Virulence and Pathogenicity Properties of Aggregatibacter actinomycetemcomitans
Abstract
1. Introduction
2. Leukotoxin (LtxA)
2.1. LtxA Production
2.2. Leukotoxin Secretion
2.3. Quantification of LtxA Production
3. Cytolethal Distending Toxin (CDT)
4. Lipopolysaccharide and Cytokine-Binding Factors
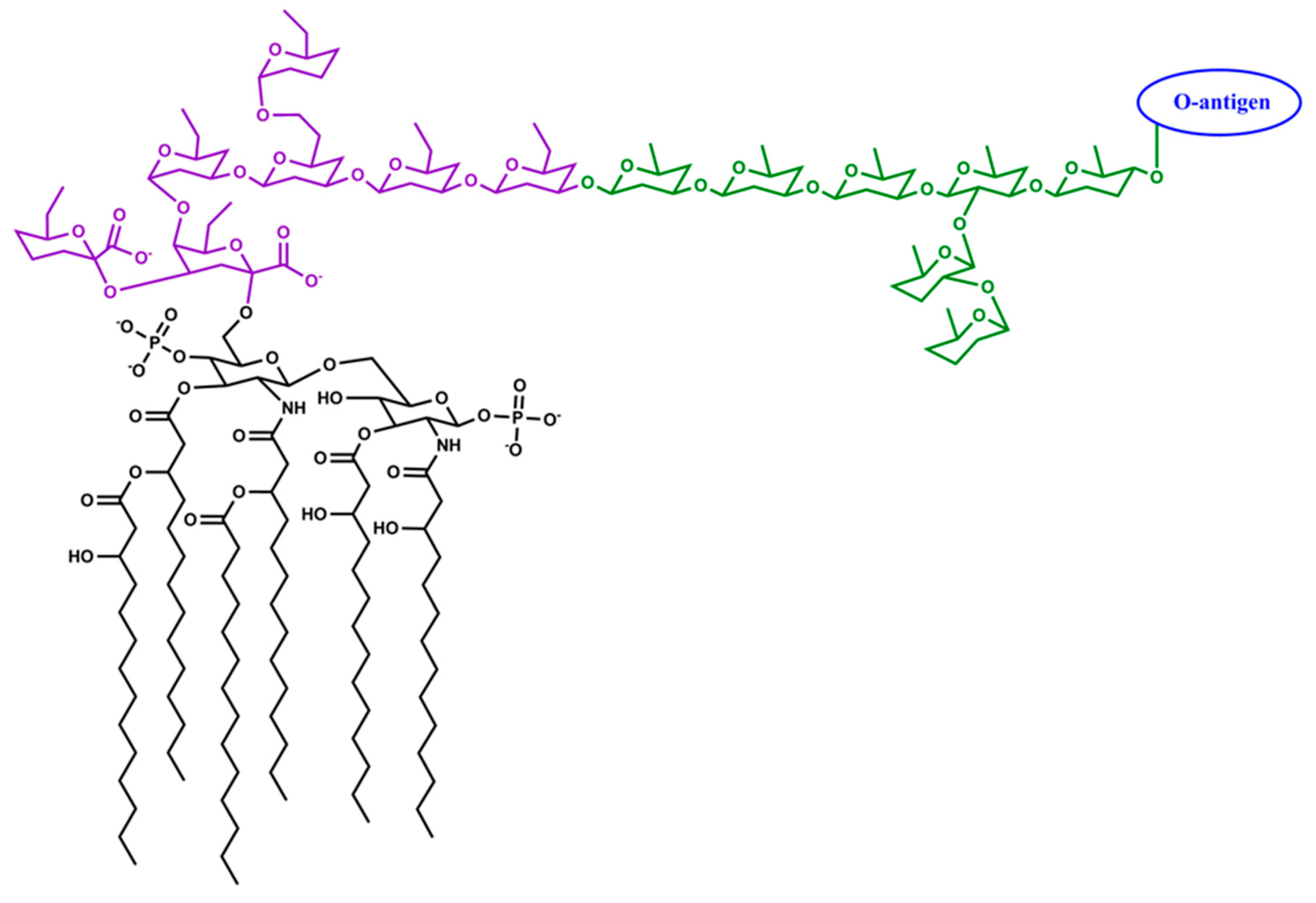
4.1. The Virulence Properties of A. actinomycetemcomitans Lipopolysaccharide
4.2. Sensing of Host Signal Molecules
5. Outer Membrane Vesicles
6. Biofilm Interactions and Proteomic Regulations
6.1. Localization in Pocket and Tissue
6.2. Localization in Biofilms and Proteomic Interactions with Other Species
7. Horizontal Gene Transfer
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henderson, B.; Ward, J.M.; Ready, D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: A triple A* periodontopathogen? Periodontology 2000 2010, 54, 78–105. [Google Scholar] [CrossRef] [PubMed]
- Åberg, C.H.; Kelk, P.; Johansson, A. Aggregatibacter actinomycetemcomitans: Virulence of its leukotoxin and association with aggressive periodontitis. Virulence 2015, 6, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Patil, A.G.; Velusamy, S.K. Aggregatibacter actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front. Immunol. 2019, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A. Aggregatibacter actinomycetemcomitans leukotoxin: A powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins 2011, 3, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Vega, B.A.; Belinka, B.A., Jr.; Kachlany, S.C. Aggregatibacter actinomycetemcomitans Leukotoxin (LtxA.; Leukothera((R))): Mechanisms of Action and Therapeutic Applications. Toxins 2019, 11, 489. [Google Scholar] [CrossRef]
- DiRienzo, J.M. Breaking the Gingival Epithelial Barrier: Role of the Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin in Oral Infectious Disease. Cells 2014, 3, 476–499. [Google Scholar] [CrossRef]
- Fujise, O.; Wang, Y.; Chen, W.; Chen, C. Adherence of Aggregatibacter actinomycetemcomitans via serotype-specific polysaccharide antigens in lipopolysaccharides. Oral Microbiol. Immunol. 2008, 23, 226–233. [Google Scholar] [CrossRef]
- Oscarsson, J.; Claesson, R.; Lindholm, M.; Höglund Åberg, C.; Johansson, A. Tools of Aggregatibacter actinomycetemcomitans to Evade the Host Response. J. Clin. Med. 2019, 8, 1079. [Google Scholar] [CrossRef]
- Thay, B.; Damm, A.; Kufer, T.A.; Wai, S.N.; Oscarsson, J. Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger NOD1- and NOD2-dependent NF-kappaB activation. Infect. Immun. 2014, 82, 4034–4046. [Google Scholar] [CrossRef]
- Kittichotirat, W.; Bumgarner, R.E.; Chen, C. Evolutionary Divergence of Aggregatibacter actinomycetemcomitans. J. Dent. Res. 2016, 95, 94–101. [Google Scholar] [CrossRef]
- Bao, K.; Bostanci, N.; Thurnheer, T.; Grossmann, J.; Wolski, W.E.; Thay, B.; Belibasakis, G.N.; Oscarsson, J. Aggregatibacter actinomycetemcomitans H-NS promotes biofilm formation and alters protein dynamics of other species within a polymicrobial oral biofilm. NPJ Biofilms Microbiomes 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Bostanci, N.; Marsh, P.D.; Zaura, E. Applications of the oral microbiome in personalized dentistry. Arch. Oral Biol. 2019, 104, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Claesson, R.; Johansson, A.; Belibasakis, G.; Hanstrom, L.; Kalfas, S. Release and activation of matrix metalloproteinase 8 from human neutrophils triggered by the leukotoxin of Actinobacillus actinomycetemcomitans. J. Periodontal Res. 2002, 37, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Claesson, R.; Hanstrom, L.; Sandstrom, G.; Kalfas, S. Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans. J. Periodontal Res. 2000, 35, 85–92. [Google Scholar] [CrossRef]
- Hirschfeld, J.; Roberts, H.M.; Chapple, I.L.; Parcina, M.; Jepsen, S.; Johansson, A.; Claesson, R. Effects of Aggregatibacter actinomycetemcomitans leukotoxin on neutrophil migration and extracellular trap formation. J. Oral Microbiol. 2016, 8, 33070. [Google Scholar] [CrossRef]
- Lopes, J.P.; Stylianou, M.; Backman, E.; Holmberg, S.; Jass, J.; Claesson, R.; Urban, C.F. Evasion of Immune Surveillance in Low Oxygen Environments Enhances Candida albicans Virulence. mBio 2018, 9. [Google Scholar] [CrossRef]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef]
- DiFranco, K.M.; Gupta, A.; Galusha, L.E.; Perez, J.; Nguyen, T.V.; Fineza, C.D.; Kachlany, S.C. Leukotoxin (Leukothera(R)) targets active leukocyte function antigen-1 (LFA-1) protein and triggers a lysosomal mediated cell death pathway. J. Biol. Chem. 2012, 287, 17618–17627. [Google Scholar] [CrossRef]
- Kelk, P.; Abd, H.; Claesson, R.; Sandstrom, G.; Sjostedt, A.; Johansson, A. Cellular and molecular response of human macrophages exposed to Aggregatibacter actinomycetemcomitans leukotoxin. Cell Death Dis. 2011, 2, e126. [Google Scholar] [CrossRef]
- Haubek, D.; Johansson, A. Pathogenicity of the highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans and its geographic dissemination and role in aggressive periodontitis. J. Oral Microbiol. 2014, 6. [Google Scholar] [CrossRef]
- Kononen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Lally, E.T.; Golub, E.E.; Kieba, I.R. Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. J. Biol. Chem. 1994, 269, 31289–31295. [Google Scholar]
- Lally, E.T.; Kieba, I.R.; Sato, A.; Green, C.L.; Rosenbloom, J.; Korostoff, J.; Wang, J.F.; Shenker, B.J.; Ortlepp, S.; Robinson, M.K.; et al. RTX toxins recognize a beta2 integrin on the surface of human target cells. J. Biol. Chem. 1997, 272, 30463–30469. [Google Scholar] [CrossRef]
- Dileepan, T.; Kachlany, S.C.; Balashova, N.V.; Patel, J.; Maheswaran, S.K. Human CD18 is the functional receptor for Aggregatibacter actinomycetemcomitans leukotoxin. Infect. Immun. 2007, 75, 4851–4856. [Google Scholar] [CrossRef]
- Kieba, I.R.; Fong, K.P.; Tang, H.Y.; Hoffman, K.E.; Speicher, D.W.; Klickstein, L.B.; Lally, E.T. Aggregatibacter actinomycetemcomitans leukotoxin requires beta-sheets 1 and 2 of the human CD11a beta-propeller for cytotoxicity. Cell. Microbiol. 2007, 9, 2689–2699. [Google Scholar] [CrossRef]
- Ristow, L.C.; Tran, V.; Schwartz, K.J.; Pankratz, L.; Mehle, A.; Sauer, J.D.; Welch, R.A. The Extracellular Domain of the beta2 Integrin beta Subunit (CD18) Is Sufficient for Escherichia coli Hemolysin and Aggregatibacter actinomycetemcomitans Leukotoxin Cytotoxic Activity. mBio 2019, 10. [Google Scholar] [CrossRef]
- Lally, E.T.; Hill, R.B.; Kieba, I.R.; Korostoff, J. The interaction between RTX toxins and target cells. Trends Microbiol. 1999, 7, 356–361. [Google Scholar] [CrossRef]
- Kittichotirat, W.; Bumgarner, R.E.; Asikainen, S.; Chen, C. Identification of the pangenome and its components in 14 distinct Aggregatibacter actinomycetemcomitans strains by comparative genomic analysis. PLoS ONE 2011, 6, e22420. [Google Scholar] [CrossRef]
- Haubek, D. The highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans: Evolutionary aspects, epidemiology and etiological role in aggressive periodontitis. APMIS Suppl. 2010, 1–53. [Google Scholar] [CrossRef]
- Zambon, J.J. Actinobacillus actinomycetemcomitans in adult periodontitis. J. Periodontol. 1994, 65, 892–893. [Google Scholar] [CrossRef]
- Haubek, D.; Ennibi, O.K.; Poulsen, K.; Vaeth, M.; Poulsen, S.; Kilian, M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: A prospective longitudinal cohort study. Lancet 2008, 371, 237–242. [Google Scholar] [CrossRef]
- Höglund Åberg, C.; Kwamin, F.; Claesson, R.; Dahlen, G.; Johansson, A.; Haubek, D. Progression of attachment loss is strongly associated with presence of the JP2 genotype of Aggregatibacter actinomycetemcomitans: A prospective cohort study of a young adolescent population. J. Clin. Periodontol. 2014, 41, 232–241. [Google Scholar] [CrossRef]
- Brogan, J.M.; Lally, E.T.; Poulsen, K.; Kilian, M.; Demuth, D.R. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: Analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect. Immun. 1994, 62, 501–508. [Google Scholar]
- Tsai, C.C.; Ho, Y.P.; Chou, Y.S.; Ho, K.Y.; Wu, Y.M.; Lin, Y.C. Aggregatibacter (Actinobacillus) actimycetemcomitans leukotoxin and human periodontitis—A historic review with emphasis on JP2. Kaohsiung J. Med. Sci. 2018, 34, 186–193. [Google Scholar] [CrossRef]
- Zambon, J.J.; Haraszthy, V.I.; Hariharan, G.; Lally, E.T.; Demuth, D.R. The Microbiology of Early-Onset Periodontitis: Association of Highly Toxic Actinobacillus actinomycetemcomitans Strains with Localized Juvenile Periodontitis. J. Periodontol. 1996, 67 (Suppl. 3S), 282–290. [Google Scholar] [CrossRef]
- Aberg, C.H.; Kwamin, F.; Claesson, R.; Johansson, A.; Haubek, D. Presence of JP2 and Non-JP2 Genotypes of Aggregatibacter actinomycetemcomitans and attachment loss in adolescents in Ghana. J. Periodontol. 2012, 83, 1520–1528. [Google Scholar] [CrossRef]
- Claesson, R.; Lagervall, M.; Hoglund-Aberg, C.; Johansson, A.; Haubek, D. Detection of the highly leucotoxic JP2 clone of Aggregatibacter actinomycetemcomitans in members of a Caucasian family living in Sweden. J. Clin. Periodontol. 2011, 38, 115–121. [Google Scholar] [CrossRef]
- Claesson, R.; Höglund-Åberg, C.; Haubek, D.; Johansson, A. Age-related prevalence and characteristics of Aggregatibacter actinomycetemcomitans in periodontitis patients living in Sweden. J. Oral Microbiol. 2017, 9, 1334504. [Google Scholar] [CrossRef]
- He, T.; Nishihara, T.; Demuth, D.R.; Ishikawa, I. A novel insertion sequence increases the expression of leukotoxicity in Actinobacillus actinomycetemcomitans clinical isolates. J. Periodontol. 1999, 70, 1261–1268. [Google Scholar] [CrossRef]
- Claesson, R.; Gudmundson, J.; Åberg, C.H.; Haubek, D.; Johansson, A. Detection of a 640-bp deletion in the Aggregatibacter actinomycetemcomitans leukotoxin promoter region in isolates from an adolescent of Ethiopian origin. J. Oral Microbiol. 2015, 7, 26974. [Google Scholar] [CrossRef]
- Höglund Åberg, C.; Haubek, D.; Kwamin, F.; Johansson, A.; Claesson, R. Leukotoxic activity of Aggregatibacter actinomycetemcomitans and periodontal attachment loss. PLoS ONE 2014, 9, e104095. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Claesson, R.; Höglund Åberg, C.; Haubek, D.; Lindholm, M.; Jasim, S.; Oscarsson, J. Genetic Profiling of Aggregatibacter actinomycetemcomitans Serotype B Isolated from Periodontitis Patients Living in Sweden. Pathogenes 2019, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Claesson, R.; Höglund Åberg, C.; Haubek, D.; Oscarsson, J. The cagE gene sequence as a diagnostic marker to identify JP2 and non-JP2 highly leukotoxic Aggregatibacter actinomycetemcomitans serotype b strains. J. Periodontal Res. 2017, 52, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Banuelos, E.; Mukherjee, A.; Darrah, E.; Andrade, F. Rheumatoid Arthritis-Associated Mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J. Clin. Med. 2019, 8, 1309. [Google Scholar] [CrossRef]
- Skals, M.; Greve, A.S.; Fagerberg, S.K.; Johnsen, N.; Christensen, M.G.; Praetorius, H.A. P2X1 receptor blockers reduce the number of circulating thrombocytes and the overall survival of urosepsis with haemolysin-producing Escherichia coli. Purinergic Signal. 2019, 15, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Kachlany, S.C. Aggregatibacter actinomycetemcomitans leukotoxin: From threat to therapy. J. Dent. Res. 2010, 89, 561–570. [Google Scholar] [CrossRef]
- Linhartova, I.; Bumba, L.; Masin, J.; Basler, M.; Osicka, R.; Kamanova, J.; Prochazkova, K.; Adkins, I.; Hejnova-Holubova, J.; Sadilkova, L.; et al. RTX proteins: A highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 2010, 34, 1076–1112. [Google Scholar] [CrossRef]
- Harding, C.M.; Pulido, M.R.; Di Venanzio, G.; Kinsella, R.L.; Webb, A.I.; Scott, N.E.; Pachon, J.; Feldman, M.F. Pathogenic Acinetobacter species have a functional type I secretion system and contact-dependent inhibition systems. J. Biol. Chem. 2017, 292, 9075–9087. [Google Scholar] [CrossRef]
- Crosby, J.A.; Kachlany, S.C. TdeA, a TolC-like protein required for toxin and drug export in Aggregatibacter (Actinobacillus) actinomycetemcomitans. Gene 2007, 388, 83–92. [Google Scholar] [CrossRef]
- Gallant, C.V.; Sedic, M.; Chicoine, E.A.; Ruiz, T.; Mintz, K.P. Membrane morphology and leukotoxin secretion are associated with a novel membrane protein of Aggregatibacter actinomycetemcomitans. J. Bacteriol. 2008, 190, 5972–5980. [Google Scholar] [CrossRef]
- Berthold, P.; Forti, D.; Kieba, I.R.; Rosenbloom, J.; Taichman, N.S.; Lally, E.T. Electron immunocytochemical localization of Actinobacillus actinomycetemcomitans leukotoxin. Oral Microbiol. Immunol. 1992, 7, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Kowashi, Y.; Demuth, D.R. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb. Pathog. 2002, 32, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Kato, K.; Kokeguchi, S.; Hara, H.; Fukui, K.; Murayama, Y. Nuclease-sensitive binding of an Actinobacillus actinomycetemcomitans leukotoxin to the bacterial cell surface. Infect. Immun. 1991, 59, 4599–4605. [Google Scholar] [PubMed]
- Ohta, H.; Hara, H.; Fukui, K.; Kurihara, H.; Murayama, Y.; Kato, K. Association of Actinobacillus actinomycetemcomitans leukotoxin with nucleic acids on the bacterial cell surface. Infect. Immun. 1993, 61, 4878–4884. [Google Scholar]
- Johansson, A.; Hanstrom, L.; Kalfas, S. Inhibition of Actinobacillus actinomycetemcomitans leukotoxicity by bacteria from the subgingival flora. Oral Microbiol. Immunol. 2000, 15, 218–225. [Google Scholar] [CrossRef]
- Johansson, A.; Claesson, R.; Hanstrom, L.; Kalfas, S. Serum-mediated release of leukotoxin from the cell surface of the periodontal pathogen Actinobacillus actinomycetemcomitans. Eur. J. Oral Sci. 2003, 111, 209–215. [Google Scholar] [CrossRef]
- Balashova, N.V.; Diaz, R.; Balashov, S.V.; Crosby, J.A.; Kachlany, S.C. Regulation of Aggregatibacter (Actinobacillus) actinomycetemcomitans leukotoxin secretion by iron. J. Bacteriol. 2006, 188, 8658–8661. [Google Scholar] [CrossRef]
- Reinholdt, J.; Poulsen, K.; Brinkmann, C.R.; Hoffmann, S.V.; Stapulionis, R.; Enghild, J.J.; Jensen, U.B.; Boesen, T.; Vorup-Jensen, T. Monodisperse and LPS-free Aggregatibacter actinomycetemcomitans leukotoxin: Interactions with human beta2 integrins and erythrocytes. Biochim. Biophys. Acta 2013, 1834, 546–558. [Google Scholar] [CrossRef]
- Brage, M.; Holmlund, A.; Johansson, A. Humoral immune response to Aggregatibacter actinomycetemcomitans leukotoxin. J. Periodontal Res. 2011, 46, 170–175. [Google Scholar] [CrossRef]
- Johansson, A.; Buhlin, K.; Sorsa, T.; Pussinen, P.J. Systemic Aggregatibacter actinomycetemcomitans Leukotoxin-Neutralizing Antibodies in Periodontitis. J. Periodontol. 2017, 88, 122–129. [Google Scholar] [CrossRef]
- Johansson, A.; Claesson, R.; Belibasakis, G.; Makoveichuk, E.; Hanstrom, L.; Olivecrona, G.; Sandstrom, G.; Kalfas, S. Protease inhibitors, the responsible components for the serum-dependent enhancement of Actinobacillus actinomycetemcomitans leukotoxicity. Eur. J. Oral Sci. 2001, 109, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Balashova, N.V.; Park, D.H.; Patel, J.K.; Figurski, D.H.; Kachlany, S.C. Interaction between leukotoxin and Cu, Zn superoxide dismutase in Aggregatibacter actinomycetemcomitans. Infect. Immun. 2007, 75, 4490–4497. [Google Scholar] [CrossRef] [PubMed]
- McArthur, W.P.; Tsai, C.C.; Baehni, P.C.; Genco, R.J.; Taichman, N.S. Leukotoxic effects of Actinobacillus actinomycetemcomitans. Modulation by serum components. J. Periodontal Res. 1981, 16, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Claesson, R.; Belibasakis, G.; Makoveichuk, E.; Hanstrom, L.; Olivecrona, G.; Kalfas, S. Lack of lipoprotein-dependent effects on the cytotoxic interactions of Actinobacillus actinomycetemcomitans leukotoxin with human neutrophils. APMIS 2002, 110, 857–862. [Google Scholar] [CrossRef]
- Hritz, M.; Fisher, E.; Demuth, D.R. Differential regulation of the leukotoxin operon in highly leukotoxic and minimally leukotoxic strains of Actinobacillus actinomycetemcomitans. Infect. Immun. 1996, 64, 2724–2729. [Google Scholar]
- Tsai, C.C.; McArthur, W.P.; Baehni, P.C.; Hammond, B.F.; Taichman, N.S. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect. Immun. 1979, 25, 427–439. [Google Scholar]
- Zambon, J.J.; DeLuca, C.; Slots, J.; Genco, R.J. Studies of leukotoxin from Actinobacillus actinomycetemcomitans using the promyelocytic HL-60 cell line. Infect. Immun. 1983, 40, 205–212. [Google Scholar]
- Sampathkumar, V.; Velusamy, S.K.; Godboley, D.; Fine, D.H. Increased leukotoxin production: Characterization of 100 base pairs within the 530 base pair leukotoxin promoter region of Aggregatibacter actinomycetemcomitans. Sci. Rep. 2017, 7, 1887. [Google Scholar] [CrossRef]
- Kelk, P.; Claesson, R.; Chen, C.; Sjostedt, A.; Johansson, A. IL-1beta secretion induced by Aggregatibacter (Actinobacillus) actinomycetemcomitans is mainly caused by the leukotoxin. Int. J. Med. Microbiol. IJMM 2008, 298, 529–541. [Google Scholar] [CrossRef]
- Umeda, J.E.; Longo, P.L.; Simionato, M.R.; Mayer, M.P. Differential transcription of virulence genes in Aggregatibacter actinomycetemcomitans serotypes. J. Oral Microbiol. 2013, 5. [Google Scholar] [CrossRef]
- Lara-Tejero, M.; Galan, J.E. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 2000, 290, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Boesze-Battaglia, K.; Besack, D.; McKay, T.; Zekavat, A.; Otis, L.; Jordan-Sciutto, K.; Shenker, B.J. Cholesterol-rich membrane microdomains mediate cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal-distending toxin. Cell Microbiol. 2006, 8, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Boesze-Battaglia, K.; Alexander, D.; Dlakic, M.; Shenker, B.J. A Journey of Cytolethal Distending Toxins through Cell Membranes. Front. Cell. Infect. Microbiol. 2016, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Fais, T.; Delmas, J.; Serres, A.; Bonnet, R.; Dalmasso, G. Impact of CDT Toxin on Human Diseases. Toxins 2016, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.; Johansson, A.; Wang, Y.; Claesson, R.; Chen, C.; Asikainen, S.; Kalfas, S. Inhibited proliferation of human periodontal ligament cells and gingival fibroblasts by Actinobacillus actinomycetemcomitans: Involvement of the cytolethal distending toxin. Eur. J. Oral Sci. 2002, 110, 366–373. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Mattsson, A.; Wang, Y.; Chen, C.; Johansson, A. Cell cycle arrest of human gingival fibroblasts and periodontal ligament cells by Actinobacillus actinomycetemcomitans: Involvement of the cytolethal distending toxin. APMIS 2004, 112, 674–685. [Google Scholar] [CrossRef]
- Kang, P.; Korostoff, J.; Volgina, A.; Grzesik, W.; DiRienzo, J.M. Differential effect of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans on co-cultures of human oral cells. J. Med. Microbiol. 2005, 54, 785–794. [Google Scholar] [CrossRef]
- Alaoui-El-Azher, M.; Mans, J.J.; Baker, H.V.; Chen, C.; Progulske-Fox, A.; Lamont, R.J.; Handfield, M. Role of the ATM-checkpoint kinase 2 pathway in CDT-mediated apoptosis of gingival epithelial cells. PLoS ONE 2010, 5, e11714. [Google Scholar] [CrossRef]
- Damek-Poprawa, M.; Haris, M.; Volgina, A.; Korostoff, J.; DiRienzo, J.M. Cytolethal distending toxin damages the oral epithelium of gingival explants. J. Dent. Res. 2011, 90, 874–879. [Google Scholar] [CrossRef]
- Kanno, F.; Korostoff, J.; Volgina, A.; DiRienzo, J.M. Resistance of human periodontal ligament fibroblasts to the cytolethal distending toxin of Actinobacillus actinomycetemcomitans. J. Periodontol. 2005, 76, 1189–1201. [Google Scholar] [CrossRef]
- Damek-Poprawa, M.; Korostoff, J.; Gill, R.; DiRienzo, J.M. Cell junction remodeling in gingival tissue exposed to a microbial toxin. J. Dent. Res. 2013, 92, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Miyauchi, M.; Tsuruda, K.; Takata, T.; Sugai, M. Topical application of Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces cell cycle arrest in the rat gingival epithelium in vivo. J. Periodontal Res. 2011, 46, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; McKay, T.; Datar, S.; Miller, M.; Chowhan, R.; Demuth, D. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J. Immunol. 1999, 162, 4773–4780. [Google Scholar] [PubMed]
- Sato, T.; Koseki, T.; Yamato, K.; Saiki, K.; Konishi, K.; Yoshikawa, M.; Ishikawa, I.; Nishihara, T. p53-independent expression of p21(CIP1/WAF1) in plasmacytic cells during G(2) cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Infect. Immun. 2002, 70, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Akifusa, S.; Poole, S.; Lewthwaite, J.; Henderson, B.; Nair, S.P. Recombinant Actinobacillus actinomycetemcomitans cytolethal distending toxin proteins are required to interact to inhibit human cell cycle progression and to stimulate human leukocyte cytokine synthesis. Infect. Immun. 2001, 69, 5925–5930. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Bostanci, N. Inflammatory and bone remodeling responses to the cytolethal distending toxins. Cells 2014, 3, 236–246. [Google Scholar] [CrossRef]
- Oscarsson, J.; Karched, M.; Thay, B.; Chen, C.; Asikainen, S. Proinflammatory effect in whole blood by free soluble bacterial components released from planktonic and biofilm cells. BMC Microbiol. 2008, 8, 206. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Johansson, A. Aggregatibacter actinomycetemcomitans targets NLRP3 and NLRP6 inflammasome expression in human mononuclear leukocytes. Cytokine 2012, 59, 124–130. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N. The RANKL-OPG system in clinical periodontology. J. Clin. Periodontol. 2012, 39, 239–248. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Johansson, A.; Wang, Y.; Chen, C.; Kalfas, S.; Lerner, U.H. The cytolethal distending toxin induces receptor activator of NF-kappaB ligand expression in human gingival fibroblasts and periodontal ligament cells. Infect. Immun. 2005, 73, 342–351. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Johansson, A.; Wang, Y.; Chen, C.; Lagergård, T.; Kalfas, S.; Lerner, U.H. Cytokine responses of human gingival fibroblasts to Actinobacillus actinomycetemcomitans cytolethal distending toxin. Cytokine 2005, 30, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Brage, M.; Lagergard, T.; Johansson, A. Cytolethal distending toxin upregulates RANKL expression in Jurkat T-cells. APMIS 2008, 116, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, D.; Ando-Suguimoto, E.S.; Bueno-Silva, B.; DiRienzo, J.M.; Mayer, M.P. Alteration of Homeostasis in Pre-osteoclasts Induced by Aggregatibacter actinomycetemcomitans CDT. Front. Cell. Infect. Microbiol. 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Perry, M.B.; MacLean, L.L.; Furgang, D.; Wilson, M.E.; Fine, D.H. Structural and genetic analyses of O polysaccharide from Actinobacillus actinomycetemcomitans serotype f. Infect. Immun. 2001, 69, 5375–5384. [Google Scholar] [CrossRef]
- Perry, M.B.; MacLean, L.M.; Brisson, J.R.; Wilson, M.E. Structures of the antigenic O-polysaccharides of lipopolysaccharides produced by Actinobacillus actinomycetemcomitans serotypes a, c, d and e. Eur. J. Biochem. 1996, 242, 682–688. [Google Scholar] [CrossRef]
- Perry, M.B.; MacLean, L.L.; Gmur, R.; Wilson, M.E. Characterization of the O-polysaccharide structure of lipopolysaccharide from Actinobacillus actinomycetemcomitans serotype b. Infect. Immun. 1996, 64, 1215–1219. [Google Scholar]
- Masoud, H.; Weintraub, S.T.; Wang, R.; Cotter, R.; Holt, S.C. Investigation of the structure of lipid A from Actinobacillus actinomycetemcomitans strain Y4 and human clinical isolate PO 1021-7. Eur. J. Biochem. 1991, 200, 775–781. [Google Scholar] [CrossRef]
- Suzuki, N.; Nakano, Y.; Yoshida, Y.; Ikeda, D.; Koga, T. Identification of Actinobacillus actinomycetemcomitans serotypes by multiplex PCR. J. Clin. Microbiol. 2001, 39, 2002–2005. [Google Scholar] [CrossRef]
- Brondz, I.; Olsen, I. Chemical differences in lipopolysaccharides from Actinobacillus (Haemophilus) actinomycetemcomitans and Haemophilus aphrophilus: Clues to differences in periodontopathogenic potential and taxonomic distinction. Infect. Immun. 1989, 57, 3106–3109. [Google Scholar]
- Brondz, I.; Olsen, I. Determination of acids in whole lipopolysaccharide and in free lipid A from Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. J. Chromatogr. 1984, 308, 19–29. [Google Scholar] [CrossRef]
- Bryn, K.; Jantzen, E. Analysis of lipopolysaccharides by methanolysis, trifluoroacetylation, and gas chromatography on a fused-silica capillary column. J. Chromatogr. 1982, 240, 405–413. [Google Scholar] [CrossRef]
- Kiley, P.; Holt, S.C. Characterization of the lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4 and N27. Infect. Immun. 1980, 30, 862–873. [Google Scholar] [PubMed]
- Schneider, B.; Weigel, W.; Sztukowska, M.; Demuth, D.R. Identification and functional characterization of type II toxin/antitoxin systems in Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2018, 33, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, P.K.; Meyer, D.H.; Fives-Taylor, P.M. Factors influencing the growth and viability of Actinobacillus actinomycetemcomitans. Oral Microbiol. Immunol. 1993, 8, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Brondz, I.; Olsen, I. Differentiation between Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus based on carbohydrates in lipopolysaccharide. J. Chromatogr. 1984, 310, 261–272. [Google Scholar] [CrossRef]
- Brondz, I.; Olsen, I. Multivariate analyses of carbohydrate data from lipopolysaccharides of Actinobacillus (Haemophilus) actinomycetemcomitans, Haemophilus aphrophilus, and Haemophilus paraphrophilus. Int. J. Syst. Bacteriol. 1990, 40, 405–408. [Google Scholar] [CrossRef]
- Patil, C.; Rossa, C., Jr.; Kirkwood, K.L. Actinobacillus actinomycetemcomitans lipopolysaccharide induces interleukin-6 expression through multiple mitogen-activated protein kinase pathways in periodontal ligament fibroblasts. Oral Microbiol. Immunol. 2006, 21, 392–398. [Google Scholar] [CrossRef]
- Rogers, J.E.; Li, F.; Coatney, D.D.; Rossa, C.; Bronson, P.; Krieder, J.M.; Giannobile, W.V.; Kirkwood, K.L. Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J. Periodontol. 2007, 78, 550–558. [Google Scholar] [CrossRef]
- Morishita, M.; Ariyoshi, W.; Okinaga, T.; Usui, M.; Nakashima, K.; Nishihara, T. A. actinomycetemcomitans LPS enhances foam cell formation induced by LDL. J. Dent. Res. 2013, 92, 241–246. [Google Scholar] [CrossRef]
- Park, O.J.; Cho, M.K.; Yun, C.H.; Han, S.H. Lipopolysaccharide of Aggregatibacter actinomycetemcomitans induces the expression of chemokines MCP-1, MIP-1alpha, and IP-10 via similar but distinct signaling pathways in murine macrophages. Immunobiology 2015, 220, 1067–1074. [Google Scholar] [CrossRef]
- Valerio, M.S.; Herbert, B.A.; Basilakos, D.S.; Browne, C.; Yu, H.; Kirkwood, K.L. Critical role of MKP-1 in lipopolysaccharide-induced osteoclast formation through CXCL1 and CXCL2. Cytokine 2015, 71, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Reese, T.A. Making Mouse Models That Reflect Human Immune Responses. Trends Immunol. 2017, 38, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Mitani, A.; Mogi, M.; Kikuchi, T.; Fujimura, T.; Takeda, H.; Hishikawa, T.; Yamamoto, G.; Hayashi, J.; Ishihara, Y.; et al. Aggregatibacter actinomycetemcomitans lipopolysaccharide stimulated epithelial cells produce interleukin-15 that regulates T cell activation. Arch. Oral Biol. 2013, 58, 1541–1548. [Google Scholar] [CrossRef]
- Takahashi, N.; Kobayashi, M.; Takaki, T.; Takano, K.; Miyata, M.; Okamatsu, Y.; Hasegawa, K.; Nishihara, T.; Yamamoto, M. Actinobacillus actinomycetemcomitans lipopolysaccharide stimulates collagen phagocytosis by human gingival fibroblasts. Oral Microbiol. Immunol. 2008, 23, 259–264. [Google Scholar] [CrossRef]
- Xiao, Y.; Bunn, C.L.; Bartold, P.M. Effect of lipopolysaccharide from periodontal pathogens on the production of tissue plasminogen activator and plasminogen activator inhibitor 2 by human gingival fibroblasts. J. Periodontal Res. 2001, 36, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.R.; Fordham, J.B.; Khan, A.; Nares, S. MicroRNAs responsive to Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis LPS modulate expression of genes regulating innate immunity in human macrophages. Innate Immun. 2014, 20, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef]
- Gocek, E.; Wang, X.; Liu, X.; Liu, C.G.; Studzinski, G.P. MicroRNA-32 upregulation by 1,25-dihydroxyvitamin D3 in human myeloid leukemia cells leads to Bim targeting and inhibition of AraC-induced apoptosis. Cancer Res. 2011, 71, 6230–6239. [Google Scholar] [CrossRef]
- Sosroseno, W.; Bird, P.S.; Seymour, G.J. Nitric oxide production by a human osteoblast cell line stimulated with Aggregatibacter actinomycetemcomitans lipopolysaccharide. Oral Microbiol. Immunol. 2009, 24, 50–55. [Google Scholar] [CrossRef]
- Ralston, S.H.; Grabowski, P.S. Mechanisms of cytokine induced bone resorption: Role of nitric oxide, cyclic guanosine monophosphate, and prostaglandins. Bone 1996, 19, 29–33. [Google Scholar] [CrossRef]
- Pollanen, M.T.; Salonen, J.I.; Grenier, D.; Uitto, V.J. Epithelial cell response to challenge of bacterial lipoteichoic acids and lipopolysaccharides in vitro. J. Med. Microbiol. 2000, 49, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ohguchi, Y.; Ishihara, Y.; Ohguchi, M.; Koide, M.; Shirozu, N.; Naganawa, T.; Nishihara, T.; Noguchi, T. Capsular polysaccharide from Actinobacillus actinomycetemcomitans inhibits IL-6 and IL-8 production in human gingival fibroblast. J. Periodontal Res. 2003, 38, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Hahn, C.L.; Tanaka, S.; Barbour, S.E.; Schenkein, H.A.; Tew, J.G. Dendritic cells stimulated with Actinobacillus actinomycetemcomitans elicit rapid gamma interferon responses by natural killer cells. Infect. Immun. 2004, 72, 5089–5096. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diaz-Zuniga, J.; Yanez, J.P.; Alvarez, C.; Melgar-Rodriguez, S.; Hernandez, M.; Sanz, M.; Vernal, R. Serotype-dependent response of human dendritic cells stimulated with Aggregatibacter actinomycetemcomitans. J. Clin. Periodontol. 2014, 41, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Aida, Y.; Kukita, T.; Takada, H.; Maeda, K.; Pabst, M.J. Lipopolysaccharides from periodontal pathogens prime neutrophils for enhanced respiratory burst: Differential effect of a synthetic lipid a precursor IVA (LA-14-PP). J. Periodontal Res. 1995, 30, 116–123. [Google Scholar] [CrossRef]
- Yoshimura, A.; Hara, Y.; Kaneko, T.; Kato, I. Secretion of IL-1 beta, TNF-alpha, IL-8 and IL-1ra by human polymorphonuclear leukocytes in response to lipopolysaccharides from periodontopathic bacteria. J. Periodontal Res. 1997, 32, 279–286. [Google Scholar] [CrossRef]
- Schytte Blix, I.J.; Helgeland, K.; Hvattum, E.; Lyberg, T. Lipopolysaccharide from Actinobacillus actinomycetemcomitans stimulates production of interleukin-1beta, tumor necrosis factor-alpha, interleukin-6 and interleukin-1 receptor antagonist in human whole blood. J. Periodontal Res. 1999, 34, 34–40. [Google Scholar] [CrossRef]
- Blix, I.J.; Helgeland, K.; Kahler, H.; Lyberg, T. LPS from Actinobacillus actinomycetemcomitans and the expression of beta2 integrins and L-selectin in an ex vivo human whole blood system. Eur. J. Oral Sci. 1999, 107, 14–20. [Google Scholar] [CrossRef]
- Grenier, D.; Leduc, A.; Mayrand, D. Interaction between Actinobacillus actinomycetemcomitans lipopolysaccharides and human hemoglobin. FEMS Microbiol. Lett. 1997, 151, 77–81. [Google Scholar] [CrossRef][Green Version]
- Ahlstrand, T.; Kovesjoki, L.; Maula, T.; Oscarsson, J.; Ihalin, R. Aggregatibacter actinomycetemcomitans LPS binds human interleukin-8. J. Oral Microbiol. 2019, 11, 1549931. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S162–S170. [Google Scholar] [CrossRef]
- Barbour, S.E.; Ishihara, Y.; Fakher, M.; Al-Darmaki, S.; Caven, T.H.; Shelburne, C.P.; Best, A.M.; Schenkein, H.A.; Tew, J.G. Monocyte differentiation in localized juvenile periodontitis is skewed toward the dendritic cell phenotype. Infect. Immun. 2002, 70, 2780–2786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diaz-Zuniga, J.; Melgar-Rodriguez, S.; Alvarez, C.; Monasterio, G.; Benitez, A.; Ciuchi, P.; Diaz, C.; Mardones, J.; Escobar, A.; Sanz, M.; et al. T-lymphocyte phenotype and function triggered by Aggregatibacter actinomycetemcomitans is serotype-dependent. J. Periodontal Res. 2015, 50, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Nicu, E.A.; Loos, B.G. Polymorphonuclear neutrophils in periodontitis and their possible modulation as a therapeutic approach. Periodontology 2000 2016, 71, 140–163. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Kawai, T.; Komatsuzawa, H.; Mintz, K.P. Lipopolysaccharides mediate leukotoxin secretion in Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2012, 27, 70–82. [Google Scholar] [CrossRef]
- Ahlstrand, T.; Torittu, A.; Elovaara, H.; Valimaa, H.; Pollanen, M.T.; Kasvandik, S.; Hogbom, M.; Ihalin, R. Interactions between the Aggregatibacter actinomycetemcomitans secretin HofQ and host cytokines indicate a link between natural competence and interleukin-8 uptake. Virulence 2018, 9, 1205–1223. [Google Scholar] [CrossRef]
- Paino, A.; Tuominen, H.; Jaaskelainen, M.; Alanko, J.; Nuutila, J.; Asikainen, S.E.; Pelliniemi, L.J.; Pollanen, M.T.; Chen, C.; Ihalin, R. Trimeric form of intracellular ATP synthase subunit beta of Aggregatibacter actinomycetemcomitans binds human interleukin-1beta. PLoS ONE 2011, 6, e18929. [Google Scholar] [CrossRef]
- Ahlstrand, T.; Tuominen, H.; Beklen, A.; Torittu, A.; Oscarsson, J.; Sormunen, R.; Pollanen, M.T.; Permi, P.; Ihalin, R. A novel intrinsically disordered outer membrane lipoprotein of Aggregatibacter actinomycetemcomitans binds various cytokines and plays a role in biofilm response to interleukin-1beta and interleukin-8. Virulence 2017, 8, 115–134. [Google Scholar] [CrossRef]
- Paino, A.; Lohermaa, E.; Sormunen, R.; Tuominen, H.; Korhonen, J.; Pollanen, M.T.; Ihalin, R. Interleukin-1beta is internalised by viable Aggregatibacter actinomycetemcomitans biofilm and locates to the outer edges of nucleoids. Cytokine 2012, 60, 565–574. [Google Scholar] [CrossRef]
- Paino, A.; Ahlstrand, T.; Nuutila, J.; Navickaite, I.; Lahti, M.; Tuominen, H.; Valimaa, H.; Lamminmaki, U.; Pollanen, M.T.; Ihalin, R. Identification of a novel bacterial outer membrane interleukin-1Beta-binding protein from Aggregatibacter actinomycetemcomitans. PLoS ONE 2013, 8, e70509. [Google Scholar] [CrossRef]
- Kanangat, S.; Postlethwaite, A.; Cholera, S.; Williams, L.; Schaberg, D. Modulation of virulence gene expression in Staphylococcus aureus by interleukin-1beta: Novel implications in bacterial pathogenesis. Microbes Infect. 2007, 9, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guina, T.; Brittnacher, M.; Nguyen, H.; Eng, J.; Miller, S.I. The Pseudomonas aeruginosa proteome during anaerobic growth. J. Bacteriol. 2005, 187, 8185–8190. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, J.; Royer, P.J.; Sjolinder, H.S.; Azimi, S.; Self, T.; Stoof, J.; Wheldon, L.M.; Brannstrom, K.; Wilson, R.; Moreton, J.; et al. Pro-inflammatory cytokines can act as intracellular modulators of commensal bacterial virulence. Open Biol. 2013, 3, 130048. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, V.; Torres, A.G.; Kaper, J.B. Quorum sensing Escherichia coli regulators B and C (QseBC): A novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 2002, 43, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.G.; Sperandio, V. The Epinephrine/Norepinephrine/Autoinducer-3 Interkingdom Signaling System in Escherichia coli O157:H7. Adv. Exp. Med. Biol. 2016, 874, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Weigel, W.A.; Demuth, D.R.; Torres-Escobar, A.; Juarez-Rodriguez, M.D. Aggregatibacter actinomycetemcomitans QseBC is activated by catecholamines and iron and regulates genes encoding proteins associated with anaerobic respiration and metabolism. Mol. Oral Microbiol. 2015, 30, 384–398. [Google Scholar] [CrossRef]
- Novak, E.A.; Shao, H.; Daep, C.A.; Demuth, D.R. Autoinducer-2 and QseC control biofilm formation and in vivo virulence of Aggregatibacter actinomycetemcomitans. Infect. Immun. 2010, 78, 2919–2926. [Google Scholar] [CrossRef]
- Deatherage, B.L.; Cookson, B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957. [Google Scholar] [CrossRef]
- Uhlin, B.E.; Oscarsson, J.; Wai, S.N. Haemolysins. In Pathogenic Escherichia Coli: Molecular and Cellular Microbiology; Morabito, S., Ed.; Caister Academic Press: Norfolk, UK, 2014; pp. 161–180. [Google Scholar]
- Rompikuntal, P.K.; Thay, B.; Khan, M.K.; Alanko, J.; Penttinen, A.M.; Asikainen, S.; Wai, S.N.; Oscarsson, J. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect. Immun. 2012, 80, 31–42. [Google Scholar] [CrossRef]
- Demuth, D.R.; James, D.; Kowashi, Y.; Kato, S. Interaction of Actinobacillus actinomycetemcomitans outer membrane vesicles with HL60 cells does not require leukotoxin. Cell. Microbiol. 2003, 5, 111–121. [Google Scholar] [CrossRef]
- Goulhen, F.; Hafezi, A.; Uitto, V.J.; Hinode, D.; Nakamura, R.; Grenier, D.; Mayrand, D. Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect. Immun. 1998, 66, 5307–5313. [Google Scholar] [PubMed]
- Karched, M.; Ihalin, R.; Eneslätt, K.; Zhong, D.; Oscarsson, J.; Wai, S.N.; Chen, C.; Asikainen, S.E. Vesicle-independent extracellular release of a proinflammatory outer membrane lipoprotein in free-soluble form. BMC Microbiol. 2008, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Kieselbach, T.; Zijnge, V.; Granström, E.; Oscarsson, J. Proteomics of Aggregatibacter actinomycetemcomitans Outer Membrane Vesicles. PLoS ONE 2015, 10, e0138591. [Google Scholar] [CrossRef] [PubMed]
- Kieselbach, T.; Oscarsson, J. Dataset of the proteome of purified outer membrane vesicles from the human pathogen Aggregatibacter actinomycetemcomintans. Data Brief 2017, 10, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Rochester, D.F. Does respiratory muscle rest relieve fatigue or incipient fatigue? Am. Rev. Respir. Dis. 1988, 138, 516–517. [Google Scholar] [CrossRef]
- Iino, Y.; Hopps, R.M. The bone-resorbing activities in tissue culture of lipopolysaccharides from the bacteria Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Capnocytophaga ochracea isolated from human mouths. Arch. Oral Biol. 1984, 29, 59–63. [Google Scholar] [CrossRef]
- Reddi, K.; Meghji, S.; Wilson, M.; Henderson, B. Comparison of the osteolytic activity of surface-associated proteins of bacteria implicated in periodontal disease. Oral Dis. 1995, 1, 26–31. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, S.C.; Hong, S.H.; Lee, H.J. Secretable Small RNAs via Outer Membrane Vesicles in Periodontal Pathogens. J. Dent. Res. 2017, 96, 458–466. [Google Scholar] [CrossRef]
- O’Donoghue, E.J.; Krachler, A.M. Mechanisms of outer membrane vesicle entry into host cells. Cell. Microbiol. 2016, 18, 1508–1517. [Google Scholar] [CrossRef]
- Kesty, N.C.; Mason, K.M.; Reedy, M.; Miller, S.E.; Kuehn, M.J. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004, 23, 4538–4549. [Google Scholar] [CrossRef]
- Nice, J.B.; Balashova, N.V.; Kachlany, S.C.; Koufos, E.; Krueger, E.; Lally, E.T.; Brown, A.C. Aggregatibacter actinomycetemcomitans Leukotoxin Is Delivered to Host Cells in an LFA-1-Indepdendent Manner When Associated with Outer Membrane Vesicles. Toxins 2018, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Ximenez-Fyvie, L.A.; Haffajee, A.D.; Socransky, S.S. Microbial composition of supra- and subgingival plaque in subjects with adult periodontitis. J. Clin. Periodontol. 2000, 27, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Noiri, Y.; Li, L.; Ebisu, S. The localization of periodontal-disease-associated bacteria in human periodontal pockets. J. Dent. Res. 2001, 80, 1930–1934. [Google Scholar] [CrossRef] [PubMed]
- Christersson, L.A.; Albini, B.; Zambon, J.J.; Wikesjo, U.M.; Genco, R.J. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. I. Light, immunofluorescence and electron microscopic studies. J. Periodontol. 1987, 58, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.V.; da Silva, C.M.; Haffajee, A.; Colombo, A.P. Identification of intracellular oral species within human crevicular epithelial cells from subjects with chronic periodontitis by fluorescence in situ hybridization. J. Periodontal Res. 2007, 42, 236–243. [Google Scholar] [CrossRef]
- Mendes, L.; Rocha, R.; Azevedo, A.S.; Ferreira, C.; Henriques, M.; Pinto, M.G.; Azevedo, N.F. Novel strategy to detect and locate periodontal pathogens: The PNA-FISH technique. Microbiol. Res. 2016, 192, 185–191. [Google Scholar] [CrossRef]
- Willi, M.; Belibasakis, G.N.; Bostanci, N. Expression and regulation of triggering receptor expressed on myeloid cells 1 in periodontal diseases. Clin. Exp. Immunol. 2014, 178, 190–200. [Google Scholar] [CrossRef]
- Thurnheer, T.; Belibasakis, G.N. Integration of non-oral bacteria into in vitro oral biofilms. Virulence 2015, 6, 258–264. [Google Scholar] [CrossRef]
- Bao, K.; Bostanci, N.; Selevsek, N.; Thurnheer, T.; Belibasakis, G.N. Quantitative proteomics reveal distinct protein regulations caused by Aggregatibacter actinomycetemcomitans within subgingival biofilms. PLoS ONE 2015, 10, e0119222. [Google Scholar] [CrossRef]
- Llama-Palacios, A.; Potupa, O.; Sanchez, M.C.; Figuero, E.; Herrera, D.; Sanz, M. Aggregatibacter actinomycetemcomitans Growth in Biofilm versus Planktonic State: Differential Expression of Proteins. J. Proteome Res. 2017, 16, 3158–3167. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Rathsam, C.; Eaton, R.E.; Simpson, C.L.; Browne, G.V.; Berg, T.; Harty, D.W.; Jacques, N.A. Up-regulation of competence- but not stress-responsive proteins accompanies an altered metabolic phenotype in Streptococcus mutans biofilms. Microbiology 2005, 151, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, K.; Fujise, O.; Miura, M.; Hamachi, T.; Maeda, K. Porphyromonas gingivalis displays a competitive advantage over Aggregatibacter actinomycetemcomitans in co-cultured biofilm. J. Periodontal Res. 2013, 48, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.P.; Voogt, R.D.; Ruiz, T.; Mintz, K.P. The conserved carboxyl domain of MorC, an inner membrane protein of Aggregatibacter actinomycetemcomitans, is essential for membrane function. Mol. Oral Microbiol. 2016, 31, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.P.; Fields, J.G.; Voogt, R.D.; Deng, B.; Lam, Y.W.; Mintz, K.P. Alteration in abundance of specific membrane proteins of Aggregatibacter actinomycetemcomitans is attributed to deletion of the inner membrane protein MorC. Proteomics 2015, 15, 1859–1867. [Google Scholar] [CrossRef]
- Zijnge, V.; Kieselbach, T.; Oscarsson, J. Proteomics of protein secretion by Aggregatibacter actinomycetemcomitans. PLoS ONE 2012, 7, e41662. [Google Scholar] [CrossRef]
- Rylev, M.; Abduljabar, A.B.; Reinholdt, J.; Ennibi, O.K.; Haubek, D.; Birkelund, S.; Kilian, M. Proteomic and immunoproteomic analysis of Aggregatibacter actinomycetemcomitans JP2 clone strain HK1651. J. Proteom. 2011, 74, 2972–2985. [Google Scholar] [CrossRef]
- Tendeng, C.; Bertin, P.N. H-NS in Gram-negative bacteria: A family of multifaceted proteins. Trends Microbiol. 2003, 11, 511–518. [Google Scholar] [CrossRef]
- Juarez, A.; Nieto, J.M.; Prenafeta, A.; Miquelay, E.; Balsalobre, C.; Carrascal, M.; Madrid, C. Interaction of the nucleoid-associated proteins Hha and H-NS to modulate expression of the hemolysin operon in Escherichia coli. Adv. Exp. Med. Biol. 2000, 485, 127–131. [Google Scholar] [CrossRef]
- Li, H.; Granat, A.; Stewart, V.; Gillespie, J.R. RpoS, H-NS, and DsrA influence EHEC hemolysin operon (ehxCABD) transcription in Escherichia coli O157:H7 strain EDL933. FEMS Microbiol. Lett. 2008, 285, 257–262. [Google Scholar] [CrossRef]
- Wang, H.; Ayala, J.C.; Benitez, J.A.; Silva, A.J. RNA-seq analysis identifies new genes regulated by the histone-like nucleoid structuring protein (H-NS) affecting Vibrio cholerae virulence, stress response and chemotaxis. PLoS ONE 2015, 10, e0118295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bao, K.; Belibasakis, G.N.; Selevsek, N.; Grossmann, J.; Bostanci, N. Proteomic profiling of host-biofilm interactions in an oral infection model resembling the periodontal pocket. Sci. Rep. 2015, 5, 15999. [Google Scholar] [CrossRef] [PubMed]
- Maughan, H.S.S.; Wilson, L.; Redfield, R. Competence, DNA Uptake and Transformation in Pasteurellaceae. In Pasteurellaceae Biology, Genomics and Molecular Aspects; Kuhnert, P., Christensen, H., Eds.; Caister Academic Press: Norfolk, UK, 2008; pp. 79–88. [Google Scholar]
- Fujise, O.; Lakio, L.; Wang, Y.; Asikainen, S.; Chen, C. Clonal distribution of natural competence in Actinobacillus actinomycetemcomitans. Oral Microbiol. Immunol. 2004, 19, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Jorth, P.; Whiteley, M. An evolutionary link between natural transformation and CRISPR adaptive immunity. mBio 2012, 3. [Google Scholar] [CrossRef]
- Redfield, R.J.; Findlay, W.A.; Bosse, J.; Kroll, J.S.; Cameron, A.D.; Nash, J.H. Evolution of competence and DNA uptake specificity in the Pasteurellaceae. BMC Evol. Biol. 2006, 6, 82. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, W.; Chen, W.; Chen, C. Type IV pilus gene homologs pilABCD are required for natural transformation in Actinobacillus actinomycetemcomitans. Gene 2003, 312, 249–255. [Google Scholar] [CrossRef]
- Tanaka, A.; Fujise, O.; Chen, C.; Miura, M.; Hamachi, T.; Maeda, K. A novel gene required for natural competence in Aggregatibacter actinomycetemcomitans. J. Periodontal Res. 2012, 47, 129–134. [Google Scholar] [CrossRef]
- Hisano, K.; Fujise, O.; Miura, M.; Hamachi, T.; Matsuzaki, E.; Nishimura, F. The pga gene cluster in Aggregatibacter actinomycetemcomitans is necessary for the development of natural competence in Ca2+-promoted biofilms. Mol. Oral Microbiol. 2014, 29, 79–89. [Google Scholar] [CrossRef]
- Wang, Y.; Goodman, S.D.; Redfield, R.J.; Chen, C. Natural transformation and DNA uptake signal sequences in Actinobacillus actinomycetemcomitans. J. Bacteriol. 2002, 184, 3442–3449. [Google Scholar] [CrossRef]


| Target | Effect | Reference |
|---|---|---|
| Epithelial cells | IL-15 expression, widening of the intercellular spaces | [113,121] |
| Gingival fibroblasts | Enhancement of collagen phagocytosis, increase the production of IL-8, IL-6, t-PA, PAI-2 | [114,115,116,122] |
| Macrophages | Upregulation of miR-146a and downregulation of miR-32 and miR-29b microRNAs | |
| Osteoblasts | Increases iNOS activity and induce NO production | [119,123,124] |
| Dendritic cells | Production of IL-12, IFN-γ, TNF-α, IL-1β, IL-6, and IL-23 | |
| PMN | Induce ROS production, stimulate IL-1β, TNF-α, and IL-6 production, downregulate surface expression of L-selectin, upregulate the expression of β2-integrins | [125,126,127,128] |
| Human hemoglobin | Binding | [129] |
| Human IL-8 | Binding | [130] |
| Author | Year (Ref) | Brief Description | Identified Proteins * | Proteomic Application, Label Free Quantification | Cutoff | PMID |
|---|---|---|---|---|---|---|
| Llama-Palacios et al. | 2017 [171] | A.a planktonic and mono-species biofilm cultures | 50 | 2DE, MALDI-TOF MS | N/A | 28707473 |
| Kieselbach T et al. | 2016 [155] | A.a outer membrane vesicles dataset | 501 | In solution digestion, LC-MS/MS | protein FDR < 1% | 28050585 |
| Kieselbach T et al. | 2015 [154] | A.a outer membrane vesicles | 151 | In solution digestion, LC-MS/MS | protein FDR < 1% | 26381655 |
| Smith KP et al. | 2015 [175] | A.a membrane proteins related to morphogenesis protein C | 613 | Stable-isotope dimethyl labeling, nanoscale LC-MS | FP < 1% | 25684173 |
| Smith KP et al. | 2015 [176] | A.a membrane proteins | 648 | Stable-isotope dimethyl labeling, nanoscale LC-MS | FP < 1% | 25055881 |
| Zijnge V et al. | 2012 [177] | A.a secreted proteins from mono-species biofilm | 179 | 2DE, HCT-Ultra ETD II IT-MS | Peptide ion score > 30 | 22848560 |
| Rylev M et al. | 2011 [178] | A.a JP2 strain HK1651 | 114 | 2DE, MALDI-TOF MS | N/A | 21867783 |
| Author | Year (Ref) | Brief Description | Identified Proteins * | Proteomic Application | Peptide Cutoff | PMID |
|---|---|---|---|---|---|---|
| Bao et al. | 2018 [169] | A. a hns + 10 species biofilms ** | 3352 | Orbitrap Fusion, LFQ | 2≥ | 25483866 |
| Bao et al. | 2015 [183] | 10-species biofilm model * Vs 3D culture ** | 3363 | Q-Exactive MS, LFQ | 2≥ | 26525412 |
| Bao et al. | 2015 [170] | A. a + 10 species biofilms ** | 3352 | Q-Exactive MS, LFQ | 2≥ | 25756960 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belibasakis, G.N.; Maula, T.; Bao, K.; Lindholm, M.; Bostanci, N.; Oscarsson, J.; Ihalin, R.; Johansson, A. Virulence and Pathogenicity Properties of Aggregatibacter actinomycetemcomitans. Pathogens 2019, 8, 222. https://doi.org/10.3390/pathogens8040222
Belibasakis GN, Maula T, Bao K, Lindholm M, Bostanci N, Oscarsson J, Ihalin R, Johansson A. Virulence and Pathogenicity Properties of Aggregatibacter actinomycetemcomitans. Pathogens. 2019; 8(4):222. https://doi.org/10.3390/pathogens8040222
Chicago/Turabian StyleBelibasakis, Georgios N., Terhi Maula, Kai Bao, Mark Lindholm, Nagihan Bostanci, Jan Oscarsson, Riikka Ihalin, and Anders Johansson. 2019. "Virulence and Pathogenicity Properties of Aggregatibacter actinomycetemcomitans" Pathogens 8, no. 4: 222. https://doi.org/10.3390/pathogens8040222
APA StyleBelibasakis, G. N., Maula, T., Bao, K., Lindholm, M., Bostanci, N., Oscarsson, J., Ihalin, R., & Johansson, A. (2019). Virulence and Pathogenicity Properties of Aggregatibacter actinomycetemcomitans. Pathogens, 8(4), 222. https://doi.org/10.3390/pathogens8040222








