Duck and Chicken Reovirus Infections" Pathogens 8, no. 3: 140. https://doi.org/10.3390/pathogens8030140
APA StyleChen, X., Li, T., Chen, X., Li, C., Lin, W., Liu, H., Song, S., Bai, X., & Zhang, Y. (2019). Characterization of Monoclonal Antibodies against σA Protein and Cross-Reactive Epitope Identification and Application for Detection of Duck and Chicken Reovirus Infections. Pathogens, 8(3), 140. https://doi.org/10.3390/pathogens8030140




