Genetic Analysis of Avian Gyrovirus 2 Variant-Related Gyrovirus Detected in Farmed King Ratsnake (Elaphe carinata): The First Report from China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Virus Detection
2.2. Whole-Genome Sequencing of AGV2
2.3. Recombination and Phylogenetic Analysis
3. Results
3.1. Positive Rates of AGV2 in the Snakes in China
3.2. Whole-Genome Sequencing of AGV2 in Snakes
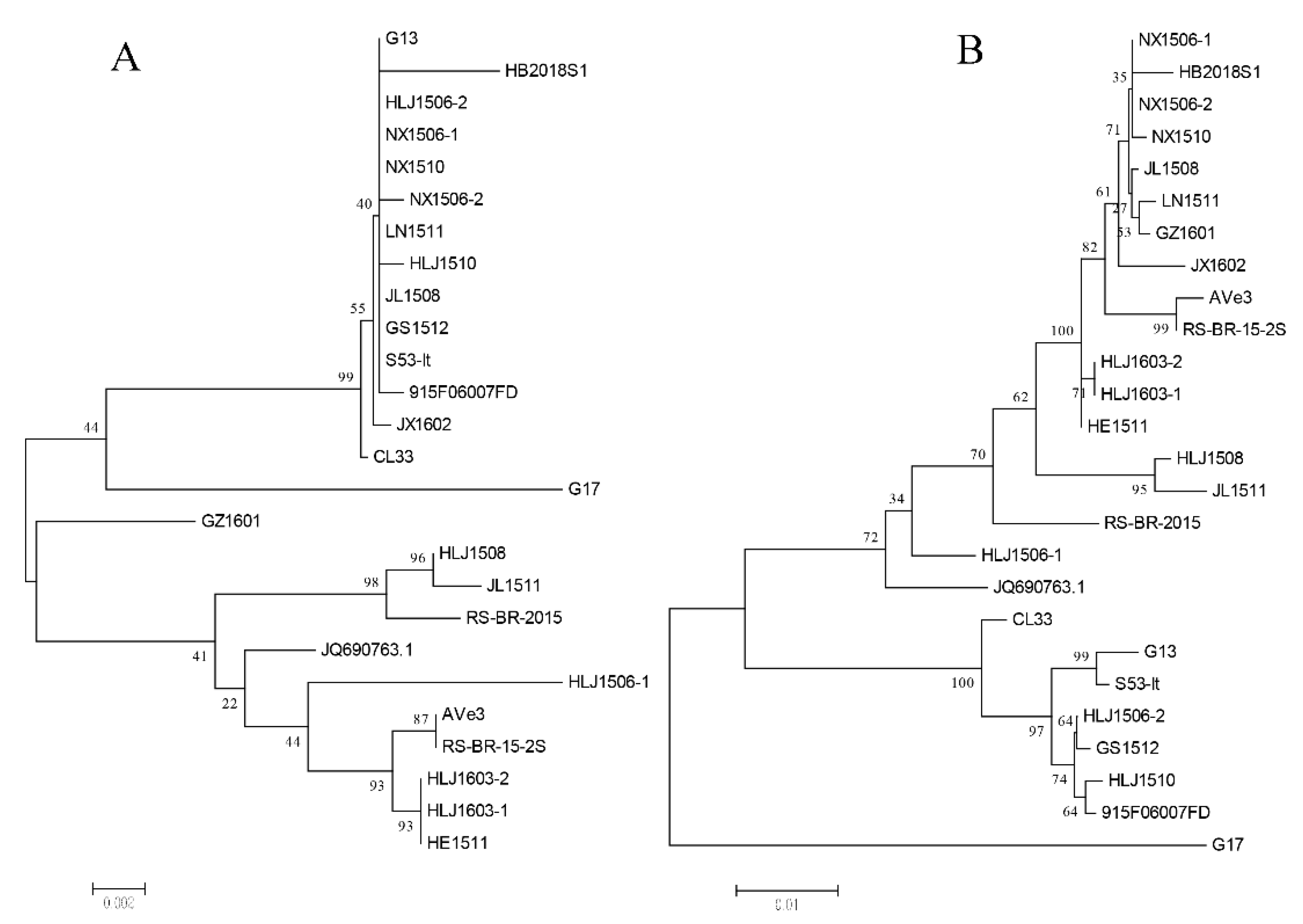
3.3. Phylogenetic Analysis
3.4. Analysis of the Mutated Amino Acids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosario, K.; Breitbart, M.; Harrach, B.; Segalés, J.; Delwart, E.; Biagini, P.; Varsani, A. Revisiting the taxonomy of the family Circoviridae: Establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Arch. Virol. 2017, 162, 1447–1463. [Google Scholar] [CrossRef]
- Schat, K.A. Chicken anemia virus. Curr. Top. Microbiol. Immunol. 2009, 331, 151–183. [Google Scholar] [PubMed]
- Rijsewijk, F.A.; Dos, S.H.F.; Teixeira, T.F.; Cibulski, S.P.; Varela, A.P.; Dezen, D.; Franco, A.C.; Roehe, P.M. Discovery of a genome of a distant relative of chicken anemia virus reveals a new member of the genus Gyrovirus. Arch. Virol. 2011, 156, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Tuo, T.; Gao, X.; Han, C.; Li, Y.; Gao, Y.; Zhang, Y.; Liu, C.; Qi, X.; Gao, H.; et al. Avian gyrovirus 2 in poultry, China, 2015–2016. Emerg. Microbes Infect. 2016, 5, e112. [Google Scholar] [CrossRef] [PubMed]
- Dos, S.H.F.; Knak, M.B.; de Castro, F.L.; Slongo, J.; Ritterbusch, G.A.; Klein, T.A.; Esteves, P.A.; Silva, A.D.; Trevisol, I.M.; Claassen, E.A.; et al. Variants of the recently discovered avian gyrovirus 2 are detected in Southern Brazil and The Netherlands. Vet. Microbiol. 2012, 155, 230–236. [Google Scholar]
- Varela, A.P.; Dos, S.H.F.; Cibulski, S.P.; Scheffer, C.M.; Schmidt, C.; Sales, L.F.E.; Silva, A.D.; Esteves, P.A.; Franco, A.C.; Roehe, P.M. Chicken anemia virus and avian gyrovirus 2 as contaminants in poultry vaccines. Biologicals 2014, 42, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Macera, L.; Focosi, D.; Vatteroni, M.L.; Boggi, U.; Antonelli, G.; Eloit, M.; Pistello, M. Human gyrovirus DNA in human blood, Italy. Emerg. Infect. Dis. 2012, 18, 956–959. [Google Scholar] [CrossRef]
- Phan, T.G.; Li, L.; O’Ryan, M.G.; Cortes, H.; Mamani, N.; Bonkoungou, I.J.; Wang, C.; Leutenegger, C.M.; Delwart, E. A third gyrovirus species in human faeces. J. Gen. Virol. 2012, 93, 1356–1361. [Google Scholar] [CrossRef]
- Abolnik, C.; Wandrag, D.B. Avian gyrovirus 2 and avirulent Newcastle disease virus coinfection in a chicken flock with neurologic symptoms and high mortalities. Avian Dis. 2014, 58, 90–94. [Google Scholar] [CrossRef]
- Feher, E.; Pazar, P.; Kovacs, E.; Farkas, S.L.; Lengyel, G.; Jakab, F.; Martella, V.; Banyai, K. Molecular detection and characterization of human gyroviruses identified in the ferret fecal virome. Arch. Virol. 2014, 159, 3401–3406. [Google Scholar] [CrossRef] [Green Version]
- Sauvage, V.; Cheval, J.; Foulongne, V.; Gouilh, M.A.; Pariente, K.; Manuguerra, J.C.; Richardson, J.; Dereure, O.; Lecuit, M.; Burguiere, A.; et al. Identification of the first human gyrovirus, a virus related to chicken anemia virus. J. Virol. 2011, 85, 7948–7950. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Tian, X.; Xie, Q.; Zhang, Y.; Sheng, Y.; Zhang, Z.; Wang, C.; Zhu, H.; Wang, Y.; Shao, H.; et al. Avian Gyrovirus 2 DNA in fowl from live poultry markets and in healthy humans, China. Emerg. Infect. Dis. 2015, 21, 1486–1488. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, H.; Gao, S.; Lercher, M.J.; Chen, W.H.; Hu, S. Evolview v2: An online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016, 44, W236–W241. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noteborn, M.H.; de Boer, G.F.; van Roozelaar, D.J.; Karreman, C.; Kranenburg, O.; Vos, J.G.; Jeurissen, S.H.; Hoeben, R.C.; Zantema, A.; Koch, G.; et al. Characterization of cloned chicken anemia virus DNA that contains all elements for the infectious replication cycle. J. Virol. 1991, 65, 3131–3139. [Google Scholar] [PubMed]
- Phenix, K.V.; Meehan, B.M.; Todd, D.; McNulty, M.S. Transcriptional analysis and genome expression of chicken anaemia virus. J. Gen. Virol. 1994, 75, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.H.; Nishizawa, T.; Takahashi, M.; Ishikawa, T.; Yoshikawa, A.; Okamoto, H. Analysis of the entire genomes of thirteen TT virus variants classifiable into the fourth and fifth genetic groups, isolated from viremic infants. Arch. Virol. 2002, 147, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.A.; Jackson, D.C.; Crabb, B.S.; Browning, G.F. Chicken anemia virus VP2 is a novel dual specificity protein phosphatase. J. Biol. Chem. 2002, 277, 39566–39573. [Google Scholar] [CrossRef]
- Takahashi, K.; Iwasa, Y.; Hijikata, M.; Mishiro, S. Identification of a new human DNA virus (TTV-like mini virus, TLMV) intermediately related to TT virus and chicken anemia virus. Arch. Virol. 2000, 145, 979–993. [Google Scholar] [CrossRef]
- Noteborn, M.H.; Verschueren, C.A.; Koch, G.; Van der Eb, A.J. Simultaneous expression of recombinant baculovirus-encoded chicken anaemia virus (CAV) proteins VP1 and VP2 is required for formation of the CAV-specific neutralizing epitope. J. Gen. Virol. 1998, 79, 3073–3077. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sheu, S.C.; Lien, Y.Y.; Lee, M.S.; Chen, H.J.; Su, W.H.; Lee, M.S. Identification of the NLS and NES motifs of VP2 from chicken anemia virus and the interaction of VP2 with mini-chromosome maintenance protein 3. BMC Vet. Res. 2012, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Taebunpakul, P.; Sayan, B.S.; Flinterman, M.; Klanrit, P.; Gaken, J.; Odell, E.W.; Melino, G.; Tavassoli, M. Apoptin induces apoptosis by changing the equilibrium between the stability of TAp73 and DeltaNp73 isoforms through ubiquitin ligase PIR2. Apoptosis 2012, 17, 762–776. [Google Scholar] [CrossRef] [PubMed]



| Farm | Province | Sample Quantity | Positive Rates | |
|---|---|---|---|---|
| Fecal Sample | Liver Tissue Sample | |||
| A | Henan | 10 | 0/9 | 0/1 |
| B | Hubei | 13 | 5/11 | 1/2 |
| C | Hubei | 5 | 0/5 | 0/0 |
| D | Hubei | 21 | 0/20 | 0/1 |
| E | Henan | 19 | 0/17 | 0/2 |
| F | Hubei | 11 | 0/10 | 0/1 |
| G | Hubei | 12 | 0/11 | 0/1 |
| Total | 91 | 5/83 | 1/8 | |
| Recombination Event | No. | Recombinant Sequence | Breakpoint Positions | Major Parent | Minor Parent | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Begin End | Similarity | Similarity | |||||||
| 1 | 7 | HB2018S1 | 100 | 1542 | HLJ1603-2 | 99% | G13 | 99.5% | 2.71 × 10−11 |
| 2 | 1 | HLJ1506-1 | 1948 | 2556 | GZ1601 | 99.3% | G17 | 99.8% | 4.50 × 10−7 |
| 3 | 1 | GZ1601 | 1110 | 1542 | JL1511 | 97.3% | S53-It | 100% | 3.38 × 10−6 |
| 4 | 3 | JL1511 | 52 | 639 | JQ690763.1 | 98.8% | G13 | 99.3% | 1.89 × 10−4 |
| Strains | Substitution of Amino Acid Residues | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 36 | 44 | 74 | 95 | 154 | 212 | 242 | 256 | 270 | 279 | 288 | 293 | 310 | 311 | 314 | 373 | 383 | 401 | 416 | 459 | |
| HB2018S1 | G | K a | A | K | A | K | R | R | S | Q | V | G | E | D | R | A | P | V | L | N |
| NX1510 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | N |
| NX1506-2 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | N |
| NX1506-1 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | T |
| JL1508 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | N |
| LN1511 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | T |
| JX1602 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | N |
| GZ1601 | S | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | N |
| HLJ1603-2 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | N |
| HLJ1603-1 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | T |
| HE1511 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | T |
| HLJ1506-2 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | N |
| GS1512 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | K | V | P | V | L | N |
| HLJ1508 | G | R | T | T | S | K | R | C | S | L | Q | Q | E | D | R | V | P | V | L | N |
| G13 | G | R | T | K | A | K | R | C | S | L | V | G | E | E | R | V | P | V | L | N |
| JL1511 | G | R | T | T | S | K | R | C | S | L | Q | Q | E | D | R | V | P | V | L | N |
| HM590588 | G | R | T | T | S | R | G | C | A | L | V | G | Q | D | R | V | Q | M | I | N |
| JQ690763 | G | R | T | K | A | K | R | C | S | L | Q | Q | E | D | R | V | P | V | L | N |
| RS/BR/15/2S | G | R | T | K | S | K | R | C | A | L | V | G | Q | D | R | V | Q | M | I | N |
| HLJ1506-1 | S | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | N |
| 915F06007FD | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | N |
| G17 | S | R | T | K | S | K | R | C | S | L | Q | Q | E | D | K | V | P | V | L | N |
| CL33 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | N |
| S53/It | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | N |
| HLJ1510 | G | R | T | K | A | K | R | C | S | L | V | G | E | D | R | V | P | V | L | N |
| Strains | Substitution of Amino Acid Residues | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 | 60 | 125 | 141 | 156–158 | 161 | 162 | 165 | 167 | 174–175 | 179 | 213 | 215 | |
| HB2018S1 | N | T a | I | R | GKR | Y | - | A | T | EE | A | N | S |
| NX1510 | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| NX1506-2 | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| NX1506-1 | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| JL1508 | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| LN1511 | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| JX1602 | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| GZ1601 | T | I | T | Q | RRG | H | - | T | T | DD | V | D | L |
| HLJ1603-2 | T | I | T | Q | RRG | H | - | T | T | DD | V | D | L |
| HLJ1603-1 | T | I | T | Q | RRG | H | - | T | T | DD | V | D | L |
| HE1511 | T | I | T | Q | RRG | H | - | T | T | DD | V | D | L |
| HLJ1506-2 | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| GS1512 | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| HLJ1508 | N | I | T | Q | RRG | H | - | A | T | DD | V | D | L |
| G13 | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| JL1511 | N | I | T | Q | RRG | H | - | A | T | DD | V | D | L |
| HM590588 | T | I | T | Q | RRG | H | - | T | T | DD | V | D | L |
| JQ690763 | N | I | T | Q | RRG | H | - | A | T | DD | V | D | L |
| RS/BR/15/2S | T | I | T | Q | RRG | H | - | T | T | DD | V | D | L |
| HLJ1506-1 | T | I | T | Q | RRG | H | - | T | T | DD | V | D | L |
| 915F06007FD | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| G17 | T | I | T | R | RRG | Y | S | A | S | EE | A | D | L |
| CL33 | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| S53/It | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| HLJ1510 | N | I | T | R | GKR | Y | - | A | T | EE | A | D | L |
| Strains | Substitution of Amino Acid Residues | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 9 | 12 | 14 | 28 | 54 | 65 | 69 | 71 | 79 | 81 | 83 | 85 | 99 | 101 | 103 | 104 | 115 | 120 | 122 | 124 | |
| HB2018S1 | R | R | T | Q | S | Y | A | D | G | V | L | Y a | R | A | K | Q | Q | N | K | - | L |
| NX1510 | R | R | T | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
| NX1506-2 | R | R | I | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
| NX1506-1 | R | R | T | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
| JL1508 | R | R | T | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
| LN1511 | R | R | T | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
| JX1602 | R | R | T | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
| GZ1601 | R | Q | T | R | C | Y | A | D | G | A | S | H | R | S | K | R | R | E | K | - | L |
| HLJ1603-2 | R | Q | T | R | C | Y | A | D | G | A | S | H | R | S | K | R | R | E | K | - | L |
| HLJ1603-1 | R | Q | T | R | C | Y | A | D | G | A | S | H | R | S | K | R | R | E | K | - | L |
| HE1511 | R | Q | T | R | C | Y | A | D | G | A | S | H | R | S | K | R | R | E | K | - | L |
| HLJ1506-2 | R | R | T | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
| GS1512 | R | R | T | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
| HLJ1508 | R | R | T | Q | C | S | V | A | E | V | L | H | R | S | R | Q | R | E | K | - | L |
| G13 | R | R | T | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
| JL1511 | R | R | T | Q | C | S | V | A | E | V | L | H | R | S | R | Q | R | E | K | - | L |
| HM590588 | R | Q | T | R | C | Y | A | D | G | A | S | H | R | S | K | Q | R | E | K | - | L |
| JQ690763 | R | Q | T | R | C | Y | A | D | G | V | L | H | R | S | K | Q | R | E | K | - | L |
| RS/BR/15/2S | R | Q | T | R | C | Y | A | D | G | A | S | H | R | S | K | Q | R | E | K | - | L |
| HLJ1506-1 | R | Q | T | R | C | Y | A | D | G | A | S | H | R | S | K | Q | R | E | K | - | L |
| 915F06007FD | R | R | T | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
| G17 | H | Q | T | R | S | Y | A | D | G | V | L | H | K | A | K | Q | R | E | R | R | V |
| CL33 | R | R | T | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
| S53/It | R | R | T | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
| HLJ1510 | R | R | T | Q | S | Y | A | D | G | V | L | H | R | A | K | Q | Q | N | K | - | L |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Xu, X.; Chen, Q.; Ji, J.; Kan, Y.; Yao, L.; Xie, Q. Genetic Analysis of Avian Gyrovirus 2 Variant-Related Gyrovirus Detected in Farmed King Ratsnake (Elaphe carinata): The First Report from China. Pathogens 2019, 8, 185. https://doi.org/10.3390/pathogens8040185
Wu Q, Xu X, Chen Q, Ji J, Kan Y, Yao L, Xie Q. Genetic Analysis of Avian Gyrovirus 2 Variant-Related Gyrovirus Detected in Farmed King Ratsnake (Elaphe carinata): The First Report from China. Pathogens. 2019; 8(4):185. https://doi.org/10.3390/pathogens8040185
Chicago/Turabian StyleWu, Qianqian, Xin Xu, Qinxi Chen, Jun Ji, Yunchao Kan, Lunguang Yao, and Qingmei Xie. 2019. "Genetic Analysis of Avian Gyrovirus 2 Variant-Related Gyrovirus Detected in Farmed King Ratsnake (Elaphe carinata): The First Report from China" Pathogens 8, no. 4: 185. https://doi.org/10.3390/pathogens8040185
APA StyleWu, Q., Xu, X., Chen, Q., Ji, J., Kan, Y., Yao, L., & Xie, Q. (2019). Genetic Analysis of Avian Gyrovirus 2 Variant-Related Gyrovirus Detected in Farmed King Ratsnake (Elaphe carinata): The First Report from China. Pathogens, 8(4), 185. https://doi.org/10.3390/pathogens8040185






