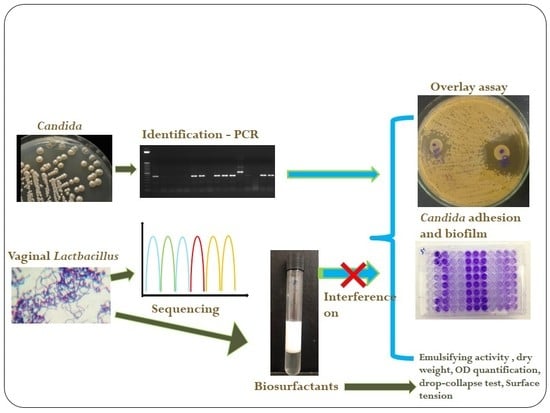
Antifungal and Antivirulence Activity of Vaginal Lactobacillus Spp. Products against Candida Vaginal Isolates
Abstract
:1. Introduction
2. Results
2.1. Microbial Isolation and Identification
2.2. Antagonism Assay
2.3. BS-Producing Lactobacilli and BS Properties
2.4. Surface Tension Determination
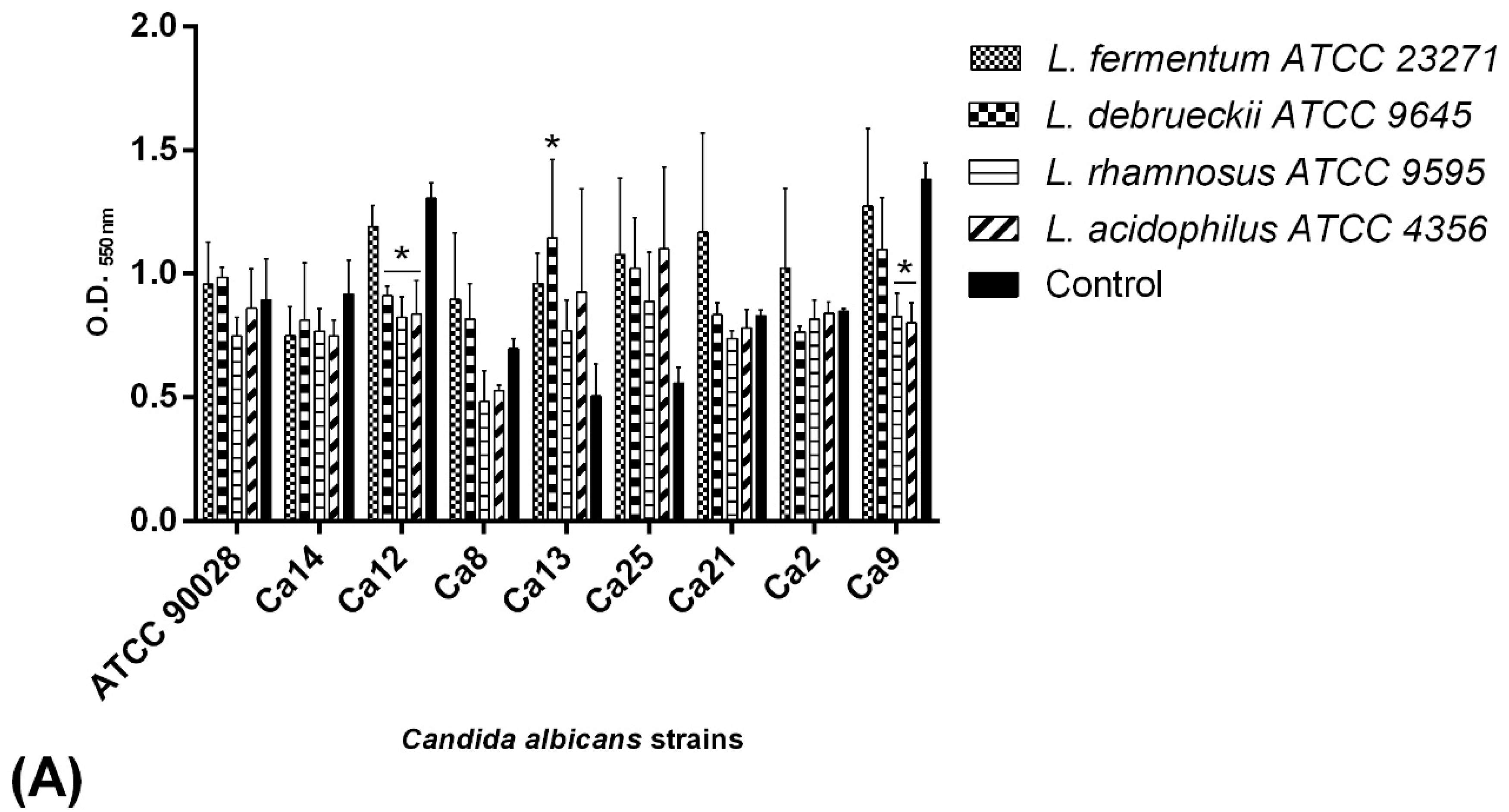
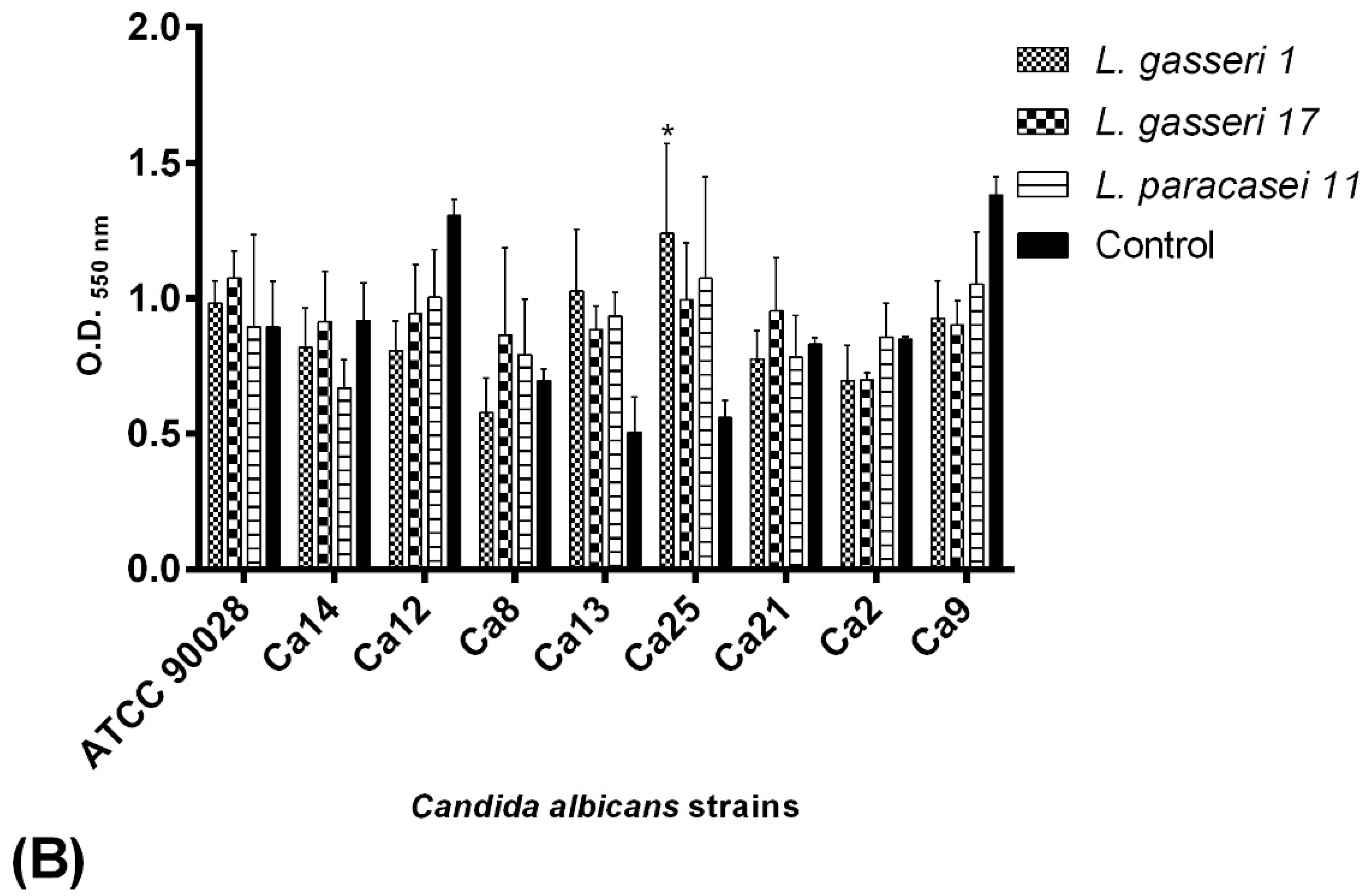
2.5. BS Interference on C. albicans Adhesion
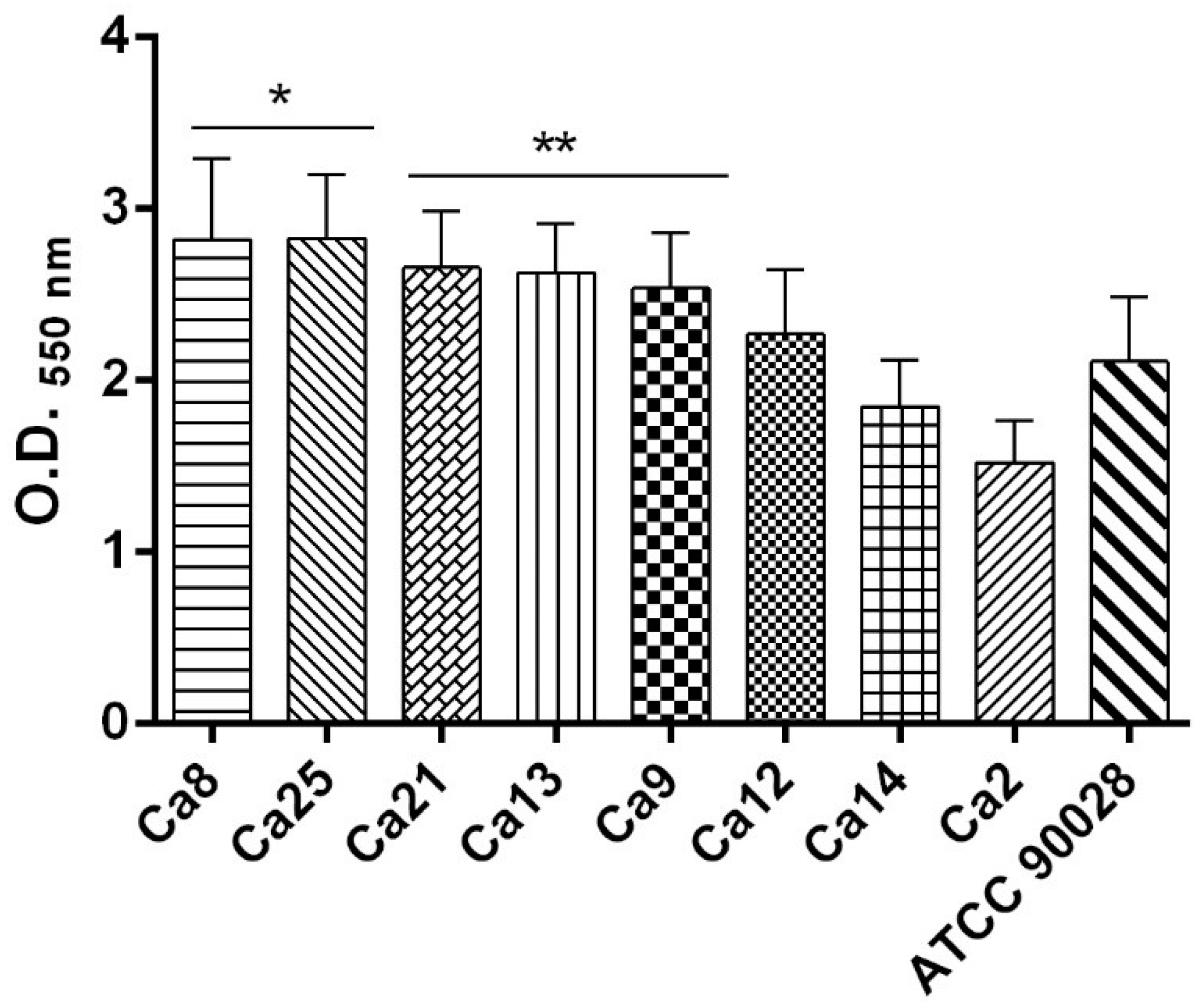
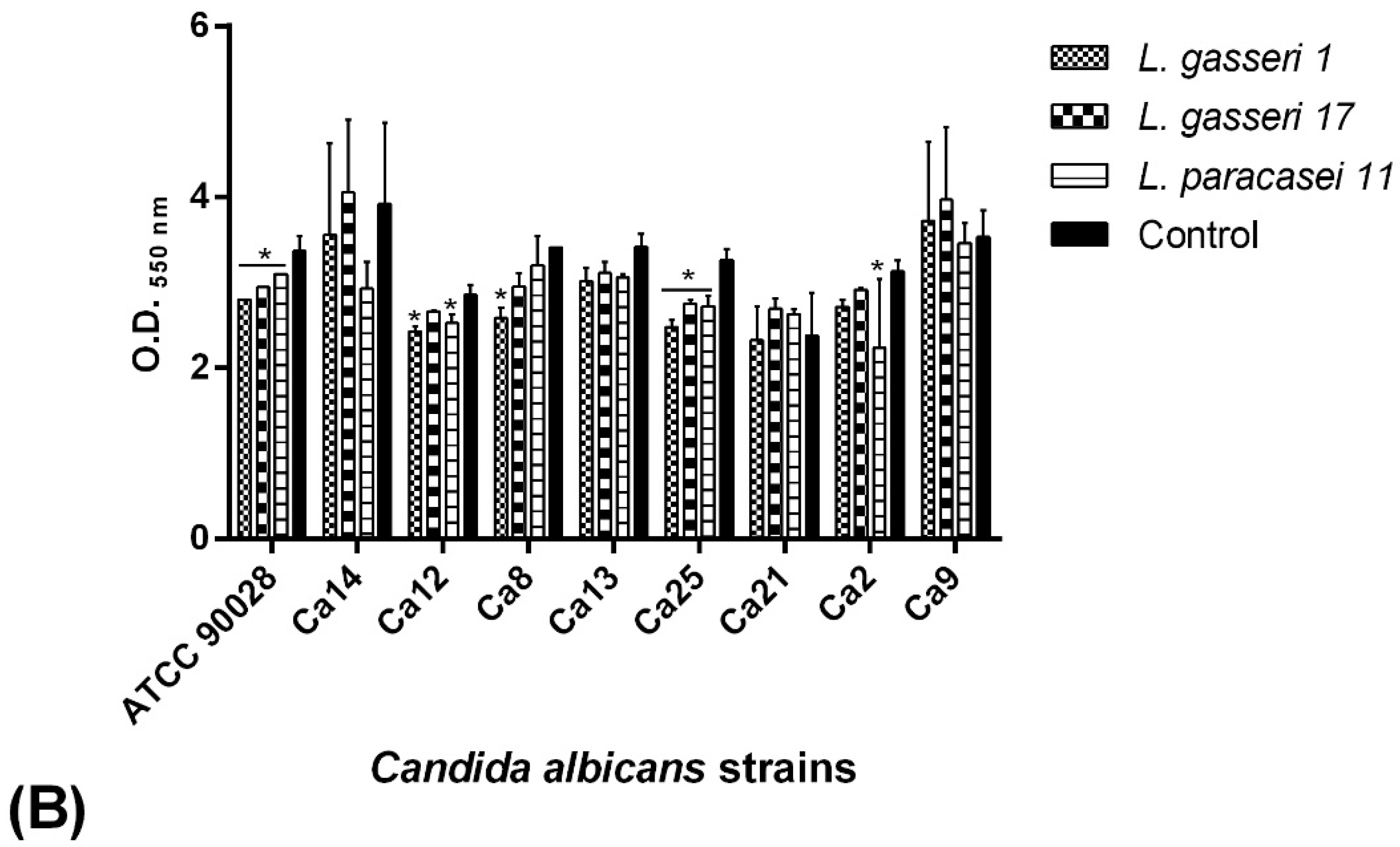
2.6. C. albicans Biofilm Formation Ability
2.7. BS Interference in the Biofilm Formation of C. albicans Isolates
2.7.1. Co-Incubation Assay
2.7.2. Pre-Incubation Assay
2.8. Antifungal Susceptibility Testing
3. Discussion
4. Materials and Methods
4.1. Patients and Ethical Aspects
4.2. Statement
4.3. Isolation and Identification of Vaginal Microorganisms
4.4. Antagonism Assay
4.5. BS Production by Lactobacillus Spp.
4.5.1. Emulsifying Activity Determination
4.5.2. Dry Weight and OD640 nm Measurements
4.5.3. Drop-Collapse Method
4.5.4. ST Determination
4.6. Selection of C. albicans Biofilm Producers
4.7. BS Interference on C. albicans Adhesion
4.8. BS Interference on C. albicans Biofilm Formation
4.9. Antifungal Susceptibility Testing
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larsen, B.; Monif, G.R.G. Understanding the Bacterial Flora of the Female Genital Tract. Clin. Infect. Dis. 2001, 32, e69–e77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolin, C.; Marangoni, A.; Laghi, L.; Foschi, C.; Ñahui Palomino, R.A.; Calonghi, N.; Cevenini, R.; Vitali, B. Isolation of Vaginal Lactobacilli and Characterization of Anti-Candida Activity. PLoS ONE 2015, 10, e0131220. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Xie, H.Y.; Feng, D.; Wei, D.M.; Mei, L.; Chen, H.; Wang, X.; Fang, F. Probiotics for vulvovaginal candidiasis in non-pregnant women. Cochrane Database Syst. Rev. 2017, 11, CD010496. [Google Scholar] [CrossRef] [PubMed]
- Holzer, I.; Farr, A.; Kiss, H.; Hagmann, M.; Petricevic, L. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch. Gynecol. Obstet. 2017, 295, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Kolecka, A.; Boekhout, T.; Zarrinfar, H.; Ghanbari Nahzag, M.A.; Badiee, P.; Rezaei-Matehkolaei, A.; Fata, A.; Dolatabadi, S.; Najafzadeh, M.J. Identification of Candida species isolated from vulvovaginitis using matrix assisted laser desorption ionizationtime of flight mass spectrometry. Curr. Med. Mycol. 2017, 3, 21–25. [Google Scholar] [CrossRef]
- Nejat, Z.A.; Farahyar, S.; Falahati, M.; Khozani, M.A.; Hosseini, A.F.; Faiazy, A.; Ekhtiari, M.; Hashemi-Hafshenjani, S. Molecular Identification and Antifungal Susceptibility Pattern of Non-albicans Candida Species Isolated from Vulvovaginal Candidiasis. Iran. Biomed. J. 2018, 22, 33–41. [Google Scholar] [CrossRef]
- Alves, M.B.; Silva, I.M.O.; Santos, C.I.; França, Y.R.; Oliveira, S.K.R.; Monteiro, S.G.; Monteiro, C.A. Prevalence of Candida spp. from samples of vaginal secretion and its relationship to factors associated with vulvovaginitis. Rev. Investig. Bioméd. 2015, 7, 58–68. [Google Scholar] [CrossRef]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef]
- Ishida, K.; Ueda-Yamaguchi, M.; Ogatta, S.F.Y.; Ueda-Nakamura, T.; Svidizinski, T.I.E.; Nakamura, C.V. Characterization of Candida spp. isolated from vaginal fluid: Identification, antifungal susceptibility, and virulence profile. Acta S. Health Sci. 2013, 35, 1–8. [Google Scholar] [CrossRef]
- Siddiqi, R.; Mendiratta1, D.K.; Rukadikar, A.; Gadre, S. Study of Virulence Markers and Antifungal Susceptibility by Vitek-2 in Various Candida Species Isolated from Cases of Vulvovaginal Candidiasis. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 3593–3605. [Google Scholar] [CrossRef]
- Sherry, L.; Kean, R.; McKloud, E.; O’Donnell, L.E.; Metcalfe, R.; Jones, B.L.; Ramage, G. Biofilms formed by isolates from recurrent vulvovaginal candidiasis patients are heterogeneous and insensitive to fluconazole. Antimicrob. Agents Chemother. 2017, 61, e01065–e01117. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Pascual, L.M.; Daniele, M.B.; Ruiz, F.; Giordano, W.; Pájaro, C.; Barberis, L. Lactobacillus rhamnosus L60, a potential probiotic isolated from the human vagina. J. Gen. Appl. Microbiol. 2008, 54, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Younes, J.A.; Vander Mei, H.C.; Gloor, G.B.; Knight, R.; Busscher, H.J. Microbiota restoration: Natural and supplemented recovery of human microbial communities. Nat. Rev. Microbiol. 2011, 9, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Babula, O.; Lazdane, G.; 2 Kroica, J.; Linhares, I.M.; Ledger, W.J.; Witkin, S.S. Frequency of Interleukin-4 (IL-4) 589 Gene Polymorphism and Vaginal Concentrations of IL-4, Nitric Oxide, and Mannose-Binding Lectin in Women with Recurrent Vulvovaginal Candidiasis. Clin. Infect. Dis. 2005, 40, 1258–1262. [Google Scholar] [CrossRef]
- Rodrigues, L.R.; Teixeira, J.A.; Van der Mei, H.C.; Oliveira, R. Physicochemical and functional characterization of a biosurfactant produced by Lactobacillus lactis 53. Colloids Surf. B Biointerfaces 2006, 49, 79–86. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Rodrigues, L.; Van der Mei, H.; Teixeira, J.A.; Oliveira, R. Biosurfactant from Lactococcus lactis 53 inhibits microbial adhesion on silicone rubber. Appl. Microbiol. Biotechnol. 2004, 66, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Rocha, V.; Teixeira, J.A.; Rodrigues, L.R. Antimicrobial and antiadhesive properties of a biosurfactant isolated from Lactobacillus paracasei ssp. paracasei A20. Lett. Appl. Microbiol. 2010, 50, 419–424. [Google Scholar] [CrossRef]
- Thavasi, R.; Jayalakshmi, S.; Banat, I.M. Application of biosurfactant produced from peanut oil cake by Lactobacillus delbrueckii in biodegradation of crude oil. Bioresour. Technol. 2011, 102, 3366–3372. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Saharan, B.S.; Chauhan, N.; Procha, S.; Lal, S. Isolation and functional characterization of novel biosurfactant produced by Enterococcus faecium. Springer Plus 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Silva, S.S.E. Recent food applications of microbial surfactants. Crit. Rev. Food Sci. Nutr. 2017, 58, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Zalai, C.; Gardiner, G. Urogenital lactobacilli probiotics, reliability, and regulatory issues. J. Dairy Sci. 2001, 84, E164–E169. [Google Scholar] [CrossRef]
- Saharan, B.S.; Sahu, R.K.; Sharma, D. A review on biosurfactants: Fermentation, current developments and perspectives. Genet. Eng. Biotechnol. J. 2011, 2011, 1–14. [Google Scholar]
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant Producing Microbes and their Potential Applications: A Review. Crit. Rev. Environ. Sci. Technol. 2014, 45, 1522–1554. [Google Scholar] [CrossRef]
- Santos, D.K.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Salgueiro, A.A.; Sarubbo, L.A. Synthesis and evaluation of biosurfactant produced by Candida lipolytica using animal fat and corn steep liquor. J. Pet. Sci. Eng. 2013, 105, 43–50. [Google Scholar] [CrossRef]
- Chew, S.Y.; Cheah, Y.K.; Seow, H.F.; Sandai, D.; Than, L.T.L. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J. Appl. Microbiol. 2015, 118, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Q.; Yang, E.; Yan, L.; Li, T.; Zhuang, H. Antimicrobial Compounds Produced by Vaginal Lactobacillus crispatus Are Able to Strongly Inhibit Candida albicans Growth, Hyphal Formation and Regulate Virulence-related Gene Expressions. Front. Microbiol. 2017, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- Carmo, M.S.; Noronha, F.M.; Arruda, M.O.; Costa, Ê.P.; Bomfim, M.R.; Monteiro, A.S.; Ferro, T.A.; Fernandes, E.S.; Girón, J.A.; Monteiro-Neto, V. Lactobacillus fermentum ATCC 23271 displays in vitro inhibitory activities against Candida spp. Front. Microbiol. 2016, 7, 1722. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Fernandes, E.C.; Teixeira, J.A.; Rodrigues, L.R. Antimicrobial and anti-adhesive activities of cell-bound biosurfactant from Lactobacillus agilis CCUG31450. RSC Adv. 2015, 5, 90960–90968. [Google Scholar] [CrossRef]
- Tahmourespour, A.; Salehi, R.; Kermanshahi, R.K. Lactobacillus acidophilus-derived biosurfactant effect on gtfB and gtfC expression level in Streptococcus mutans biofilm cells. Braz. J. Microbiol. 2011, 42, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Al Kassaa, I.; Hamze, M.; Hober, D.; Chihib, N.E.; Drider, D. Identification of vaginal lactobacilli with potential probiotic properties isolated from women in North Lebanon. Microbial. Ecol. 2014, 67, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Tessarolo, F.; Caola, I.; Nollo, G.; Cavallo, M.; Rinaldi, M.; Fracchia, L. Inhibition of Candida albicans adhesion on medical-grade silicone by a Lactobacillus-derived biosurfactant. J. Appl. Microbiol. 2015, 118, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Morais, I.M.C.; Cordeiro, A.L.; Teixeira, G.S.; Domingues, V.S.; Nardi, R.M.D.; Monteiro, A.S.; Alves, R.J.; Siqueira, E.P.; Santos, V.L. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P6A and Lactobacillus gasseri P65. Microb. Cell Fact. 2017, 16, 155. [Google Scholar] [CrossRef]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Characterization of Biosurfactant Produced during Degradation of Hydrocarbons Using Crude Oil as Sole Source of Carbon. Front. Microbiol. 2017, 8, 279. [Google Scholar] [CrossRef]
- Madhu, N.; Prapulla, S.G. Evaluation and functional characterization of a biosurfactant produced by Lactobacillus plantarum CFR 2194. Appl. Biochem. Biotechnol. 2014, 172, 1777–1789. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Nair, A.; Dubey, K.V.; Singh, S.K.; Devotta, S. Biosurfactant technology for remediation of cadmium and lead contaminated soils. Chemosphere 2007, 68, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbial. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant-Producing Lactobacilli: Screening, Production Profiles, and Effect of Medium Composition. Appl. Environ. Soil Sci. 2011. [Google Scholar] [CrossRef]
- Monteiro, A.S.; Miranda, T.T.; Lula, I.; Denadai, Â.M.; Sinisterra, R.D.; Santoro, M.M.; Santos, V.L. Inhibition of Candida albicans CC biofilms formation in polystyrene plate surfaces by biosurfactant produced by Trichosporon montevideense CLOA72. Colloids Surf. B Biointerfaces 2011, 84, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R.; Marshall, K.C. Bacterial polymers: Physicochemical aspects of their interactions at interfaces. J. Biomater. Appl. 1990, 5, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Maingault, M. Utilization of Sophorolipids as Therapeutically Active Substances or Cosmetic Products, in Particular for the Treatment of the Skin. U.S. Patent No. 5,981,497, 29 January 1999. [Google Scholar]
- Stipcevic, T.; Piljac, A.; Piljac, G. Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns 2006, 32, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piljac, A.; Stipčević, T.; Piljac-Žegarac, J.; Piljac, G. Successful treatment of chronic decubitus ulcer with 0.1% dirhamnolipid ointment. J. Cutan. Med. Surg. 2008, 12, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Vilela, S.F.; Barbosa, J.O.; Rossoni, R.D.; Santos, J.D.; Prata, M.C.; Anbinder, A.L.; Jorge, A.O.; Junqueira, J.C. Lactobacillus acidophilus ATCC 4356 inhibits biofilm formation by C. albicans and attenuates the experimental candidiasis in Galleria mellonella. Virulence 2015, 6, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Meylheuc, T.; Methivier, C.; Renault, M.; Herry, J.M.; Pradier, C.M.; Bellon-Fontaine, M.N. Adsorption on stainless steel surfaces of biosurfactants produced by gram-negative and gram-positive bacteria: Consequence on the bioadhesive behavior of Listeria monocytogenes. Colloids Surf. B Biointerfaces 2006, 52, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Velraeds, M.M.; Van de Belt-Gritter, B.; Van der Mei, H.C.; Reid, G.; Busscher, H.J. Interference in initial adhesion of uropathogenic bacteria and yeasts to silicone rubber by a Lactobacillus acidophilus biosurfactant. J. Med. Microbiol. 1998, 47, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Fracchia, L.; Cavallo, M.; Allegrone, G.; Martinotti, M.G.A. Lactobacillus-derived biosurfactant inhibits biofilm formation of human pathogenic Candida albicans biofilm producers. Appl. Microbiol. Biotechnol. 2010, 2, 827–837. [Google Scholar]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids Surf. B Biointerfaces 2010, 76, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute [CLSI]. M27-A3 Reference method for broth dilution antifungal susceptibility testing of yeast; approved standard- third edition. Clin. Lab. Stand. Instit. 2008, 28, 6–12. [Google Scholar]




| Patient ID | Condition | Candida Identification | Lactobacillus Identification | |
|---|---|---|---|---|
| Multiplex PCR | 16S rRNA Sequencing (% homology) | No. Access | ||
| V2 | VVC | C. albicans | L. gasseri (100%) | MK982449 |
| V3 | C. glabrata | |||
| V5 | C. glabrata | |||
| V6 | C. glabrata | |||
| V8 | C. albicans | |||
| V10 | C. glabrata | |||
| V12 | C. albicans | |||
| V14 | C. albicans | |||
| V15 | L. gasseri (100%) | MK982454 | ||
| V17 | L. gasseri (100%) | MK982456 | ||
| V19 | C. glabrata | L. vaginalis (99%) and L. gasseri (100%) | MN019109MK982457 | |
| V21 | C. albicans | L. vaginalis (100%) | MK982458 | |
| V23 | L. crispatus (100%) | MK982461 | ||
| V24 | L. paracasei (99%) | MK982460 | ||
| A1 | Asymptomatic | L. gasseri (100%) | MK982448 | |
| A4 | C. glabrata | L. gasseri (97.49%) | MK982450 | |
| A7 | L. vaginalis (99%) | MK982451 | ||
| A9 | C. albicans | L. rhamnosus (100%) | MK982452 | |
| A11 | L. paracasei (99.37%) | MK982453 | ||
| A13 | C. albicans | |||
| A16 | L. gasseri (100%) | MK982455 | ||
| A18 | C. krusei | |||
| A20 | C. parapsilosis | |||
| A22 | L. gasseri (100%) | MK982459 | ||
| A25 | C. albicans | |||
| Lactobacillus1 | Candida albicans Isolates 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Ca2 | Ca8 | Ca9 | Ca12 | Ca13 | Ca14 | Ca21 | Ca25 | |
| L. gasseri V17 | NZ | NZ | NZ | 13 ± 0 | NZ | NZ | NZ | NZ |
| L. vaginalis V19.1 | 10 ± 0 | 12 ± 1.41 | 12.5 ± 0.70 | NZ | 11 ± 0 | NZ | NZ | NZ |
| L. gasseri V19.2 | NZ | NZ | NZ | NZ | NZ | NZ | NZ | NZ |
| L. vaginalis V21 | 18.5 ± 2.12 | NZ | 13.5 ± 3.53 | 12.5 ± 0.70 | 15.5 ± 0.70 | 15.5 ± 2.12 | 15.5 ± 0.70 | 14.5 ± 2.12 |
| L. crispatus V23 | NZ | 13 ± 1.41 | 11.5 ± 0.70 | NZ | NZ | NZ | NZ | 11 ± 0 |
| L. paracasei V24 | 9.5 ± 0.70 | 13 ± 1.41 | 11 ± 1.41 | 15 ± 0 | NZ | 13.5 ± 0.70 | NZ | 11 ± 0 |
| L. gasseri A1 | 15.5 ± 0.70 | NZ | NZ | 10.5 ± 0.70 | NZ | 14 ± 1.41 | NZ | NZ |
| L. gasseri A4 | NZ | 12.5 ± 0.70 | 16 ± 1.41 | NZ | 14 ± 1.41 | 14 ± 1.41 | NZ | 13.5 ± 0.70 |
| L. vaginalis A7 | NZ | 16 ± 1.41 | 16 ± 0 | 19.5 ± 2.12 | 22.5 ± 3.53 | 20 ± 0 | 17 ± 1.41 | 16.5 ± 4.94 |
| L. paracasei A11 | 26 ± 1.41 | 19.5 ± 0.7 | 18.5 ± 2.12 | 13.5 ± 3.53 | 17 ± 8.48 | 21.5 ± 4.94 | 19.5 ± 2.12 | 16 ± 1.41 |
| L. gasseri A16 | NZ | 15.5 ± 0.70 | NZ | NZ | 20.5 ± 2.12 | 13.5 ± 0.70 | 16 ± 0 | 11.5 ± 0.70 |
| L. gasseri A22 | NZ | NZ | NZ | NZ | 12.5 ± 0.70 | NZ | NZ | NZ |
| L. acidophilus ATCC 4356 | 21.5 ± 2.12 | 16 ± 0 | 13 ± 1.41 | 21.5 ± 2.12 | 17 ± 2.82 | 15.5 ± 6.36 | 15.5 ± 0.70 | 19.5 ± 0.70 |
| L. debrueckii ATCC 9645 | NZ | NZ | NZ | NZ | NZ | 13 ± 1.41 | NZ | 11.5 ± 0.70 |
| L. fermentum ATCC 23271 | 17 ± 1.41 | 16 ± 1.41 | 13 ± 0 | NZ | 15 ± 4.24 | 12 ± 1.41 | 12.5 ± 0.70 | 14.5 ± 3.53 |
| L. paracasei ATCC 335 | 28.5 ± 2.12 | 17.5 ± 0.70 | 15 ± 5.65 | NZ | 17.5 ± 0.70 | 17.5 ± 3.53 | 15.5 ± 0.70 | 16 ± 1.41 |
| L. rhamnosus ATCC 9595 | 22.5 ± 0.70 | 20 ± 0 | 18.5 ± 0.70 | 21 ± 4.24 | 20 ± 0 | 19.5 ± 0.70 | 25 ± 7.07 | 19.5 ± 0.70 |
| % Inhibited Candida albicans Isolates | |
|---|---|
| VVC | Asymptomatic |
| 12.5 | 37.5 |
| 50 | 62.5 |
| 0 | 87.5 |
| 87.5 | 100 |
| 37.5 | 62.5 |
| 75 | 12.5 |
| % means: 43.75 | 60.42 |
| χ2 = 11.26 p = 0.046 | |
| Microorganisms | Surface Tension (mN/m) ± Standard Deviation |
|---|---|
| L. gasseri 17 | 52.25 ± 0.10 |
| L. paracasei 11 | 55.25 ± 0.07 |
| L. gasseri 1 | 49.34 ± 0.09 |
| L. acidophilus ATCC 4356 | 61.79 ± 0.17 |
| L. delbrueckii ATCC 9645 | 64.46 ± 0.06 |
| L. rhamnosus ATCC 9595 | 64.99 ± 0.06 |
| L. fermentum ATCC 23271 | 53.85 ± 0.14 |
| PBS | 71.90 ± 0.10 |
| Biosurfactant Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lactobacillus | Patients Groups | Ability to Inhibit Candida Growth (Antagonism Test; %) | E24 | Dry Weight | OD640nm | ST | Ability to Inhibit Candida Adhesion (%) | Ability to Inhibit Candida Biofilm (Pre-Coating Assay; %) | Ability to Inhibit Candida Biofilm (Co-Incubation Assay; %) |
| L. acidophilus ATCC 4356 | - | 100 | 44.80 | 0.015 | 2.028 | 61.79 | 78 | 89 | 67 |
| L. debrueckii ATCC 9645 | - | 25 | 48.20 | 0.007 | 1.985 | 64.46 | 44 | 78 | 78 |
| L. fermentum ATCC 23271 | - | 87.5 | 50 | 0.005 | 1.898 | 53.85 | 33 | 100 | 67 |
| L. rhamnosus ATCC 9595 | - | 100 | 25 | 0.012 | 2.060 | 64.99 | 78 | 100 | 67 |
| L. paracasei 11 | Asymptomatic | 100 | 51.80 | 0.009 | 2.026 | 35.25 | 44 | 89 | 89 |
| L. gasseri 1 | Asymptomatic | 37.5 | 51.80 | 0.013 | 1.970 | 49.34 | 67 | 89 | 89 |
| L. gasseri 17 | VVC | 12.5 | 39.20 | 0.007 | 2.033 | 52.25 | 22.2 | 67 | 67 |
| PATIENTS GROUPS | STRAINS | SPECIES | MIC100 (µg/mL) | ||
|---|---|---|---|---|---|
| FLC | ITC | AMB | |||
| VVC | Ca2 | C. albicans | 8 | 0.5 | 1 |
| Ca8 | 16 | 8 | 0.5 | ||
| Ca12 | 4 | 0.5 | 0.25 | ||
| Ca14 | 8 | 0.062 | 0.5 | ||
| Ca21 | 8 | 0.125 | 0.5 | ||
| Control | Ca9 | C. albicans | 8 | 1 | 0.5 |
| Ca13 | 16 | 8 | 1 | ||
| Ca25 | 16 | 0.25 | 0.5 | ||
| Ca18 | 32 | 0.25 | 1 | ||
| - | ATCC 90028 | C. albicans | 2 | 0.5 | 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itapary dos Santos, C.; Ramos França, Y.; Duarte Lima Campos, C.; Quaresma Bomfim, M.R.; Oliveira Melo, B.; Assunção Holanda, R.; Santos, V.L.; Gomes Monteiro, S.; Buozzi Moffa, E.; Souza Monteiro, A.; et al. Antifungal and Antivirulence Activity of Vaginal Lactobacillus Spp. Products against Candida Vaginal Isolates. Pathogens 2019, 8, 150. https://doi.org/10.3390/pathogens8030150
Itapary dos Santos C, Ramos França Y, Duarte Lima Campos C, Quaresma Bomfim MR, Oliveira Melo B, Assunção Holanda R, Santos VL, Gomes Monteiro S, Buozzi Moffa E, Souza Monteiro A, et al. Antifungal and Antivirulence Activity of Vaginal Lactobacillus Spp. Products against Candida Vaginal Isolates. Pathogens. 2019; 8(3):150. https://doi.org/10.3390/pathogens8030150
Chicago/Turabian StyleItapary dos Santos, Camilla, Yasmine Ramos França, Carmem Duarte Lima Campos, Maria Rosa Quaresma Bomfim, Bruna Oliveira Melo, Rodrigo Assunção Holanda, Vera Lucia Santos, Sílvio Gomes Monteiro, Eduardo Buozzi Moffa, Andrea Souza Monteiro, and et al. 2019. "Antifungal and Antivirulence Activity of Vaginal Lactobacillus Spp. Products against Candida Vaginal Isolates" Pathogens 8, no. 3: 150. https://doi.org/10.3390/pathogens8030150
APA StyleItapary dos Santos, C., Ramos França, Y., Duarte Lima Campos, C., Quaresma Bomfim, M. R., Oliveira Melo, B., Assunção Holanda, R., Santos, V. L., Gomes Monteiro, S., Buozzi Moffa, E., Souza Monteiro, A., Andrade Monteiro, C., & Monteiro-Neto, V. (2019). Antifungal and Antivirulence Activity of Vaginal Lactobacillus Spp. Products against Candida Vaginal Isolates. Pathogens, 8(3), 150. https://doi.org/10.3390/pathogens8030150