A Review of Strongyloides spp. Environmental Sources Worldwide
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Animals
3.1.1. Canids
3.1.2. Primates
3.1.3. Ruminants
3.1.4. Rodents
3.1.5. Insects
3.2. Water
3.3. Fruit and Vegetables
3.4. Soil
3.5. PCR and Microscopy
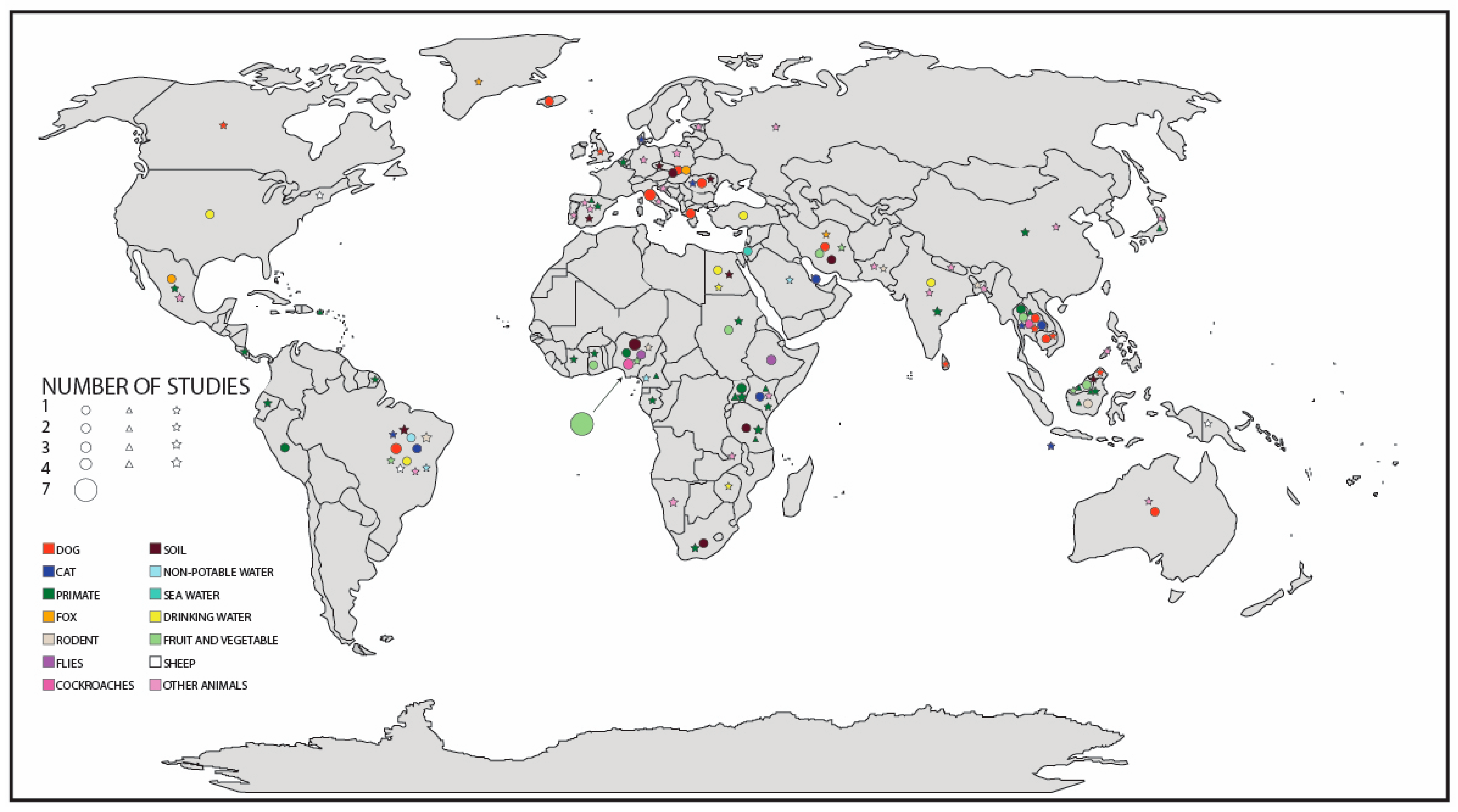
3.6. Global Distribution
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species Parasite | Prevalence | Sample Size | Detection Method | Country | Reference | Source |
|---|---|---|---|---|---|---|
| Strongyloides stercoralis | 1% | 3465 | Microscopy | Romania | (Ardelean et al., 2005) [60] | Dog |
| Strongyloides stercoralis | 49% | 35 | Molecular | Australia | (Beknazarova et al., 2017) [59] | Dog |
| Strongyloides stercoralis | <1% | 3208 | Microscopy | Iceland | (Eydal and Skirnisson 2016) [88] | Dog |
| Strongyloides stercoralis | <1% | 215 | Microscopy | Brazil | (Ferreira et al., 2006) [89] | Dog |
| Strongyloides spp. | <1% | 457 | Microscopy | Canada | (Gaunt and Carr 2011) [90] | Dog |
| Strongyloides stercoralis | <1% | 181 | Microscopy | Brazil | (Goncalves et al., 2007) [91] | Dog |
| Strongyloides stercoralis | 87% | 88 | Molecular | Cambodia | (Jaleta et al., 2017) [6] | Dog |
| Strongyloides stercoralis | <1% | 879 | Microscopy | Greece | (Kostopoulou et al., 2017) [48] | Dog |
| Strongyloides stercoralis | <1% | 189 | Microscopy | Thailand | (Leelayoova et al., 2009) [49] | Dog |
| Strongyloides stercoralis | 45% | 171 | Microscopy | Brazil | (Martins et al., 2012) [58] | Dog |
| Strongyloides stercoralis | 4% | 52 | Microscopy | Romania | (Mircean et al., 2012) [92] | Dog |
| Strongyloides spp. | 5% | 175 | Microscopy | Malaysia | (Noor Azian et al., 2008) [40] | Dog |
| Strongyloides stercoralis | 2% | 281 | Microscopy | Greece | (Papazahariadouet al., 2007) [50] | Dog |
| Strongyloides stercoralis | 2% | 272 | Microscopy | Italy | (Paradies et al., 2017) [93] | Dog |
| Strongyloides spp. | 11% | 90 | Microscopy | Sri Lanka | (Perera et al., 2013) [57] | Dog |
| Strongyloides stercoralis | 6% | 174 | Microscopy | Iran | (Razmi et al., 2009) [52] | Dog |
| Strongyloides stercoralis | <1% | 239 | Microscopy | Italy | (Riggio et al., 2013) [53] | Dog |
| Strongyloides stercoralis | <1% | 639 | Microscopy | Italy | (Sauda et al., 2018) [55] | Dog |
| Strongyloides spp. | 15% | 94 | Microscopy | Cambodia | (Schär et al.,2014) [87] | Dog |
| Strongyloides stercoralis | 10% | 60 | Microscopy | Slovakia | (Štrkolcová et al., 2017) [45] | Dog |
| Strongyloides spp. | 2% | 171 | Microscopy | England | (Wright et al., 2016) [56] | Dog |
| Strongyloides stercoralis | <1% | 463 | Microscopy | Italy | (Zanzani et al., 2014) [94] | Dog |
| Strongyloides spp. | 2% | 197 | Microscopy | Thailand | (Pumidonming et al., 2016) [51] | Dog |
| Strongyloides stercoralis | 18% | 824 | Microscopy | Qatar | (Abu-Madi et al., 2007) [95] | Cat |
| Strongyloides spp. | 47% | 28 | Microscopy | Christmas Island | (Adams et al., 2008) [96] | Cat |
| Strongyloides spp. | 54% | 37 | Microscopy | Brazil | (Lima et al., 2017) [97] | Cat |
| Strongyloides spp. | 3% | 414 | Microscopy | Romania | (Mircean et al., 2010) [98] | Cat |
| Strongyloides stercoralis | 14% | 173 | Microscopy | Brazil | (Monteiro et al., 2016) [99] | Cat |
| Strongyloides stercoralis | 44% | 103 | Microscopy | Kenya | (Njuguna et al., 2017) [100] | Cat |
| Strongyloides spp. | <1% | 300 | Microscopy | Thailand | (Rojekittikhun et al., 2014) [101] | Cat |
| Strongyloides stercoralis | 21% | 38 | Microscopy | Thailand | (Sedionoto and Anamnart 2018) [102] | Cat |
| Strongyloides spp. | 99 1.0% | 99 | Microscopy | Denmark | (Takeuchi-Storm et al., 2015) [103] | Cat |
| Strongyloides fuelleborni | UNK | UNK | Molecular and Microscopy | Japan | (Arizono et al., 2012) [13] | Primate |
| Strongyloides spp. | 41 44% | 41 | Microscopy | Uganda | (Bezjian et al., 2008) [11] | Primate |
| Strongyloides spp. | 37% | 24 | Microscopy | French Guiana | (De Thoisy et al., 2001) [14] | Primate |
| Strongyloides spp. | 21% | 125 | Microscopy | India | (Ekanayake et al., 2006) [104] | Primate |
| Strongyloides fuelleborni | 28% | 293 | Microscopy | Uganda | (Gillespie et al., 2004) [105] | Primate |
| Strongyloides fuelleborni and Strongyloides stercoralis | <1% S. stercoralis, 4% S. fuelleborni | 2103 | Microscopy | Uganda | (Gillespie et al., 2005) [106] | Primate |
| Strongyloides fuelleborni | 84% | 153 | Microscopy | Tanzania | (Gillespie et al., 2010) [107] | Primate |
| Strongyloides fuelleborni and Strongyloides spp. | 11% S. fulleborni, 15% S. spp | 27 | Microscopy | Spain | (Gomez et al., 1996) [15] | Primate |
| Strongyloides fuelleborni | 23% | 401 | Microscopy | Japan | (Gotoh 2000) [108] | Primate |
| Strongyloides fuelleborni and Strongyloides stercoralis | 100% | 7 | Molecular | Uganda | (Hasegawa et al., 2016) [61] | Primate |
| Strongyloides spp. | 88% | 96 | Microscopy | Ecuador | (Helenbrook et al., 2015) [64] | Primate |
| Strongyloides spp. | 4% | 238 | Microscopy | Uganda | (Hodder and Chapman 2012) [109] | Primate |
| Strongyloides spp. | 7% | 40 | Microscopy | Kenya | (Karere and Munene 2002) [12] | Primate |
| Strongyloides spp. | 41% | 624 | Microscopy | Borneo | (Klaus et al., 2018) [62] | Primate |
| Strongyloides fuelleborni | 32% | 652 | Microscopy | Borneo | (Klaus et al., 2017) [110] | Primate |
| Strongyloides fuelleborni | 57% | 141 | Microscopy | Puerto Rico | (Knezevich et al., 1998) [111] | Primate |
| Strongyloides spp. | 43% | 686 | Microscopy | Tanzania | (Kooriyama et al., 2012) [112] | Primate |
| Strongyloides spp. | 74% | 3142 | Microscopy | Côte d’Ivoire | (Kouassi et al., 2015 [113] | Primate |
| Strongyloides spp. | 13% | 366 | Microscopy | India | (Kumar et al., 2018) [114] | Primate |
| Strongyloides fuelleborni | 44% S. fuelleborni, 4% S. spp. | 25 | Microscopy | Malaysia | (Kuze et al., 2010) [115] | Primate |
| Strongyloides fuelleborni | 95% | 20 | Molecular and Microscopy | Indonesia | (Labes et al., 2011) [116] | Primate |
| Strongyloides spp. | 37% | 59 | Microscopy | Ethiopia | (Legesse and Erko 2004) [117] | Primate |
| Strongyloides spp. | 5% | 222 | Microscopy | Belgium | (Levecke et al., 2007) [118] | Primate |
| Strongyloides spp. | 6% | 3349 | microscopy | China | (Li et al., 2017) [65] | Primate |
| Strongyloides stercoralis | 6% | 46 | Microscopy | Nigeria | (Mafuyai et al., 2013) [119] | Primate |
| Strongyloides spp. | 50% | 134 | Microscopy | Costa Rica | (Maldonado-Lopez et al., 2014) [120] | Primate |
| Strongyloides spp. | 77% | 78 | Microscopy | Ecuador | (Martin-Solano et al., 2017) [121] | Primate |
| Strongyloides spp. | 17% | 53 | Microscopy | Uganda | (Matsubayashi et al., 1992) [122] | Primate |
| Strongyloides fuelleborni | 58% | 432 | Molecular | Uganda | (McLennan et al., 2017) [123] | Primate |
| Strongyloides spp. | 84% | 121 | Microscopy | Uganda | (Muehlenbein et al., 2005) [124] | Primate |
| Strongyloides fuelleborni | 45% | 315 | Microscopy | Kenya | (Munene et al., 1998) [125] | Primate |
| Strongyloides fuelleborni | 21% | 297 | Microscopy | Kenya | (Muriuki et al., 1998) [126] | Primate |
| Strongyloides spp. | 76% | 83 | Microscopy | Costa Rica | (Parr et al., 2013) [127] | Primate |
| Strongyloides spp. | 13% | 366 | Microscopy | Tanzania | (Petrasova et al., 2010) [128] | Primate |
| Strongyloides spp. | 44% | 130 | Microscopy | Tanzania | (Petrzelkova et al., 2010) [129] | Primate |
| Strongyloides stercoralis | 15% | 86 | Microscopy | Peru | (Phillips et al., 2004) [130] | Primate |
| Strongyloides spp. | 43% | 47 | Microscopy | Gabon | (Pouillevet et al., 2017) [131] | Primate |
| Strongyloides fuelleborni | 6% | 125 | Microscopy | Cameroon | (Pourrut et al., 2011) [132] | Primate |
| Strongyloides spp. | 53% | 55 | Microscopy | Ghana | (Ryan et al., 2012) [133] | Primate |
| Strongyloides spp. | 8% | 420 | Molecular | Mexico | (Solorzano-Garcia and de Leon 2017) [134] | Primate |
| Strongyloides fuelleborni | 39% | 243 | Molecular | Thailand and Laos | (Thanchomnang et al., 2018) [135] | Primate |
| Strongyloides spp. | 35% | 283 | Microscopy | India | (Tiwari et al., 2017) [136] | Primate |
| Strongyloides stercoralis | 31% | 135 | Microscopy | Thailand | (Wenz-Mucke et al., 2013) [137] | Primate |
| Strongyloides spp. | 24% | 272 | Microscopy | South Africa | (Wren et al., 2015) [138] | Primate |
| Strongyloides spp. | 24% | 332 | Microscopy | South Africa | (Wren et al., 2016) [139] | Primate |
| Strongyloides fuelleborni and Strongyloides spp. | UNK | 14 | Molecular | Malaysian Borneo | (Frias et al., 2018) [140] | Primate |
| Strongyloides spp. | 29% | 64 | Microscopy | Brazil | (De Souza et al., 2012) [66] | Sheep |
| Strongyloides spp. | 8% | 165 | Microscopy | Papua New Guinea | (Koinari et al., 2013) [141] | Sheep |
| Strongyloides spp. | <1% | 27 | Microscopy | New England | (MacGlaflin et al., 2011) [142] | Sheep |
| Strongyloides spp. | UNK | 1798 | Microscopy | Brazil | (McManus et al., 2009) [143] | Sheep |
| Strongyloides spp. | 2% | 275 | Microscopy | Greenland | (Andreassen et al., 2017) [144] | Fox |
| Strongyloides spp. | 4% | 22 | Microscopy | Iran | (Dalimi et al., 2006) [145] | Fox |
| Strongyloides stercoralis | 16% | 249 | Microscopy | Mexico | (Hernandez-Camacho et al., 2011) [146] | Fox |
| Strongyloides stercoralis | 2% | 1198 | Microscopy | Slovakia | (Miterpakova et al., 2009) [147] | Fox |
| Strongyloides spp. | 65% | 60 | Microscopy | Pakistan | (Afshan et al., 2013) [16] | Rat |
| Strongyloides spp. | 97% | 299 | Microscopy | Brazil | (Carvalho-Pereira et al., 2018) [17] | Rat |
| Strongyloides spp. | 40% | 25 | Microscopy | Brazil | (Lima et al., 2017) [97] | Rat |
| Strongyloides spp. | 13% | 76 | Microscopy | Bangladesh | (Fuehrer et al., 2012) [18] | Rat |
| Strongyloides spp. | 10% | 502 | Microscopy | Nigeria | (Isaac et al., 2018) [19] | Mouse and rat |
| Strongyloides stercoralis | 53% | 98 | Microscopy | Indonesia | (Prasetyo et al., 2016) [20] | House rat |
| Strongyloides spp. | 10% | 10 | Microscopy | Brazil | (Souza et al., 2015) [21] | Capybaras |
| Strongyloides spp. | 10% | 31 | Microscopy | Brazil | (Gioia-Di Chiacchio et al., 2014) [22] | Capybaras |
| Strongyloides stercoralis | 2% | 6530 | Microscopy | Ethiopia | (Fetene and Worku 2009) [23] | Flies |
| Strongyloides stercoralis | <1% | 9950 | Microscopy | Ethiopia | (Getachew et al., 2007) [24] | Flies |
| Strongyloides stercoralis | 2% | 5000 | Microscopy | Nigeria | (Umeche 1989b) [25] | Flies |
| Strongyloides stercoralis | 12% | 749 | Microscopy | Nigeria | (Adenusi et al., 2018) [26] | Cockroaches |
| Strongyloides stercoralis | 1% | 920 | Microscopy | Thailand | (Chamavit et al., 2010) [27] | Cockroaches |
| Strongyloides stercoralis | 81% | 70 | Microscopy | Nigeria | (Morenikeji et al., 2016) [28] | Cockroaches |
| Strongyloides stercoralis | UNK | 234 | Microscopy | Nigeria | (Tatfeng et al., 2005) [29] | Cockroaches |
| Strongyloides stercoralis | 2% | 125 | Microscopy | Nigeria | (Adesewa and Morenikeji, 2017) [82] | Soil |
| Strongyloides spp. | 3% | 625 | Microscopy | Spain | (Dado et al., 2012) [38] | Soil |
| Strongyloides spp. | 8% | 120 | Microscopy | Egypt | (Etewa et al., 2016) [83] | Soil |
| Strongyloides stercoralis | 1% | 797 | Microscopy | Nigeria | (Ivoke et al., 2017) [85] | Geophagy |
| Strongyloides stercoralis | 2% | 1078 | Microscopy | Tanzania | (Kawai et al., 2009) [86] | Geophagy |
| Strongyloides stercoralis | 3% | 112 | Microscopy | Iran | (Motazedian et al., 2006) [39] | Soil |
| Strongyloides spp. | 7% | 182 | Microscopy | Malaysia | (Noor Azian et al., 2008) [40] | Soil |
| Strongyloides stercoralis | 20% | 102 | Microscopy | Nigeria | (Ogbolu et al., 2011) [41] | Soil |
| Strongyloides spp. | 5% | 2520 | Microscopy | Brazil | (Rocha et al., 2011) [42] | Soil |
| Strongyloides spp. | 2% | 500 | Microscopy | Czech Republic | (Valkounova 1982) [43] | Soil |
| Strongyloides spp. | 3% | 125 | Microscopy | Brazil | (Mandarino-Pereira et al., 2010) [44] | Soil |
| Strongyloides stercoralis | 14% | 14 | Microscopy | Slovakia | (Strkolcova et al., 2017) [45] | Soil |
| Strongyloides stercoralis | 12% | 17 | Microscopy | South Africa | (Sumbele et al., 2014) [84] | Soil |
| Strongyloides spp. | 4% | 45 | Microscopy | Romania | (Tudor 2015) [46] | Soil |
| Strongyloides stercoralis | 6% | 150 | Microscopy | Nigeria | (Umeche 1989a) [47] | Soil |
| Strongyloides spp. | 6% | 16 | Microscopy | Brazil | (da Silva et al., 2014) [148] | Soil |
| Strongyloides spp. | UNK | 8 | Microscopy | Cameroon | (Aghaindum and Landry, 2019) [149] | Non-potable water |
| Strongyloides spp. | 40% - 100% | 100 | Microscopy | Saudi Arabia | (Bolbol 1992) [69] | Non-potable water |
| Strongyloides stercoralis | 2%% | UNK | Microscopy | Brazil | (Bastos et al., 2008) [70] | Non-potable water |
| Strongyloides spp. | 100% | 3 | Microscopy | Brazil | (Cutolo et al., 2006) [71] | Non-potable water |
| Strongyloides stercoralis | 19% | 52 | Microscopy | Palestine | (Hilles et al., 2014) [150] | Seawater |
| Strongyloides stercoralis | 1% | 85 | Microscopy | Turkey | (Bakir et al., 2003) [151] | Drinking water |
| Strongyloides fuelleborni and Strongyloides spp. | 11% S. fuelleborni, 15% S. spp. | 9950 | Microscopy | Zimbabwe | (Dalu et al., 2011) [76] | Drinking water |
| Strongyloides spp. | UNK | UNK | Microscopy | Egypt | (El Shazly et al., 2003) [75] | Drinking water |
| Strongyloides stercoralis | 7% | 80 | Microscopy | Egypt | (El-Badry et al., 2018) [152] | Drinking water |
| Strongyloides stercoralis | 81% | 16 | Microscopy | Brazil | (Freitas et al., 2017) [153] | Drinking water |
| Strongyloides stercoralis | 51% | 232 | Microscopy | India | (Jonnalagadda and Bhat 1995) [77] | Drinking water |
| Strongyloides stercoralis | 100% | UNK | Microscopy | USA | (Klotz et al., 1992) [154] | Drinking water |
| Strongyloides stercoralis | UNK | UNK | Molecular | Malaysia | (Zeehaida et al., 2011) [155] | Fruit & vegetables |
| Strongyloides stercoralis | <1% | 1130 | Microscopy | Nigeria | (Adamu et al., 2012) [30] | Fruit & vegetables |
| Strongyloides stercoralis | <1% | 960 | Microscopy | Nigeria | (Adenusi et al., 2015) [31] | Fruit & vegetables |
| Strongyloides stercoralis | 10% | 150 | Microscopy | Nigeria | (Amaechi et al., 2016) [78] | Fruit & vegetables |
| Strongyloides stercoralis | 7% | 190 | Microscopy | Nigeria | (Amuta et al., 2017) [32] | Fruit & vegetables |
| Strongyloides stercoralis | 7% | 240 | Microscopy | Nigeria | (Dada et al., 2015) [33] | Fruit & vegetables |
| Strongyloides spp. | 1% | 453 | Microscopy | Iran | (Fallah et al., 2016) [34] | Fruit & vegetables |
| Strongyloides stercoralis | 36% | 360 | Microscopy | Ghana | (Kudah et al., 2018) [35] | Fruit & vegetables |
| Strongyloides spp. | 13% | 108 | Microscopy | Brazil | (Luz et al., 2017) [156] | Fruit & vegetables |
| Strongyloides spp. | 19% | 199 | Microscopy | Nigeria | (Maikai et al., 2012) [157] | Fruit & vegetables |
| Strongyloides spp. | 11% | 36 | Microscopy | Malaysia | (Matyusof et al., 2017) [80] | Fruit & vegetables |
| Strongyloides stercoralis | 1% | 260 | Microscopy | Sudan | (Mohamed et al., 2016) [36] | Fruit & vegetables |
| Strongyloides stercoralis | 10% | 265 | Microscopy | Thailand | (Punsawad et al., 2019) [37] | Fruit & vegetables |
| Strongyloides stercoralis | 46% | 120 | Microscopy | Nigeria | (Ogbolu et al., 2009) [81] | Fruit & vegetables |
| Strongyloides stercoralis | 14% | 140 | Microscopy | Iran | (Madadi 2010) [158] | Fruit & vegetables |
| Strongyloides stercoralis | 19% | 80 | Microscopy | Nigeria | (Ohaeri and Unogu 2011) [79] | Fruit & vegetables |
| Strongyloides spp. | 7% | 15 | Microscopy | Zambia | (Berentsen et al., 2012) [159] | Other animals |
| Strongyloides spp. | 5% | 272 | Microscopy | Nepal | (Bista et al., 2017) [160] | Other animals |
| Strongyloides spp. | 100% | 1 | Microscopy | Brazil | (Cardia et al., 2016) [161] | Other animals |
| Strongyloides spp. | 4% | 432 | Microscopy | Spain | (Cordon et al., 2008) [162] | Other animals |
| Strongyloides spp. | 40% | 52 | Microscopy | Russia | (González et al., 2007) [163] | Other animals |
| Strongyloides spp. | 2% | 956 | Microscopy | India | (Gupta et al., 2018) [164] | Other animals |
| Strongyloides spp. | <1% | 1005 | Microscopy | Germany | (Hallinger et al., 2018) [165] | Other animals |
| Strongyloides spp. | 31% | 42 | Microscopy | Japan | (Hasegawa et al., 2017) [166] | Other animals |
| Strongyloides spp. | <1% | 400 | Microscopy | Croatia | (Hermosilla et al., 2017) [167] | Other animals |
| Strongyloides spp. | 64% - 99% | 990 | Microscopy | Mexico | (Hu et al., 2018) [168] | Other animals |
| Strongyloides spp. | 4% | 821 | Microscopy | China | (Huang et al., 2014) [169] | Other animals |
| Strongyloides spp. | 15% | 2280 | Microscopy | Pakistan | (Khan et al., 2010) [67] | Other animals |
| Strongyloides spp. | UNK | 6 | Microscopy | Namibia | (Kumba et al., 2003) [170] | Other animals |
| Strongyloides spp. | 36% | 58 | Microscopy | Poland | (Mizgajska-Wiktor et al., 2010) [171] | Other animals |
| Strongyloides spp. | 67% | 12 | Microscopy | Mexico | (Mukul-Yerves et al., 2014) [172] | Other animals |
| Strongyloides spp. | 57% | 201 | Microscopy | Estonia | (Oja et al., 2017) [173] | Other animals |
| Strongyloides spp. | 47% | 383 | Microscopy | Mexico | (Ojeda-Robertos et al., 2017) [174] | Other animals |
| Strongyloides spp. | 7% | 6 | Molecular | Iberian Peninsula | (Perera et al., 2013) [175] | Other animals |
| Strongyloides spp. | 3% | 468 | Microscopy | Poland | (Pilarczyk et al., 2015) [176] | Other animals |
| Strongyloides spp. | 17% | 86 | Microscopy | Bangladesh | (Rahman et al., 2018) [177] | Other animals |
| Strongyloides spp. | 3% | 1883 | Microscopy | Italy | (Rinaldi et al., 2009) [178] | Other animals |
| Strongyloides spp. | 44% | 163 | Microscopy | Portugal | (Rosalino et al., 2006) [179] | Other animals |
| Strongyloides spp. | 45% | 82 | Microscopy | Australia | (Turni and Smales 2001) [180] | Other animals |
| Strongyloides spp. | UNK | UNK | Microscopy | Namibia | (Turner et al., 2010) [181] | Other animals |
| Strongyloides spp. | UNK | UNK | Microscopy | Namibia | (Turner et al., 2012) [182] | Other animals |
| Strongyloides spp. | <1% | 213 | Microscopy | Kenya | (VanderWaal et al., 2014) [183] | Other animals |
| Strongyloides spp. | 74% | 243 | Microscopy | Philippines | (Ybanez et al., 2018) [184] | Other animals |
References
- Olsen, A.; Van Lieshout, L.; Marti, H.; Polderman, T.; Polman, K.; Steinmann, P.; Stothard, R.; Thybo, S.; Verweij, J.J.; Magnussen, P. Strongyloidiasis—The most neglected of the neglected tropical diseases? Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Ashford, R.W.; Barnish, G.; Viney, M.E. Strongyloides fuelleborni kellyi: Infection and disease in Papua New Guinea. Parasitol. Today 1992, 8, 314–318. [Google Scholar] [CrossRef]
- Hauber, H.P.; Galle, J.; Chiodini, P.L.; Rupp, J.; Birke, R.; Vollmer, E.; Zabel, P.; Lange, C. Fatal outcome of a hyperinfection syndrome despite successful eradication of Strongyloides with subcutaneous ivermectin. Infect. 2005, 33, 383–386. [Google Scholar] [CrossRef]
- Kearns, T.M.; Currie, B.J.; Cheng, A.C.; McCarthy, J.; Carapetis, J.R.; Holt, D.C.; Page, W.; Shield, J.; Gundjirryirr, R.; Mulholland, E.; et al. Strongyloides seroprevalence before and after an ivermectin mass drug administration in a remote Australian Aboriginal community. PLoS Negl. Trop. Dis. 2017, 11, e0005607. [Google Scholar] [CrossRef] [PubMed]
- Vadlamudi, R.S.; Chi, D.S.; Krishnaswamy, G. Intestinal strongyloidiasis and hyperinfection syndrome. Clin. Mol. Allergy 2006, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Jaleta, T.G.; Zhou, S.; Bemm, F.M.; Schär, F.; Khieu, V.; Muth, S.; Odermatt, P.; Lok, J.B.; Streit, A. Different but overlapping populations of Strongyloides stercoralis in dogs and humans—Dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl. Trop. Dis. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Bisoffi, Z.; Buonfrate, D.; Montresor, A.; Requena-Méndez, A.; Muñoz, J.; Krolewiecki, A.J.; Gotuzzo, E.; Mena, M.A.; Chiodini, P.L.; Anselmi, M.; et al. Strongyloides stercoralis: A plea for action. PLoS Negl. Trop. Dis. 2013, 7, e2214. [Google Scholar] [CrossRef]
- Jourdan, P.M.; Lamberton, P.H.L.; Fenwick, A.; Addiss, D.G. Soil-transmitted helminth infections. Lancet 2018, 391, 252–265. [Google Scholar] [CrossRef] [Green Version]
- Maroto, R.; Jiménez, A.E.; Romero, J.J.; Alvarez, V.; De Oliveira, J.B.; Hernández, J. First report of anthelmintic resistance in gastrointestinal nematodes of sheep from Costa Rica. Vet. Med. Int. 2011, 2011, 145312. [Google Scholar] [CrossRef]
- Beknazarova, M.; Whiley, H.; Ross, K. Mass drug administration for the prevention human strongyloidiasis should consider concomitant treatment of dogs. PLoS Negl. Trop. Dis. 2017, 11, e0005735. [Google Scholar] [CrossRef]
- Bezjian, M.; Gillespie, T.R.; Chapman, C.A.; Greiner, E.C. Coprologic evidence of gastrointestinal helminths of forst baboons, Papio anubis, in Kibale national park, Uganda. J. Wildl. Dis. 2008, 44, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Karere, G.M.; Munene, E. Some gastro-intestinal tract parasites in wild De Brazza’s monkeys (Cercopithecus neglectus) in Kenya. Vet. Parasitol. 2002, 110, 153–157. [Google Scholar] [CrossRef]
- Arizono, N.; Yamada, M.; Tegoshi, T.; Onishi, K. Molecular identification of oesophagostomum and trichuris eggs isolated from wild Japanese macaques. Korean J. Parasitol. 2012, 50, 253–257. [Google Scholar] [CrossRef] [PubMed]
- De Thoisy, B.; Vogel, I.; Reynes, J.M.; Pouliquen, J.F.; Carme, B.; Kazanji, M.; Vié, J.C. Health evaluation of translocated free-ranging primates in French Guiana. Am. J. Primatol. 2001, 54, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.S.; Gracenea, M.; Montoliu, I.; Feliu, C.; Monleon, A.; Fernandez, J.; Ensenat, C. Intestinal parasitism—Protozoa and helminths—In primates at the Barcelona zoo. J. Med. Primatol. 1996, 25, 419–423. [Google Scholar] [CrossRef]
- Afshan, K.; Beg, M.A.; Rizvi, S.S.R.; Qayyum, M. Helminths and nematode infection in Norway rats (Rattus norvegicus) captured from Northern Punjab, Pakistan. Pak. J. Zool. 2013, 45, 1456–1459. [Google Scholar]
- Carvalho-Pereira, T.; Souza, F.N.; Santos, L.R.N.; Walker, R.; Pertile, A.C.; de Oliveira, D.S.; Pedra, G.G.; Minter, A.; Rodrigues, M.G.; Bahiense, T.C.; et al. The helminth community of a population of Rattus norvegicus from an urban Brazilian slum and the threat of zoonotic diseases. Parasitol. 2018, 145, 797–806. [Google Scholar] [CrossRef]
- Fuehrer, H.P.; Baumann, T.A.; Riedl, J.; Treiber, M.; Igel, P.; Swoboda, P.; Joachim, A.; Noedl, H. Endoparasites of rodents from the Chittagong hill tracts in Southeastern Bangladesh. Wien. Klin. Wochenschr. 2012, 124, 27–30. [Google Scholar] [CrossRef]
- Isaac, C.; Igbinosa, B.I.; Ohiolei, J.A.; Osimen, C.E. Endoparasites of small mammals in Edo State, Nigeria: Public health implications. Korean J. Parasitol. 2018, 56, 93–100. [Google Scholar] [CrossRef]
- Prasetyo, R.H. Survey of house rat intestinal parasites from Surabaya District, East Java, Indonesia that can cause opportunistic infections in humans. Southeast Asian J. Trop. Med. Public Health 2016, 47, 194–198. [Google Scholar]
- Souza, G.T.R.; Ribeiro, T.S.; Antonucci, A.M.; Ueda, B.H.; Carniel, M.K.; Karling, L.C.; Eiras, J.C.; Takemoto, R.M.; Pavanelli, G.C. Endoparasite fauna of wild capybaras (Hydrochoerus hydrochaeris) (linnaeus, 1766) from the Upper Parana River floodplain, Brazil. Aquat. Mamm. 2015, 41, 213–221. [Google Scholar] [CrossRef]
- Gioia-Di Chiacchio, R.; Prioste, F.E.S.; Vanstreels, R.E.T.; Knobl, T.; Kolber, M.; Miyashiro, S.I.; Matushima, E.R. Health evaluation and survey of zoonotic pathogens in free-ranging capybaras (Hydrochoerus hydrochaeris). J. Wildl. Dis. 2014, 50, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Fetene, T.; Worku, N. Public health importance of non-biting cyclorrhaphan flies. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Getachew, S.; Gebre-Michael, T.; Erko, B.; Balkew, M.; Medhin, G. Non-biting cyclorrhaphan flies (diptera) as carriers of intestinal human parasites in slum areas of Addis Ababa, Ethiopia. Acta Trop. 2007, 103, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Umeche, N.; Mandah, L.E. Musca domestica as a carrier of intestinal helminths in Calabar, Nigeria. East. Afr. Med. J. 1989, 66, 349–352. [Google Scholar]
- Adenusi, A.A.; Akinyemi, M.I.; Akinsanya, D. Domiciliary cockroaches as carriers of human intestinal parasites in Lagos metropolis, Southwest Nigeria: Implications for public health. J. Arthropod-Borne Dis. 2018, 12, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Chamavit, P.; Sahaisook, P.; Niamnuy, N. The majority of cockroaches from the Samutprakarn province of Thailand are carriers of parasitic organisms. EXCLI J. 2011, 10, 218–222. [Google Scholar]
- Morenikeji, O.A.; Adebiyi, A.; Oluwayiose, O.A. Parasites in cockroaches recovered from residential houses around awotan dumpsite in Ido local government area of Oyo State, Nigeria. Annu. Res. Rev. Biol. 2016, 9. [Google Scholar] [CrossRef]
- Tatfeng, Y.M.; Usuanlele, M.U.; Orukpe, A.; Digban, A.K.; Okodua, M.; Oviasogie, F.; Turay, A.A. Mechanical transmission of pathogenic organisms: The role of cockroaches. J. Vector Borne Dis. 2005, 42, 129–134. [Google Scholar]
- Adamu, N.B.; Adamu, J.Y.; Mohammed, D. Prevalence of helminth parasites found on vegetables sold in Maiduguri, Northeastern Nigeria. Food Control 2012, 25, 23–26. [Google Scholar] [CrossRef]
- Adenusi, A.A.; Abimbola, W.A.; Adewoga, T.O.S. Human intestinal helminth contamination in pre-washed, fresh vegetables for sale in major markets in Ogun State, Southwest Nigeria. Food Control 2015, 50, 843–849. [Google Scholar] [CrossRef]
- Amuta, E.U.; Obisike, V.U.; Acham, N.I. Prevalence of helminth eggs on raw vegetables and fruits sold in selected markets in Makurdi, Benue State, Nigeria. Annu. Res. Rev. Biol. 2017, 19. [Google Scholar] [CrossRef]
- Dada, A.J.; Wartu, J.R.; Auta, T.; Diya, A.W. Public health significance of helminthes eggs isolated from raw vegetables obtained from farms and those sold within Kaduna Metropolis, Nigeria. Asian J. Microbiol. Biotechnol. Environ. Sci. 2015, 17, 527–532. [Google Scholar]
- Fallah, A.A.; Makhtumi, Y.; Pirali-Kheirabadi, K. Seasonal study of parasitic contamination in fresh salad vegetables marketed in Shahrekord, Iran. Food Control 2016, 60, 538–542. [Google Scholar] [CrossRef]
- Kudah, C.; Sovoe, S.; Baiden, F. Parasitic contamination of commonly consumed vegetables in two markets in Ghana. Ghana Med. J. 2018, 52, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.A.; Siddig, E.E.; Elaagip, A.H.; Edris, A.M.M.; Nasr, A.A. Parasitic contamination of fresh vegetables sold at central markets in Khartoum State, Sudan. Ann. Clin. Microb. Antimicrob. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Punsawad, C.; Phasuk, N.; Thongtup, K.; Nagavirochana, S.; Viriyavejakul, P. Prevalence of parasitic contamination of raw vegetables in Nakhon Si Thammarat province, Southern Thailand. BMC Public Health 2019, 19. [Google Scholar] [CrossRef]
- Dado, D.; Izquierdo, F.; Vera, O.; Montoya, A.; Mateo, M.; Fenoy, S.; Galvan, A.L.; Garcia, S.; Garcia, A.; Aranguez, E.; et al. Detection of zoonotic intestinal parasites in public parks of Spain. Potential Epidemiological Role of Microsporidia. Zoonoese Public Health 2012, 59, 23–28. [Google Scholar] [CrossRef]
- Motazedian, H.; Mehrabani, D.; Tabatabaee, S.H.R.; Pakniat, A.; Tavalali, M. Prevalence of helminth ova in soil samples from public places in Shiraz. East. Med. Health J. 2006, 12, 562–565. [Google Scholar]
- Noor Azian, M.Y.; Sakhone, L.; Hakim, S.L.; Yusri, M.Y.; Nurulsyamzawaty, Y.; Zuhaizam, A.H.; Rodi, I.M.; Maslawaty, M.N. Detection of helminth infections in dogs and soil contamination in rural and urban areas. Southeast Asian J. Trop. Med. Public Health 2008, 39, 205–212. [Google Scholar]
- Ogbolu, D.O.; Alli, O.A.; Amoo, A.O.; Olaosun, I.I.; Ilozavbie, G.W.; Olusoga-Ogbolu, F.F. High-level parasitic contamination of soil sampled in Ibadan Metropolis. Afr. J. Med. Med. Sci. 2011, 40, 321–325. [Google Scholar] [PubMed]
- Rocha, S.; Pinto, R.M.F.; Floriano, A.P.; Teixeira, L.H.; Bassili, B.; Martinez, A.; da Costa, S.O.P.; Caseiro, M.M. Environmental analyses of the parasitic profile found in the sandy soil from the Santos municipality beaches, sp, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2011, 53, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Valkounová, J. Parasitological investigation of children’s sandboxes and dog faeces from public areas of housing development in Prague. Folia Parasitol. 1982, 29, 133–138. [Google Scholar] [PubMed]
- Mandarino-Pereira, A.; de Souza, F.S.; Lopes, C.W.G.; Pereira, M.J.S. Prevalence of parasites in soil and dog feces according to diagnostic tests. Vet. Parasitol. 2010, 170, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Štrkolcová, G.; Goldová, M.; Bocková, E.; Mojžišová, J. The roundworm Strongyloides stercoralis in children, dogs, and soil inside and outside a segregated settlement in Eastern Slovakia: Frequent but hardly detectable parasite. Parasitol. Res. 2017, 116, 891–900. [Google Scholar] [CrossRef]
- Tudor, P. Soil Contamination with canine intestinal parasites eggs in the parks and shelter dogs from Bucharest Area. Agric. Agric. Sci. Procedia 2015, 6, 387–391. [Google Scholar] [CrossRef]
- Umeche, N. Helminth ova in soil from children’s playgrounds in Calabar, Nigeria. Central Afr. J. Med. 1989, 35, 432–434. [Google Scholar]
- Kostopoulou, D.; Claerebout, E.; Arvanitis, D.; Ligda, P.; Voutzourakis, N.; Casaert, S.; Sotiraki, S. Abundance, zoonotic potential and risk factors of intestinal parasitism amongst dog and cat populations: The scenario of Crete, Greece. Parasit. Vectors 2017, 10. [Google Scholar] [CrossRef]
- Leelayoova, S.; Siripattanapipong, S.; Naaglor, T.; Taamasri, P.; Mungthin, M. Prevalence of intestinal parasitic infections in military personnel and military dogs, Thailand. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2009, 92 (Suppl. 1), S53–S59. [Google Scholar]
- Papazahariadou, A.; Founta, A.; Papadopoulos, E.; Chliounakis, S.; Antoniadou-Sotiriadou, K.; Theodorides, Y. Gastrointestinal parasites of shepherd and hunting dogs in the Serres Prefecture, Northern Greece. Vet. Parasitol. 2007, 148, 170–173. [Google Scholar] [CrossRef]
- Pumidonming, W.; Salman, D.; Gronsang, D.; Abdelbaset, A.E.; Sangkaeo, K.; Kawazu, S.; Igarashi, M. Prevalence of gastrointestinal helminth parasites of zoonotic significance in dogs and cats in lower Northern Thailand. J.Vet. Med. Sci. 2016, 78, 1779–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razmi, G.R. Survey of dogs’ parasites in Khorasan Razavi province, Iran. Iran. J. Parasitol. 2009, 4, 48–54. [Google Scholar]
- Riggio, F.; Mannella, R.; Ariti, G.; Perrucci, S. Intestinal and lung parasites in owned dogs and cats from central Italy. Vet. Parasitol. 2013, 193, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.C.; Magalhanes, N.B.; dos Santos, H.A.; Ribeiro, R.R.; Guimaraes, M.P. Parasites of domestic and wild canids in the region of Serra Do Cipo national park, Brazil. Rev. Bras. De Parasitol. Vet. 2012, 21, 270–277. [Google Scholar] [CrossRef]
- Sauda, F.; Malandrucco, L.; Macri, G.; Scarpulla, M.; De Liberato, C.; Terracciano, G.; Fichi, G.; Berrilli, F.; Perrucci, S. Leishmania infantum, dirofilaria spp. And other endoparasite infections in kennel dogs in central Italy. Parasite 2018, 25. [Google Scholar] [CrossRef]
- Wright, I.; Stafford, K.; Coles, G. The prevalence of intestinal nematodes in cats and dogs from Lancashire, North-West England. J. Small Anim. Pract. 2016, 57, 393–395. [Google Scholar] [CrossRef]
- Perera, P.K.; Rajapakse, R.; Rajakaruna, R.S. Gastrointestinal parasites of dogs in Hantana area in the Kandy district. J. National Sci. Found. Sri Lanka 2013, 41, 81–91. [Google Scholar] [CrossRef]
- Martins, C.M.; de Barros, C.D.; Bier, D.; Marinho, A.P.; Figueiredo, J.M.G.; Hoffmann, J.L.; Molento, M.B.; Biondo, A.W. Dog parasite incidence and risk factors, from sampling after one-year interval, in Pinhais, Brazil. Rev. Bras. De Parasitol. Vet. 2012, 21, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Beknazarova, M.; Millsteed, S.; Robertson, G.; Whiley, H.; Ross, K. Validation of dess as a DNA preservation method for the detection of Strongyloides spp. In canine feces. Int. J. Environmen. Res. Public Health 2017, 14, 624. [Google Scholar] [CrossRef]
- Ardelean, A.I.; Suteu, E.; Cozma, V. Epidemiology of digestive helminthosis from urban area of Clujnapoca, Romania. Bull. Univ. Agric. Sci. Vet. Med. Vet. Med. 2005, 62, 322–329. [Google Scholar]
- Hasegawa, H.; Kalousova, B.; McLennan, M.R.; Modry, D.; Profousova-Psenkova, I.; Shutt-Phillips, K.A.; Todd, A.; Huffman, M.A.; Petrzelkova, K.J. Strongyloides infections of humans and great apes in Dzangasangha protected areas, Central African republic and in degraded forest fragments in Bulindi, Uganda. Parasitol. Int. 2016, 65, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Strube, C.; Roper, K.M.; Radespiel, U.; Schaarschmidt, F.; Nathan, S.; Goossens, B.; Zimmermann, E. Fecal parasite risk in the endangered proboscis monkey is higher in an anthropogenically managed forest environment compared to a Riparian rain forest in Sabah, Borneo. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Grove, D.I. Strongyloidiasis: Is it transmitted from husband to wife? Br. J. Vener. Dis. 1982, 58, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Helenbrook, W.D.; Wade, S.E.; Shields, W.M.; Stehman, S.V.; Whipps, C.M. Gastrointestinal parasites of Ecuadorian mantled howler monkeys (Alouatta palliata aequatorialis) based on fecal analysis. J. Parasitol. 2015, 101, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Dong, H.J.; Wang, R.J.; Yu, F.C.; Wu, Y.Y.; Chang, Y.K.; Wang, C.R.; Qi, M.; Zhang, L.X. An investigation of parasitic infections and review of molecular characterization of the intestinal protozoa in nonhuman primates in China from 2009 to 2015. Int. J. Parasitol. Parasit. Wildl. 2017, 6, 8–15. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.D.; Pimentel-Neto, M.; da Silva, R.M.; Farias, A.C.B.; Guimaraes, M.P. Gastrointestinal parasites of sheep, municipality of Lajes, Rio Grande Do Norte, Brazil. Rev. Bras. De Parasitol. Vet. 2012, 21, 71–73. [Google Scholar] [CrossRef]
- Khan, M.N.; Sajid, M.S.; Khan, M.K.; Iqbal, Z.; Hussain, A. Gastrointestinal helminthiasis: Prevalence and associated determinants in domestic ruminants of district Toba Tek Singh, Punjab, Pakistan. Parasitol. Res. 2010, 107, 787–794. [Google Scholar] [CrossRef]
- Förster, M.; Klimpel, S.; Sievert, K. The house fly (Musca domestica) as a potential vector of metazoan parasites caught in a pig-pen in Germany. Vet. Parasitol. 2009, 160, 163–167. [Google Scholar] [CrossRef]
- Bolbol, A.S. Risk of contamination of human and agricultural environment with parasites through reuse of treated municipal wastewater in Riyadh, Saudi Arabia. J. Hyg. Epidemiol. Microbiol. Immunol. 1992, 36, 330–337. [Google Scholar]
- Bastos, R.K.X.; Bevilacqua, P.D.; Silva, C.A.B.; Silva, C.V. Wastewater irrigation of salad crops: Further evidence for the evaluation of the WHO guidelines. Water Sci. Technol. 2008, 57, 1213–1219. [Google Scholar] [CrossRef]
- Cutolo, S.A.; Matté, M.H.; Rocha, A.A. Monitoring of parasitological contamination in treated wastewater from activated sludge system. Manag. Environ. Qual. Int. J. 2006, 17, 43–56. [Google Scholar] [CrossRef]
- Saqer, A.S.; Seham, H.; Ragaa, G.; Yehia, A.E.W.; Wafaa, S. Optimum methods of inactivation of Strongyloides stercoralis larvae from reclaimed wastewater. Environ. Monit. Assess. 2007, 130, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Tonani, K.A.A.; Julião, F.C.; Trevilato, T.M.B.; Takayanagui, A.M.M.; Bocio, A.; Domingo, J.L.; Segura-Muñoz, S.I. Behavior of metals, pathogen parasites, and indicator bacteria in sewage effluents during biological treatment by activated sludge. Biol. Trace Elem. Res. 2011, 143, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Hatam-Nahavandi, K.; Mahvi, A.H.; Mohebali, M.; Keshavarz, H.; Mobedi, I.; Rezaeian, M. Detection of parasitic particles in domestic and urban wastewaters and assessment of removal efficiency of treatment plants in Tehran, Iran. J. Environ. Health Sci. Eng. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- El Shazly, A.M.; Soliman, M.; Nemr, H.I.; El Moafyo, N.; Abel Gawad, A.G.; El Bendary, M. Nematodes and water pollution in eastern part of Nile Delta. J. Egy. Soc. Parasitol. 2003, 33, 631–636. [Google Scholar]
- Dalu, T.; Barson, M.; Nhiwatiwa, T. Impact of intestinal microorganisms and protozoan parasites on drinking water quality in Harare, Zimbabwe. J. Water Sanit. Hyg. Dev. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Jonnalagadda, P.R.; Bhat, R.V. Parasitic contamination of stored water used for drinking/cooking in Hyderabad. Southeast Asian J. Trop. Med. Public Health 1995, 26, 789–794. [Google Scholar]
- Amaechi, E.C.; Ohaeri, C.C.; Ukpai, O.M.; Adegbite, R.A. Prevalence of parasitic contamination of salad vegetables in Ilorin, North Central, Nigeria. Momona Ethiop. J. Sci. 2016, 8, 136–145. [Google Scholar] [CrossRef]
- Ohaeri, C.C.; Unogu, L.O. Soil transmitted helminths of some common fruits and vegetables in Umuahia, Abia State Nigeria. Niger. J. Parasitol. 2011, 32, 305–308. [Google Scholar]
- Matyusof, A.; Mohammad, M.; Abshir Abdullahi, M.; Mohamed, Z.; Zakaria, R.; Abdul Wahab, R. Occurrence of intestinal parasitic contamination in select consumed local raw vegetables and fruits in Kuantan, Pahang. Trop. Life Sci. Res. 2017, 28, 23–32. [Google Scholar] [CrossRef]
- Ogbolu, D.O.; Alli, O.A.; Ogunleye, V.F.; Olusoga-Ogbolu, F.F.; Olaosun, I. The presence of intestinal parasites in selected vegetables from open markets in South Western Nigeria. Afr. J. Med. Med. Sci. 2009, 38, 319–324. [Google Scholar] [PubMed]
- Adesewa, A.; Morenikeji, O. Helminths and heavy metals in soils from a dumpsite in Ibadan City, Nigeria. J. Prev. Med. Hyg. 2017, 58, E328–E333. [Google Scholar] [PubMed]
- Etewa, S.E.; Abdel-Rahman, S.A.; Abd El-Aal, N.F.; Fathy, G.M.; El-Shafey, M.A.; Ewis, A.M.G. Geohelminths distribution as affected by soil properties, physicochemical factors and climate in Sharkyia Governorate Egypt. J. Parasit. Dis. 2016, 40, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Sumbele, I.U.; Ngole, V.M.; Ekosse, G.I.E. Influence of physico-chemistry and mineralogy on the occurrence of geohelminths in geophagic soils from selected communities in the Eastern cape, South Africa, and their possible implication on human health. Int. J. Environ. Health Res. 2014, 24, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Ivoke, N.; Ikpor, N.; Ivoke, O.; Ekeh, F.; Ezenwaji, N.; Odo, G.; Iyaji, F.; Onoja, U.; Eyo, J. Geophagy as risk behaviour for gastrointestinal nematode infections among pregnant women attending antenatal clinics in a humid tropical zone of Nigeria. Afr. Health Sci. 2017, 17, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Saathoff, E.; Antelman, G.; Msamanga, G.; Fawzi, W.W. Geophagy (soil-eating) in relation to anemia and helminth infection among HIV-infected pregnant women in Tanzania. Am. J. Trop. Med. Hyg. 2009, 80, 36–43. [Google Scholar] [CrossRef]
- Schär, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides stercoralis: Global distribution and risk factors. PLoS Negl. Trop. Dis. 2013, 7, e2288. [Google Scholar] [CrossRef]
- Eydal, M.; Skirnisson, K. Strongyloides stercoralis found in imported dogs, household dogs and kennel dogs in Iceland. Icel. Agric. Sci. 2016, 29, 39–51. [Google Scholar] [CrossRef]
- Ferreira, A.; Goncalves-Pires, M.R.F.; Silva, D.A.O.; Goncalves, A.L.R.; Costa-Cruz, J.M. Parasitological and serological diagnosis of Strongyloides stercoralis in domesticated dogs from Southeastern Brazil. Vet. Parasitol. 2006, 136, 137–145. [Google Scholar] [CrossRef]
- Gaunt, M.C.; Carr, A.P. A survey of intestinal parasites in dogs from Saskatoon, Saskatchewan. Can. Vet. J. Revue Vet. Can. 2011, 52, 497–500. [Google Scholar]
- Goncalves, A.L.R.; Machado, G.A.; Goncalves-Pires, M.R.F.; Ferreira-Junior, A.; Silva, D.A.O.; Costa-Cruz, J.M. Evaluation of strongyloidiasis in kennel dogs and keepers by parasitological and serological assays. Vet. Parasitol. 2007, 147, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Mircean, V.; Gyorke, A.; Cozma, V. Prevalence and risk factors of Giardia duodenalis in dogs from Romania. Vet. Parasitol. 2012, 184, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Paradies, P.; Iarussi, F.; Sasanelli, M.; Capogna, A.; Lia, R.P.; Zucca, D.; Greco, B.; Cantacessi, C.; Otranto, D. Occurrence of strongyloidiasis in privately owned and sheltered dogs: Clinical presentation and treatment outcome. Parasit. Vectors 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Zanzani, S.A.; Di Cerbo, A.R.; Gazzonis, A.L.; Genchi, M.; Rinaldi, L.; Musella, V.; Cringoli, G.; Manfredi, M.T. Canine fecal contamination in a metropolitan area (Milan, North-Western Italy): Prevalence of intestinal parasites and evaluation of health risks. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Abu-Madi, M.A.; Al-Ahbabi, D.A.; Al-Mashhadani, M.M.; Al-Ibrahim, R.; Pal, P.; Lewis, J.W. Patterns of parasitic infections in faecal samples from stray cat populations in Qatar. J. Helminthol. 2007, 81, 281–286. [Google Scholar] [CrossRef]
- Adams, P.J.; Elliot, A.D.; Algar, D.; Brazell, R.I. Gastrointestinal parasites of feral cats from Christmas Island. Aust. Vet. J. 2008, 86, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.F.S.; Ramos, R.A.N.; Lepold, R.; Borges, J.C.G.; Ferreira, C.D.; Rinaldi, L.; Cringoli, G.; Alves, L.C. Gastrointestinal parasites in feral cats and rodents from the Fernando De Noronha Archipelago, Brazil. Rev. Bras. Parasit. Vet. 2017, 26, 521–524. [Google Scholar] [CrossRef]
- Mircean, V.; Titilincu, A.; Vasile, C. Prevalence of endoparasites in household cat (felis catus) populations from Transylvania (Romania) and association with risk factors. Vet. Parasitol. 2010, 171, 163–166. [Google Scholar] [CrossRef]
- Monteiro, M.F.M.; Ramos, R.A.N.; Calado, A.M.C.; Lima, V.F.S.; Ramos, I.C.D.; Tenorio, R.F.L.; Faustino, M.A.D.; Alves, L.C. Gastrointestinal parasites of cats in Brazil: Frequency and zoonotic risk. Rev. Bras. Parasitol. Vet. 2016, 25, 254–257. [Google Scholar] [CrossRef]
- Njuguna, A.N.; Kagira, J.M.; Karanja, S.M.; Ngotho, M.; Mutharia, L.; Maina, N.W. Prevalence of Toxoplasma gondii and other gastrointestinal parasites in domestic cats from households in Thika region, Kenya. Biomed Res. Int. 2017. [Google Scholar] [CrossRef]
- Rojekittikhun, W.; Chaisiri, K.; Mahittikorn, A.; Pubampen, S.; Sa-nguankiat, S.; Kusolsuk, T.; Maipanich, W.; Udonsom, R.; Mori, H. Gastrointestinal parasites of dogs and cats in a refuge in Nakhon Nayok, Thailand. Southeast Asian J. Trop. Med. Public Health 2014, 45, 31–39. [Google Scholar] [PubMed]
- Sedionoto, B.; Anamnart, W. Prevalence of hookworm infection and strongyloidiasis in cats and potential risk factor of human diseases. E3S Web of Conf. 2018, 31, 06002. [Google Scholar] [CrossRef]
- Takeuchi-Storm, N.; Mejer, H.; Al-Sabi, M.N.S.; Olsen, C.S.; Thamsborg, S.M.; Enemark, H.L. Gastrointestinal parasites of cats in Denmark assessed by necropsy and concentration mcmaster technique. Vet. Parasitol. 2015, 214, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake, D.K.; Arulkanthan, A.; Horadagoda, N.U.; Sanjeevani, G.K.M.; Kieft, R.; Gunatilake, S.; Dittus, W.P.J. Prevalence of cryptosporidium and other enteric parasites among wild non-human primates in Polonnaruwa, Sri Lanka. Am. J. Trop. Med. Hyg. 2006, 74, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, T.R.; Greiner, E.C.; Chapman, C.A. Gastrointestinal parasites of the Guenons of Western Uganda. J. Parasitol. 2004, 90, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, T.R.; Greiner, E.C.; Chapman, C.A. Gastrointestinal parasites of the Colobus monkeys of Uganda. J. Parasitol. 2005, 91, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, T.R.; Lonsdorf, E.V.; Canfield, E.P.; Meyer, D.J.; Nadler, Y.; Raphael, J.; Pusey, A.E.; Pond, J.; Pauley, J.; Mlengeya, T.; et al. Demographic and ecological effects on patterns of parasitism in Eastern chimpanzees (Pan troglodytes schweinfurthii) in Gombe national park, Tanzania. Am. J. Phys. Anthropol. 2010, 143, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, S. Regional differences in the infection of wild Japanese macaques by gastrointestinal helminth parasites. Primates 2000, 41, 291–298. [Google Scholar] [CrossRef]
- Hodder, S.A.M.; Chapman, C.A. Do nematode infections of red colobus (Procolobus rufomitratus) and black-and-white colobus (Colobus guereza) on humanized forest edges differ from those on nonhumanized forest edges? Int. J. Primatol. 2012, 33, 845–859. [Google Scholar] [CrossRef]
- Klaus, A.; Zimmermann, E.; Roper, K.M.; Radespiel, U.; Nathan, S.; Goossens, B.; Strube, C. Co-infection patterns of intestinal parasites in arboreal primates (Proboscis monkeys, Nasalis larvatus) in Borneo. Int. J. Parasitol. Parasit. Wildl. 2017, 6, 320–329. [Google Scholar] [CrossRef]
- Knezevich, M. Geophagy as a therapeutic mediator of endoparasitism in a free-ranging group of Rhesus macaques (Macaca mulatta). Am. J. Primatol. 1998, 44, 71–82. [Google Scholar] [CrossRef]
- Kooriyama, T.; Hasegawa, H.; Shimozuru, M.; Tsubota, T.; Nishida, T.; Iwaki, T. Parasitology of five primates in Mahale mountains national park, Tanzania. Primates 2012, 53, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, R.Y.W.; McGraw, S.W.; Yao, P.K.; Abou-Bacar, A.; Brunet, J.; Pesson, B.; Bonfoh, B.; N’Goran, E.K.; Candolfi, E. Diversity and prevalence of gastrointestinal parasites in seven non-human primates of the Tai national park, Cote D’ivoire. Parasite 2015, 22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sundararaj, P.; Kumara, H.N.; Pal, A.; Santhosh, K.; Vinoth, S. Prevalence of gastrointestinal parasites in bonnet macaque and possible consequences of their unmanaged relocations. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Kuze, N.; Kanamori, T.; Malim, T.P.; Bernard, H.; Zamma, K.; Kooriyama, T.; Morimoto, A.; Hasegawa, H. Parasites found from the feces of Bornean orangutans in Danum Valley, Sabah, Malaysia, with a redescription of Pongobius hugoti and the description of a new species of Pongobius (nematoda: Oxyuridae). J. Parasitol. 2010, 96, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Labes, E.M.; Wijayanti, N.; Deplazes, P.; Mathis, A. Genetic characterization of Strongyloides spp. From captive, semi-captive and wild Bornean orangutans (Pongo pygmaeus) in central and east Kalimantan, Borneo, Indonesia. Parasitology 2011, 138, 1417–1422. [Google Scholar] [CrossRef]
- Legesse, M.; Erko, B. Zoonotic intestinal parasites in Papio anubis (Baboon) and Cercopithecus aethiops (Vervet) from four localities in Ethiopia. Acta Trop. 2004, 90, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Levecke, B.; Dorny, P.; Geurden, T.; Vercammen, F.; Vercruysse, J. Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet. Parasitol. 2007, 148, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Mafuyai, H.B.; Barshep, Y.; Audu, B.S.; Kumbak, D.; Ojobe, T.O. Baboons as potential reservoirs of zoonotic gastrointestinal parasite infections at Yankari National Park, Nigeria. Afr. Health Sci. 2013, 13, 252–254. [Google Scholar] [CrossRef]
- Maldonado-Lopez, S.; Maldonado-Lopez, Y.; Ch, A.G.T.; Cuevas-Reyes, P.; Stoner, K.E. Patterns of infection by intestinal parasites in sympatric howler monkey (Alouatta palliata) and spider monkey (Ateles geoffroyi) populations in a tropical dry forest in Costa Rica. Primates 2014, 55, 383–392. [Google Scholar] [CrossRef]
- Martin-Solano, S.; Carrillo-Bilbao, G.A.; Ramirez, W.; Celi-Erazo, M.; Huynen, M.C.; Levecke, B.; Benitez-Ortiz, W.; Losson, B. Gastrointestinal parasites in captive and free-ranging Cebus albifrons in the Western Amazon, Ecuador. Int. J. Parasitol. Parasit. Wil. 2017, 6, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, K.; Gotoh, S.; Kawamoto, Y.; Watanabe, T.; Nozawa, K.; Takasaka, M.; Narita, T.; Griffiths, O.; Stanley, M.A. Clinical examinations on crab-eating macaques in Mauritius. Primates 1992, 33, 281–288. [Google Scholar] [CrossRef]
- McLennan, M.R.; Hasegawa, H.; Bardi, M.; Huffman, M.A. Gastrointestinal parasite infections and self-medication in wild chimpanzees surviving in degraded forest fragments within an agricultural landscape mosaic in Uganda. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Muehlenbein, M.P. Parasitological analyses of the male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale national park, Uganda. Am. J. Primatol. 2005, 65, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Munene, E.; Otsyula, M.; Mbaabu, D.A.N.; Mutahi, W.T.; Muriuki, S.M.K.; Muchemi, G.M. Helminth and protozoan gastrointestinal tract parasites in captive and wild-trapped African non-human primates. Vet. Parasitol. 1998, 78, 195–201. [Google Scholar] [CrossRef]
- Muriuki, S.M.K.; Murugu, R.K.; Munene, E.; Karere, G.M.; Chai, D.C. Some gastro-intestinal parasites of zoonotic (public health) importance commonly observed in old world non-human primates in Kenya. Acta Trop. 1998, 71, 73–82. [Google Scholar] [CrossRef]
- Parr, N.A.; Fedigan, L.M.; Kutz, S.J. A coprological survey of parasites in white-faced Capuchins (Cebus capucinus) from sector Santa Rosa, acg, Costa Rica. Folia Primatol. 2013, 84, 102–114. [Google Scholar] [CrossRef]
- Petrasova, J.; Modry, D.; Huffman, M.A.; Mapua, M.I.; Bobakova, L.; Mazoch, V.; Singh, J.; Kaur, T.; Petrzelkova, K.J. Gastrointestinal parasites of Indigenous and introduced primate species of Rubondo island national park, Tanzania. Int. J. Primatol. 2010, 31, 920–936. [Google Scholar] [CrossRef]
- Petrzelkova, K.J.; Hasegawa, H.; Appleton, C.C.; Huffman, M.A.; Archer, C.E.; Moscovice, L.R.; Mapua, M.I.; Singh, J.; Kaur, T. Gastrointestinal parasites of the chimpanzee population introduced onto Rubondo island national park, Tanzania. Am. J. Primatol. 2010, 72, 307–316. [Google Scholar] [CrossRef]
- Phillips, K.A.; Haas, M.E.; Grafton, B.W.; Yrivarren, M. Survey of the gastrointestinal parasites of the primate community at Tambopata national reserve, Peru. J. Zool. 2004, 264, 149–151. [Google Scholar] [CrossRef]
- Pouillevet, H.; Dibakou, S.E.; Ngoubangoye, B.; Poirotte, C.; Charpentier, M.J.E. A comparative study of four methods for the detection of nematode eggs and large protozoan cysts in mandrill faecal material. Folia Primatol. 2017, 88, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Pourrut, X.; Diffo, J.L.D.; Somo, R.M.; Bilong, C.F.B.; Delaportee, E.; LeBreton, M.; Gonzalez, J.P. Prevalence of gastrointestinal parasites in primate bushmeat and pets in Cameroon. Vet. Parasitol. 2011, 175, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Brashares, J.S.; Walsh, C.; Milbers, K.; Kilroy, C.; Chapman, C.A. A survey of gastrointestinal parasites of olive baboons (Papio anubis) in human settlement areas of Mole national park, Ghana. J. Parasitol. 2012, 98, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Solorzano-Garcia, B.; de Leon, G.P.P. Helminth parasites of howler and spider monkeys in Mexico: Insights into molecular diagnostic methods and their importance for zoonotic diseases and host conservation. Int. J. Parasitol. Parasit. Wil. 2017, 6, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Thanchomnang, T.; Intapan, P.M.; Sanpool, O.; Rodpai, R.; Sadaow, L.; Phosuk, I.; Somboonpatarakun, C.; Laymanivong, S.; Tourtip, S.; Maleewong, W. First molecular identification of Strongyloides fuelleborni in long-tailed macaques in Thailand and Lao People’s Democratic Republic reveals considerable genetic diversity. J. Helminthol. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Reddy, D.M.; Pradheeps, M.; Sreenivasamurthy, G.S.; Umapathy, G. Prevalence and co-occurrence of gastrointestinal parasites in Nilgiri Langur (Trachypithecus johnii) of fragmented landscape in Anamalai hills, Western Ghats, India. Curr. Sci. 2017, 113, 2194–2200. [Google Scholar] [CrossRef]
- Wenz, A.; Heymann, E.W.; Petney, T.N.; Taraschewski, H.F. The influence of human settlements on the parasite community in two species of Peruvian Tamarin. Parasitology 2010, 137, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Wren, B.T.; Gillespie, T.R.; Camp, J.W.; Remis, M.J. Helminths of vervet monkeys, Chlorocebus aethiops, from Loskop Dam nature reserve, South Africa. Comp. Parasitol. 2015, 82, 101–108. [Google Scholar] [CrossRef]
- Wren, B.T.; Remis, M.J.; Camp, J.W.; Gillespie, T.R. Number of grooming partners is associated with hookworm infection in wild vervet monkeys (Chlorocebus aethiops). Folia Primatol. 2016, 87, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Frias, L.; Stark, D.J.; Lynn, M.S.; Nathan, S.K.; Goossens, B.; Okamoto, M.; MacIntosh, A.J.J. Lurking in the dark: Cryptic strongyloides in a Bornean slow loris. Int. J. Parasitol. Parasites Wil. 2018, 7, 141–146. [Google Scholar] [CrossRef]
- Koinari, M.; Karl, S.; Ryan, U.; Lymbery, A.J. Infection levels of gastrointestinal parasites in sheep and goats in Papua New Guinea. J. Helminthol. 2013, 87, 409–415. [Google Scholar] [CrossRef] [PubMed]
- MacGlaflin, C.E.; Zajac, A.M.; Rego, K.A.; Petersson, K.H. Effect of vitamin E supplementation on naturally acquired parasitic infection in lambs. Vet. Parasitol. 2011, 175, 300–305. [Google Scholar] [CrossRef]
- McManus, C.; Louvandini, H.; Paiva, S.R.; de Oliveira, A.A.; Azevedo, H.C.; de Melo, C.B. Genetic factors of sheep affecting gastrointestinal parasite infections in the Distrito Federal, Brazil. Vet. Parasitol. 2009, 166, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, P.N.S.; Schmidt, N.M.; Kapel, C.M.O.; Christensen, M.U.; Sittler, B.; Gilg, O.; Enemark, H.L.; Al-Sabi, M.N.S. Gastrointestinal parasites of two populations of arctic foxes (Vulpes lagopus) from North-East Greenland. Polar Res. 2017, 36. [Google Scholar] [CrossRef]
- Dalimi, A.; Sattari, A.; Motamedi, G. A study on intestinal helminthes of dogs, foxes and jackals in the western part of Iran. Vet. Parasitol. 2006, 142, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Camacho, N.; Pineda-Lopez, R.; Lopez-Gonzalez, C.A.; Jones, R.W. Nematodes parasites of the gray fox (Urocyon cinereoargenteus schreber, 1775) in the seasonally dry tropical highlands of central Mexico. Parasitol. Res. 2011, 108, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Miterpakova, M.; Hurnikova, Z.; Antolova, D.; Dubinsky, P. Endoparasites of red fox (Vulpes vulpes) in the Slovak Republic with the emphasis on zoonotic species Echinococcus multilocularis and Trichinella spp. Helminthologia 2009, 46, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, S.R.M.; Maldonade, I.R.; Ginani, V.C.; Lima, S.A.; Mendes, V.S.; Azevedo, M.L.X.; Gurgel-Gonçalves, R.; Machado, E.R. Detection of intestinal parasites on field-grown strawberries in the federal district of Brazil. Rev. Soc. Bras. Med. Trop. 2014, 47, 801–805. [Google Scholar] [CrossRef]
- Aghaindum, A.G.; Landry, F.K.A. Dissemination of the resistant forms of intestinal worms in the marshy areas of the city of Yaounde (Cameroon): Importance of some abiotic factors of the medium. Appl. Water Sci. 2019, 9. [Google Scholar] [CrossRef]
- Hilles, A.H.; Al Hindi, A.I.; Abu Safieh, Y.A. Assessment of parasitic pollution in the coastal seawater of Gaza city. J. Environ. Health Sci. Eng. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Tanyuksel, M.; Saylam, F.; Tanriverdi, S.; Araz, R.E.; Hacim, A.K.; Hasde, M. Investigation of waterborne parasites in drinking water sources of Ankara, Turkey. J. Microbiol. 2003, 41, 148–151. [Google Scholar]
- El-Badry, A.A.; Hamdy, D.A.; El Wahab, W.M.A. Strongyloides stercoralis larvae found for the first time in tap water using a novel culture method. J. Parasitol. Res. 2018, 117, 3775–3780. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.A.; Cabral, J.J.S.P.; Rocha, F.J.S.; Paiva, A.L.R.; Sens, M.L.; Veras, T.B. Cryptosporidium spp. and Giardia spp. removal by bank filtration at Beberibe River, Brazil. J. River Res. App. 2017, 33, 1079–1087. [Google Scholar] [CrossRef]
- Klotz, S.A.; Normand, R.E.; Kalinsky, R.G. “Through a drinking glass and what was found there”: Pseudocontamination of a hospital's drinking water. J. Infect. Control Hosp. Epidemiol. 1992, 8, 477–481. [Google Scholar]
- Zeehaida, M.; Zairi, N.Z.; Rahmah, N.; Maimunah, A.; Madihah, B. Strongyloides stercoralis in common vegetables and herbs in Kota Bharu, Keletan, Malaysia. J. Trop. Biomed. 2011, 28, 188–193. [Google Scholar]
- Luz, J.G.G.; Barbosa, M.V.; de Carvalho, A.G.; Resende, S.D.; Dias, J.V.L.; Martins, H.R. Contamination by intestinal parasites in vegetables marketed in an area of Jequitinhonha Valley, Minas Gerais, Brazil. Rev. Nutricao-Braz. J. Nutr. 2017, 30, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Maikai, B.V.; Elisha, I.A.; Baba-Onoja, E.B.T. Contamination of vegetables sold in markets with helminth eggs in Zaria metropolis, Kaduna State, Nigeria. Food Control 2012, 28, 345–348. [Google Scholar] [CrossRef]
- Madadi, M. Parasitic contamination in the table vegetables planted in Shiraz plain, Iran. Pak. J. Sci. Ind. Res. 2010, 53, 42–45. [Google Scholar]
- Berentsen, A.R.; Becker, M.S.; Stockdale-Walden, H.; Matandiko, W.; McRobb, R.; Dunbar, M.R. Survey of gastrointestinal parasite infection in African lion (Panthera leo), African wild dog (Lycaon pictus) and spotted hyaena (Crocuta crocuta) in the Luangwa valley, Zambia. Afr. Zool. 2012, 47, 363–368. [Google Scholar] [CrossRef]
- Bista, D.; Shrestha, S.; Kunwar, A.J.; Acharya, S.; Jnawali, S.R.; Acharya, K.P. Status of gastrointestinal parasites in red panda of Nepal. PeerJ 2017, 2017. [Google Scholar] [CrossRef]
- Cardia, D.F.F.; Camossi, L.G.; Fornazari, F.; Babboni, S.D.; Teixeira, C.R.; Bresciani, K.D.S. First report of Strongyloides sp. (nematoda, Strongyloididae) in Lutreolina crassicaudata (didelphimorphia: Didelphidae). Braz. J. Biol. 2016, 76, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Cordon, G.P.; Prados, A.H.; Romero, D.; Moreno, M.S.; Pontes, A.; Osuna, A.; Rosales, M.J. Intestinal parasitism in the animals of the zoological garden “Pena Escrita” (Almunecar, Spain). Vet. Parasitol. 2008, 156, 302–309. [Google Scholar] [CrossRef] [PubMed]
- González, P.; Carbonell, E.; Urios, V.; Rozhnov, V.V. Coprology of Panthera tigris altaica and Felis bengalensis euptilurus from the Russian far East. J. Parasitol. 2007, 93, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, N.K.; Singh, H.; Rath, S.S. Assessment of risk factors associated with prevalence of gastrointestinal helminths in buffaloes from Punjab State, India. Buffalo Bull. 2018, 37, 279–290. [Google Scholar]
- Hallinger, M.J.; Taubert, A.; Hermosilla, C.; Mutschmann, F. Occurrence of health-compromising protozoan and helminth infections in tortoises kept as pet animals in Germany. Parasites Vectors 2018, 11. [Google Scholar] [CrossRef]
- Hasegawa, H.; Ota, H. Parasitic Helminths Found from Polypedates leucomystax (Amphibia: Rhacophoridae) on Miyakojima Island, Ryukyu Archipelago, Japan. Curr. Herpetol. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Hermosilla, C.; Kleinertz, S.; Silva, L.M.R.; Hirzmann, J.; Huber, D.; Kusak, J.; Taubert, A. Protozoan and helminth parasite fauna of free-living Croatian wild wolves (Canis lupus) analyzed by scat collection. Vet. Parasitol. 2017, 233, 14–19. [Google Scholar] [CrossRef]
- Hu, X.L.; Liu, G.; Wei, Y.T.; Wang, Y.H.; Zhang, T.X.; Yang, S.; Hu, D.F.; Liu, S.Q. Regional and seasonal effects on the gastrointestinal parasitism of captive forest musk deer. Acta Tropica 2018, 177, 1–8. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, L.Z.; Zhao, N.N. Temporal-spatial patterns of intestinal parasites of the Hooded Crane (Grus monacha) wintering in lakes of the middle and lower Yangtze River floodplain. Avian Res. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Kumba, F.F.; Katjivena, H.; Kauta, G.; Lutaaya, E. Seasonal evolution of faecal egg output by gastrointestinal worms in goats on communal farms in eastern Namibia. Onderstepoort J. Vet. Res. 2003, 70, 265–271. [Google Scholar] [CrossRef]
- Mizgajska-Wiktor, H.; Jarosz, W. Potential risk of zoonotic infections in recreational areas visited by Sus scrofa and Vulpes vulpes. Case study--Wolin Island, Poland. Wiad. Parazytol. 2010, 56, 243–251. [Google Scholar]
- Mukul-Yerves, J.M.; Zapata-Escobedo, M.D.; Montes-Perez, R.C.; Rodriguez-Vivas, R.I.; Torres-Acosta, J.F. Gastrointestinal and ectoparasites in wildlife-ungulates under captive and free-living conditions in the Mexican tropic. Rev. Mex. Cienc. Pecu. 2014, 5, 459–469. [Google Scholar]
- Oja, R.; Velstrom, K.; Moks, E.; Jokelainen, P.; Lassen, B. How does supplementary feeding affect endoparasite infection in wild boar? Parasitol. Res. 2017, 116, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Robertos, N.; Torres-Chable, O.M.; Peralta-Torres, J.; Luna-Palomera, C.; Aguilar-Cabrales, A.; Chay-Canul, A.; Gonzalez-Garduno, R.; Machain-Williams, C.; Camara-Sarmiento, R. Study of gastrointestinal parasites in water buffalo (Bubalus bubalis) reared under Mexican humid tropical conditions. Trop. Anim. Health Prod. 2017, 49, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Perera, A.; Maia, J.; Jorge, F.; Harris, D.J. Molecular screening of nematodes in lacertid lizards from the Iberian Peninsula and Balearic Islands using 18s rRNA sequences. J. Helminthol. 2013, 87, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Pilarczyk, B.; Tomza-Marciniak, A.; Udala, J.; Kuba, J. The prevalence and control of gastrointestinal nematodes in farmed fallow deer (Dama dama l.). Vet. Arh. 2015, 85, 415–423. [Google Scholar]
- Rahman, M.; Islam, S.; Masuduzzaman, M.; Alam, M.; Chawdhury, M.N.U.; Ferdous, J.; Islam, M.N.; Hassan, M.M.; Hossain, M.A.; Islam, A. Prevalence and diversity of gastrointestinal helminths in free-ranging Asian house shrew (Suncus murinus) in Bangladesh. Vet. World 2018, 11, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Musella, V.; Veneziano, V.; Condoleo, R.U.; Cringoli, G. Helmintic infections in water buffaloes on Italian farms: A spatial analysis. Geospat. Health 2009, 3, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Rosalino, L.M.; Torres, J.; Santos-Reis, M. A survey of helminth infection in Eurasian badgers (Meles meles) in relation to their foraging behaviour in a mediterranean environment in Southwest Portugal. Eur. J. Wildl. Res. 2006, 52, 202–206. [Google Scholar] [CrossRef]
- Turni, C.; Smales, L.R. Parasites of the bridled nailtail wallaby (Onychogalea fraenata) (marsupialia : Macropodidae). Wildl. Res. 2001, 28, 403–411. [Google Scholar] [CrossRef]
- Turner, W.C.; Getz, W.M. Seasonal and demographic factors influencing gastrointestinal parasitism in ungulates of Etosha national park. J. Wildl. Dis. 2010, 46, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Turner, W.C.; Cizauskas, C.A.; Getz, W.M. Variation in faecal water content may confound estimates of gastro-intestinal parasite intensity in wild African herbivores. J. Helminthol. 2010, 84, 99–105. [Google Scholar] [CrossRef] [PubMed]
- VanderWaal, K.; Omondi, G.P.; Obanda, V. Mixed-host aggregations and helminth parasite sharing in an East African wildlife-livestock system. Vet. Parasitol. 2014, 205, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Ybañez, R.H.D.; Resuelo, K.J.G.; Kintanar, A.P.M.; Ybañez, A.P. Detection of gastrointestinal parasites in small-scale poultry layer farms in Leyte, Philippines. Vet. World 2018, 11, 1587–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Search Terms Employed to Identify Relevant Literature |
|---|
| Strongyloides OR Strongyloidiasis OR “Strongyloides stercoralis” OR “S. stercoralis” OR “Strongyloides fuelleborni” OR “S. fulleborni” OR “Strongyloides fulleborni kellyi” OR “S. fulleborni kellyi” |
| AND |
| “Tap Water” OR “Potable water” OR Water OR Soil OR Dirt OR sediment OR synanthropic OR “synanthropic insect” OR Insect OR “Musca domestica” OR flies OR “Musca vetustissima” OR Sarcophagidae OR “Chrysomya megacephala” OR “Musca sorbens” OR “Lucilia cuprina” OR “Calliphora vicina” OR “Blattella germanica” OR “Periplaneta Americana” OR Cockroach OR dog OR “Canis lupis” OR zoonotic OR Monkey OR “septic tank” OR waste OR wastewater OR rubbish OR trash OR environment |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. F. White, M.; Whiley, H.; E. Ross, K. A Review of Strongyloides spp. Environmental Sources Worldwide. Pathogens 2019, 8, 91. https://doi.org/10.3390/pathogens8030091
A. F. White M, Whiley H, E. Ross K. A Review of Strongyloides spp. Environmental Sources Worldwide. Pathogens. 2019; 8(3):91. https://doi.org/10.3390/pathogens8030091
Chicago/Turabian StyleA. F. White, Mae, Harriet Whiley, and Kirstin E. Ross. 2019. "A Review of Strongyloides spp. Environmental Sources Worldwide" Pathogens 8, no. 3: 91. https://doi.org/10.3390/pathogens8030091
APA StyleA. F. White, M., Whiley, H., & E. Ross, K. (2019). A Review of Strongyloides spp. Environmental Sources Worldwide. Pathogens, 8(3), 91. https://doi.org/10.3390/pathogens8030091





