Activation of Gingival Fibroblasts by Bacterial Cyclic Dinucleotides and Lipopolysaccharide
Abstract
:1. Introduction
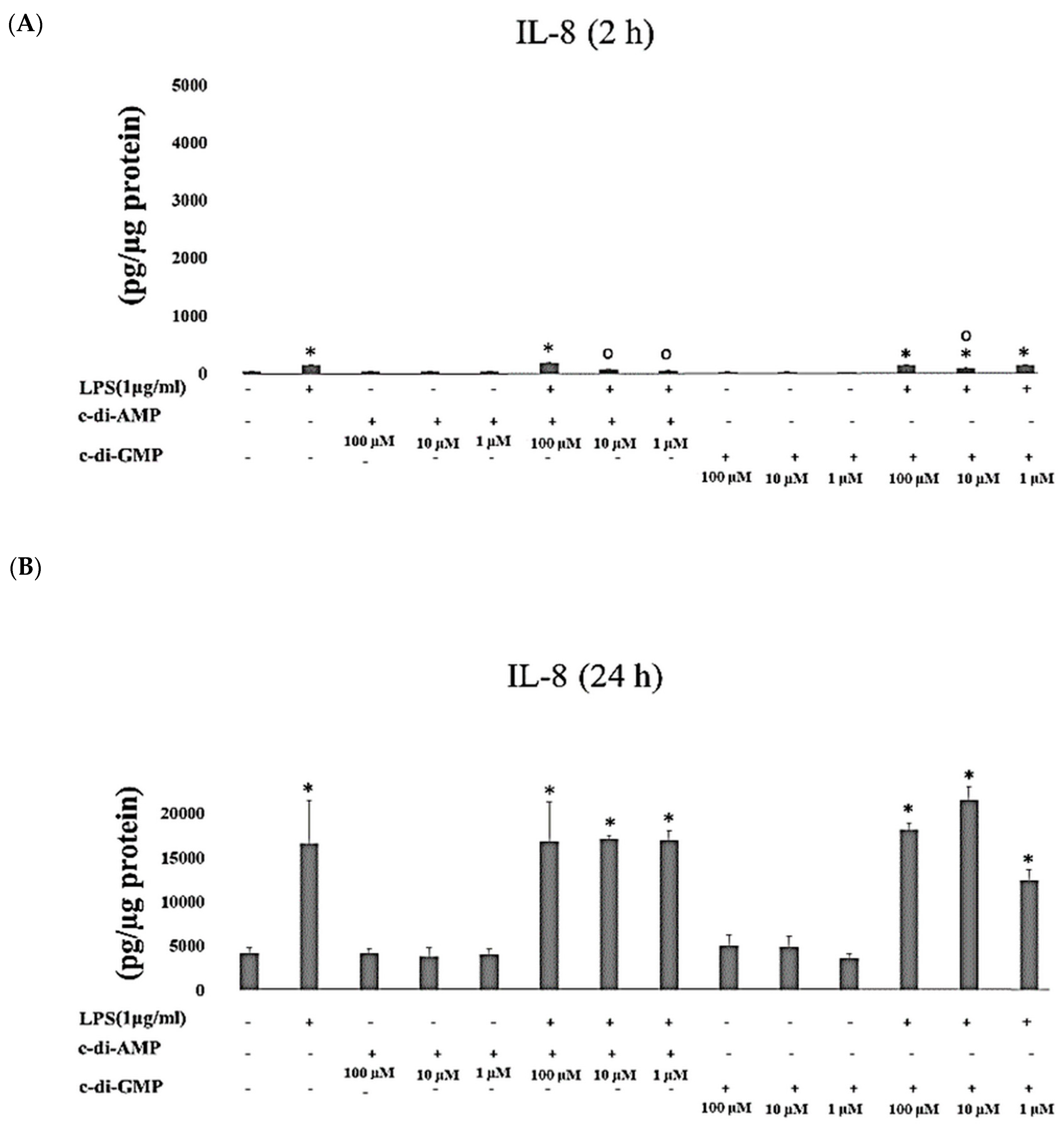
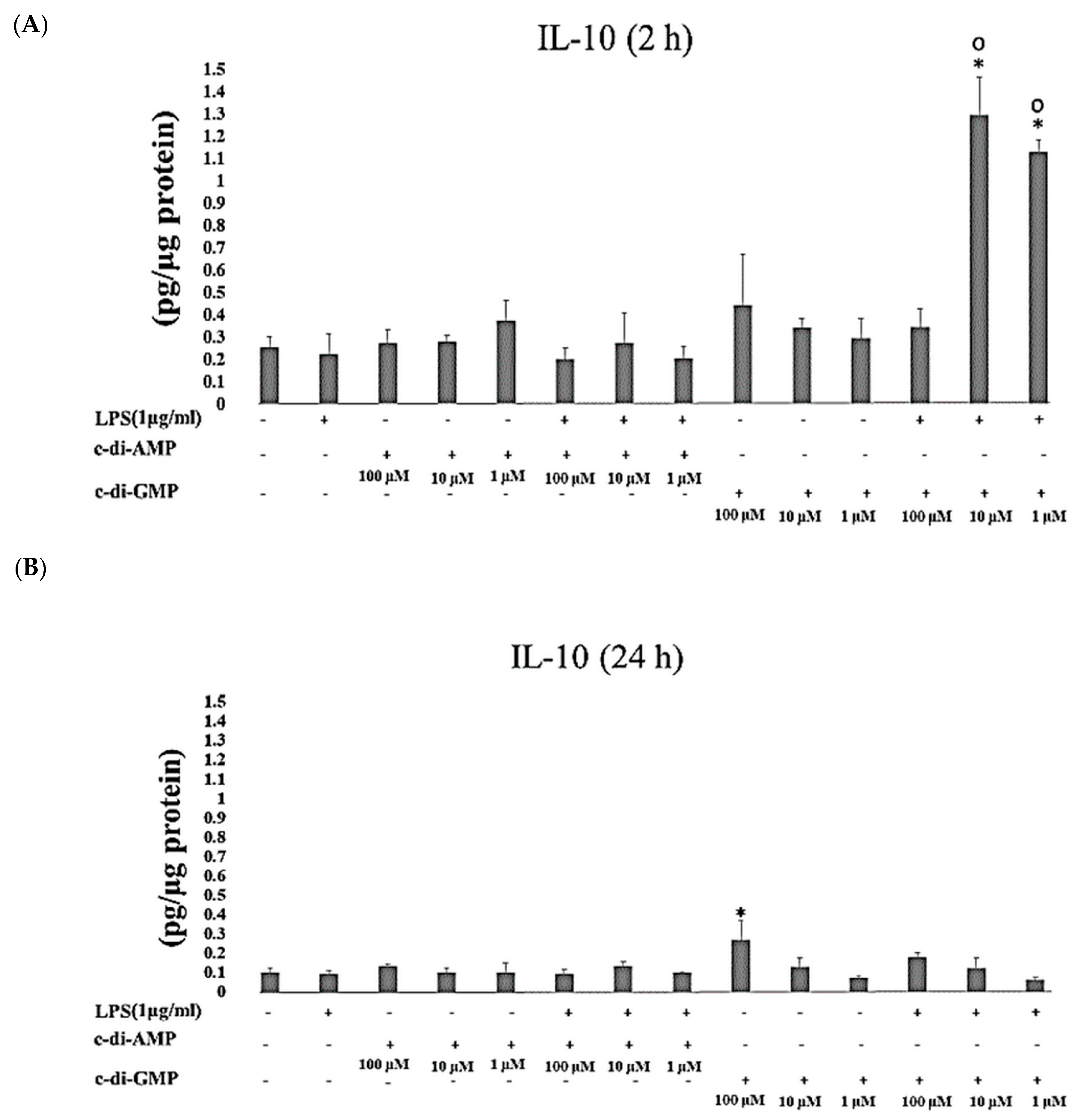
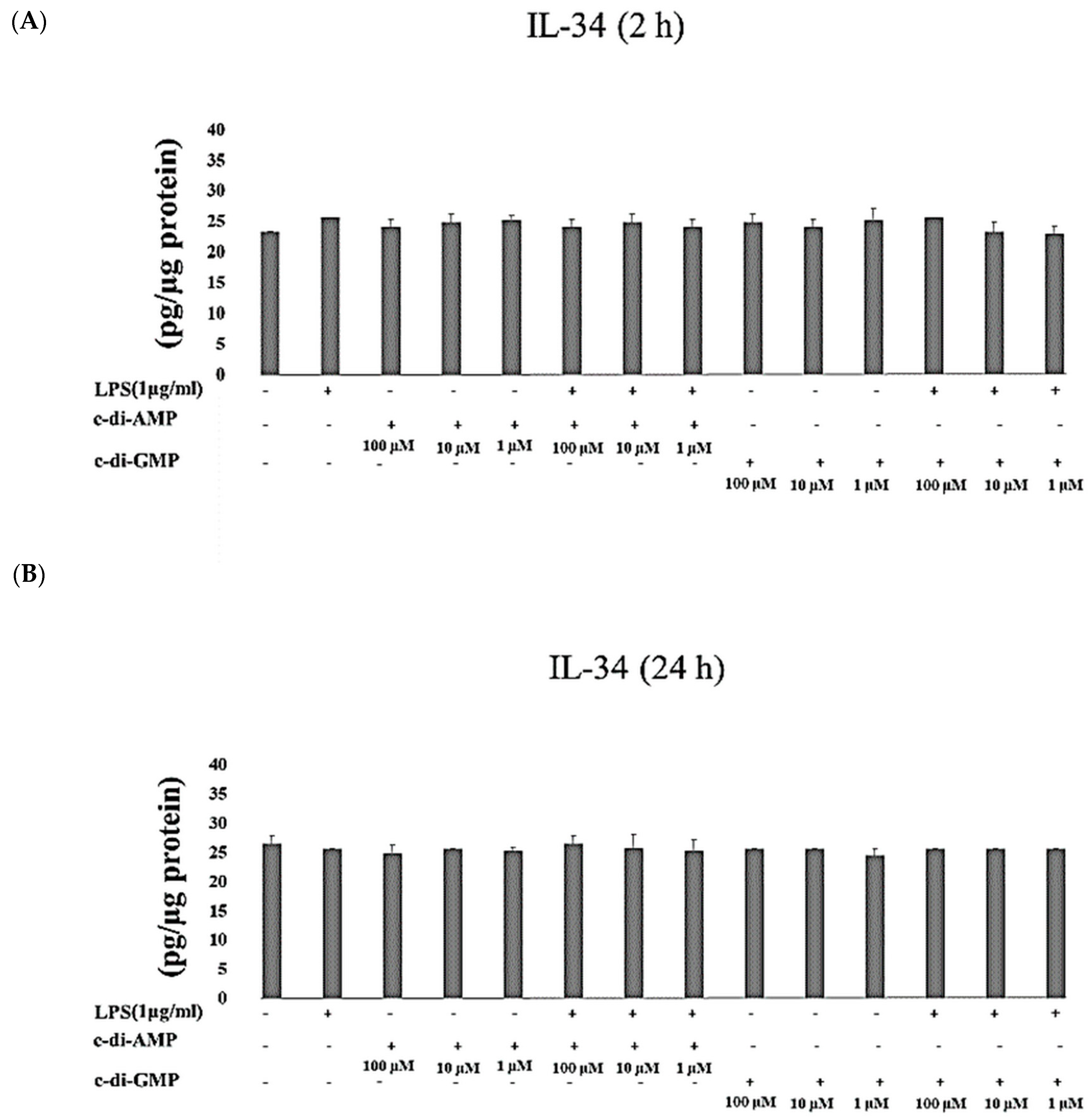
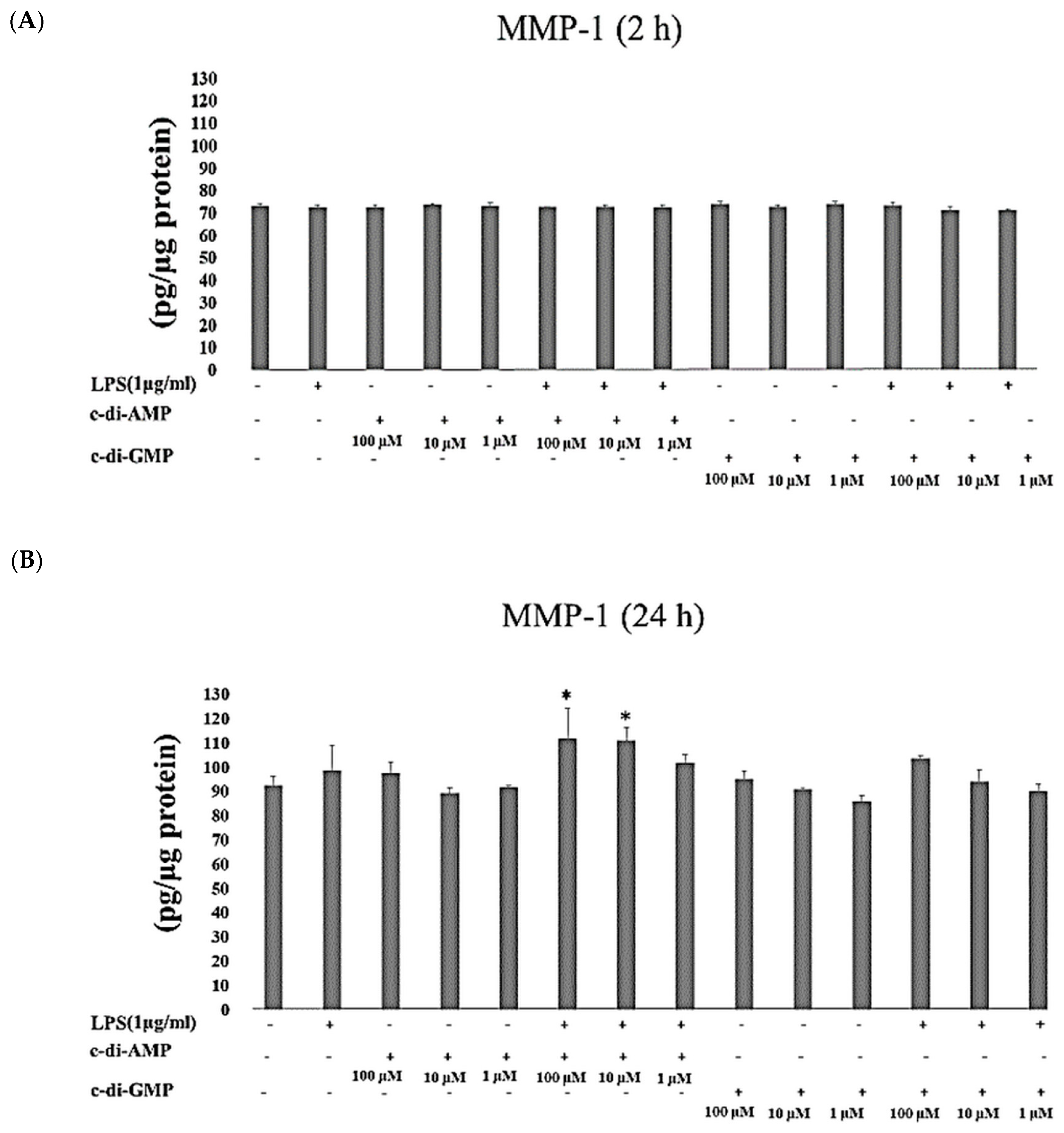
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Synthesis of c-di-GMP and c-di-AMP
4.3. Preparation of P. gingivalis LPS Stock Solution
4.4. Incubation of Fibroblasts with Cyclic Dinucleotides
4.5. Analysis of Interleukin and MMP Concentrations
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCulloch, C.A. Origins and functions of cells essential for periodontal repair: The role of fibroblasts in tissue homeostasis. Oral Dis. 1995, 1, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Han, N.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Pathogenesis of important virulence factors of Porphyromonas gingivalis via Toll-Like receptors. Front. Cell. Infect. Microbiol. 2019, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Oido-Mori, M.; Fujii, T.; Kowashi, Y.; Kikuchi, M.; Suetsugu, Y.; Tanaka, J.; Azuma, Y.; Shinohara, M.; Ohura, K. Heterogeneous expression of Toll-like receptor 4 and downregulation of Toll-like receptor 4 expression on human gingival fibroblasts by Porphyromonas gingivalis lipopolysaccharide. Biochem. Biophys. Res. Commun. 2001, 288, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Doughty, L.A. Pathogen Associated Molecular Patterns, Pattern recognition receptors and pediatric sepsis. Open Inflamm. J. 2011, 4, 31–48. [Google Scholar] [CrossRef] [Green Version]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [Green Version]
- Schletter, J.; Heine, H.; Ulmer, A.J.; Rietschel, E.T. Molecular mechanisms of endotoxin activity. Arch. Microbiol. 1995, 164, 383–389. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Hans, M.; Hans, V.M. Toll-like receptors and their dual role in periodontitis: A review. J. Oral Sci. 2011, 53, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Qu, S.; Chen, X.; Wu, Q.; Shi, M. Promising targets for cancer immunotherapy: TLRs, RLRs, and STING-mediated innate immune pathways. Int. J. Mol. Sci. 2017, 18, 404. [Google Scholar] [CrossRef]
- Molteni, M.; Gemma, S.; Rossetti, C. The role of Toll-Like Receptor 4 in infectious and noninfectious inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef] [Green Version]
- Imatani, T.; Kato, T.; Okuda, K. Production of inflammatory cytokines by human gingival fibroblasts stimulated by cell-surface preparations of Porphyromonas gingivalis. Oral Microbiol. Immunol. 2001, 1, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular remodeling, and vascular disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar]
- Gürsoy, U.K.; Gürsoy, M.; Könönen, E.; Sintim, H.O. Cyclic Dinucleotides in Oral Bacteria and in Oral Biofilms. Front. Cell. Infect. Microbiol. 2017, 21, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opoku-Temeng, C.; Zhou, J.; Zheng, Y.; Su, J.; Sintim, H.O. Cyclic dinucleotide (c-di-GMP, c-di-AMP, and cGAMP) signalings have come of age to be inhibited by small molecules. Chem. Commun. 2016, 52, 9327–9342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commichau, F.M.; Dickmanns, A.; Gundlach, J.; Ficner, R.; Stülke, J. A jack of all trades: The multiple roles of the unique essential second messenger cyclic di-AMP. Mol. Microbiol. 2015, 97, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.J.; Iavarone, A.T.; Portnoy, D.A. C-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 2010, 328, 1703–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motwani, M.; Pesiridis, S.; Fitzgerald, K.A. DNA sensing by the cGAS–STING pathway in health and disease. Nat. Rev. Genet. 2019, 20, 657–674. [Google Scholar] [CrossRef]
- Sooreshjani, M.A.; Gursoy, U.K.; Aryal, U.K.; Sintim, H.O. Proteomic analysis of RAW macrophages treated with cGAMP or c-di-GMP reveals differentially activated cellular pathways. RSC Adv. 2018, 8, 36840–36851. [Google Scholar] [CrossRef] [Green Version]
- Aryal, U.K.; Hedrick, V.; Onyedibe, K.I.; Sobreira, T.J.P.; Sooreshjani, M.A.; Wang, M.; Gürsoy, U.K.; Sintim, H.O. Global proteomic analyses of STING-positive and -negative macrophages reveal STING and Non-STING differentially regulated cellular and molecular pathways. Proteomics Clin. Appl. 2020, 14, 1900109. [Google Scholar] [CrossRef]
- Karaolis, D.K.; Means, T.K.; Yang, D.; Takahashi, M.; Yoshimura, T.; Muraille, E.; Philpott, D.; Schroeder, J.T.; Hyodo, M.; Hayakawa, Y.; et al. Bacterial c-di-GMP is an immunostimulatory molecule. J. Immunol. 2007, 178, 2171–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmanfi, S.; Zhou, J.; Sintim, H.O.; Könönen, E.; Gürsoy, M.; Gürsoy, U.K. Regulation of gingival epithelial cytokine response by bacterial cyclic dinucleotides. J. Oral Microbiol. 2019, 11, 1538927. [Google Scholar] [CrossRef] [Green Version]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar]
- Temizoz, B.; Kuroda, E.; Ohata, K.; Jounai, N.; Ozasa, K.; Kobiyama, K.; Aoshi, T.; Ishii, K.J. TLR9 and STING agonists synergistically induce innate and adaptive type-II IFN. Eur. J. Immunol. 2015, 45, 1159–1169. [Google Scholar] [PubMed]
- Scumpia, P.O.; Botten, G.A.; Norman, J.S.; Kelly-Scumpia, K.M.; Spreafico, R.; Ruccia, A.R.; Purbey, P.K.; Thomas, B.J.; Modlin, R.L.; Smile, S.T. Opposing roles of Toll-like receptor and cytosolic DNA-STING signaling pathways for Staphylococcus aureus cutaneous host defense. PLoS Pathog. 2017, 13, e1006496. [Google Scholar] [CrossRef]
- Jordana, M.; Särnstrand, B.; Sime, P.J.; Ramis, I. Immune-inflammatory functions of fibroblasts. Eur. Respir. J. 1994, 7, 2212–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.L.; Chang, M.C.; Lin, L.D.; Chan, C.P.; Wang, C.Y.; Lin, P.S.; Jeng, J.H. Stimulation of prostanoids and IL-8 production in human gingival fibroblasts by Porphyromonas gingivalis LPS is associated with MEK/ERK signaling. J. Den. Sci. 2014, 9, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Sakaki, H.; Matsumiya, T.; Kusumi, A.; Imaizumi, T.; Satoh, H.; Yoshida, H.; Satoh, K.; Kimur, H. Interleukin-1beta induces matrix metalloproteinase-1 expression in cultured human gingival fibroblasts: Role of cyclooxygenase-2 and prostaglandin E2. Oral Dis. 2004, 10, 87–93. [Google Scholar]
- Ara, T.; Kurata, K.; Hirai, K.; Uchihashi, T.; Uematsu, T.; Imamura, Y.; Furusawa, K.; Kurihara, S.; Wang, P.L. Human gingival fibroblasts are critical in sustaining inflammation in periodontal disease. J. Periodontal Res. 2009, 44, 21–27. [Google Scholar] [CrossRef]
- Yao, C.; Oh, J.H.; Lee, D.H.; Bae, J.S.; Jin, C.L.; Park, C.H.; Chung, J.H. Toll-like receptor family members in skin fibroblasts are functional and have a higher expression compared to skin keratinocytes. Int. J. Mol. Med. 2015, 35, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Yamaji, Y.; Kubota, T.; Sasaguri, K.; Sato, S.; Suzuki, Y.; Kumada, H.; Umemoto, T. Inflammatory cytokine gene expression in human periodontal ligament fibroblasts stimulated with bacterial lipopolysaccharides. Infect. Immun. 1995, 63, 3576–3581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boström, E.A.; Lundberg, P. The newly discovered cytokine IL-34 is expressed in gingival fibroblasts, shows enhanced expression by pro-inflammatory cytokines, and stimulates osteoclast differentiation. PLoS ONE 2013, 8, e81665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garlet, G.P.; Cardoso, C.R.; Silva, T.A.; Ferreira, B.R.; Avila-Campos, M.J.; Cunha, F.Q.; Silva, J.S. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol. Immunol. 2006, 21, 12–20. [Google Scholar] [CrossRef]
- Dziarski, R.; Gupta, D. Mammalian peptidoglycan recognition proteins (PGRPs) in innate immunity. Innate Immun. 2000, 16, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Lipford, G.B.; Heeg, K.; Wagner, H. Bacterial DNA as immune cell activator. Trends Microbiol. 1998, 6, 496–500. [Google Scholar] [CrossRef]
- Brown, A.G.; Parry, S.; Elovitz, M.A. Lipopolysaccharide induces cytokine production and decreases extravillous trophoblast invasion through a mitogen-activated protein kinase-mediated pathway: Possible mechanisms of first trimester placental dysfunction. Hum. Reprod. 2012, 27, 61–72. [Google Scholar]
- Liu, M.K.; Herrera-Velit, P.; Brownsey, R.W.; Reiner, N.E. CD14-dependent activation of protein kinase C and mitogen-activated protein kinases (p42 and p44) in human monocytes treated with bacterial lipopolysaccharide. J. Immunol. 1994, 153, 2642–2652. [Google Scholar]
- Mahanonda, R.; Sa-Ard-Iam, N.; Montreekachon, P.; Pimkhaokham, A.; Yongvanichit, K.; Fukuda, M.M.; Pichyangkul, S. IL-8 and IDO expression by human gingival fibroblasts via TLRs. J. Immunol. 2007, 178, 1151–1157. [Google Scholar] [CrossRef] [Green Version]
- Morandini, A.C.; Sipert, C.R.; Ramos-Junior, E.S.; Brozoski, D.T.; Santos, C.F. Periodontal ligament and gingival fibroblasts participate in the production of TGF-β, interleukin (IL)-8 and IL-10. Braz. Oral Res. 2011, 25, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Palm, E.; Khalaf, H.; Bengtsson, T. Porphyromonas gingivalis downregulates the immune response of fibroblasts. BMC Microbiol. 2013, 13, 155. [Google Scholar] [CrossRef] [Green Version]
- Sorsa, T.; Tjaderhane, L.; Salo, T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004, 10, 311–318. [Google Scholar] [CrossRef]
- Bozkurt, S.B.; Hakki, S.S.; Hakki, E.E.; Durak, Y.; Kantarci, A. Porphyromonas gingivalis Lipopolysaccharide Induces a Pro-inflammatory human gingival fibroblast phenotype. Inflammation 2017, 40, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, T.; Port, G.C.; Cho, K.H. C-di-AMP: An essential molecule in the signaling pathways that regulate the viability and virulence of gram-positive bacteria. Genes 2017, 8, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, P.; Wang, S.; Xiong, Z.; Zhu, X.; Ye, B.; Du, Y.; Meng, S.; Qu, Y.; Liu, J.; Gao, G. The ER membrane adaptor ERAdP senses the bacterial second messenger c-di-AMP and initiates anti-bacterial immunity. Nat. Immunol. 2018, 19, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lagente, V.; Boichot, E. Role of matrix metalloproteinases in the inflammatory process of respiratory diseases. J. Mol. Cell. Cardiol. 2010, 48, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Patricia, H.-R.; Timo, S.; Claudia, B.; Marcela, H. Matrix metalloproteinases as regulators of periodontal inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef] [Green Version]
- Moreau, M.; Brocheriou, I.; Petit, L.; Ninio, E.; Chapman, M.J.; Rouis, M. Interleukin-8 mediates downregulation of tissue inhibitor of metalloproteinase-1 expression in cholesterol-loaded human macrophages: Relevance to stability of atherosclerotic plaque. Circulation 1999, 99, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Ahn, S.H.; Lee, J.-S.; Song, J.-E.; Cho, S.-H.; Jung, S.; Kim, S.-K.; Kim, S.-H.; Lee, K.-P.; Kwon, K.-S.; et al. Differential Matrix Metalloprotease (MMP) Expression Profiles Found in Aged Gingiva. PLoS ONE 2016, 11, e0158777. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Hormia, M. An organotypic in vitro model that mimics the dento-epithelial junction. J. Periodontol. 2002, 73, 86–93. [Google Scholar] [CrossRef]
- Gaffney, B.L.; Veliath, E.; Zhao, J.; Jones, R.A. One-flask syntheses of c-di-GMP and the [rp,rp] and [rp,sp] thiophosphate analogues. Org. Lett. 2010, 12, 3269–3271. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmanfi, S.; Sintim, H.O.; Zhou, J.; Gürsoy, M.; Könönen, E.; Gürsoy, U.K. Activation of Gingival Fibroblasts by Bacterial Cyclic Dinucleotides and Lipopolysaccharide. Pathogens 2020, 9, 792. https://doi.org/10.3390/pathogens9100792
Elmanfi S, Sintim HO, Zhou J, Gürsoy M, Könönen E, Gürsoy UK. Activation of Gingival Fibroblasts by Bacterial Cyclic Dinucleotides and Lipopolysaccharide. Pathogens. 2020; 9(10):792. https://doi.org/10.3390/pathogens9100792
Chicago/Turabian StyleElmanfi, Samira, Herman O. Sintim, Jie Zhou, Mervi Gürsoy, Eija Könönen, and Ulvi K. Gürsoy. 2020. "Activation of Gingival Fibroblasts by Bacterial Cyclic Dinucleotides and Lipopolysaccharide" Pathogens 9, no. 10: 792. https://doi.org/10.3390/pathogens9100792
APA StyleElmanfi, S., Sintim, H. O., Zhou, J., Gürsoy, M., Könönen, E., & Gürsoy, U. K. (2020). Activation of Gingival Fibroblasts by Bacterial Cyclic Dinucleotides and Lipopolysaccharide. Pathogens, 9(10), 792. https://doi.org/10.3390/pathogens9100792





