Morphological Description of the Early Events during the Invasion of Acanthamoeba castellanii Trophozoites in a Murine Model of Skin Irradiated under UV-B Light
Abstract
1. Introduction
2. Results
2.1. Murine Skin Infection
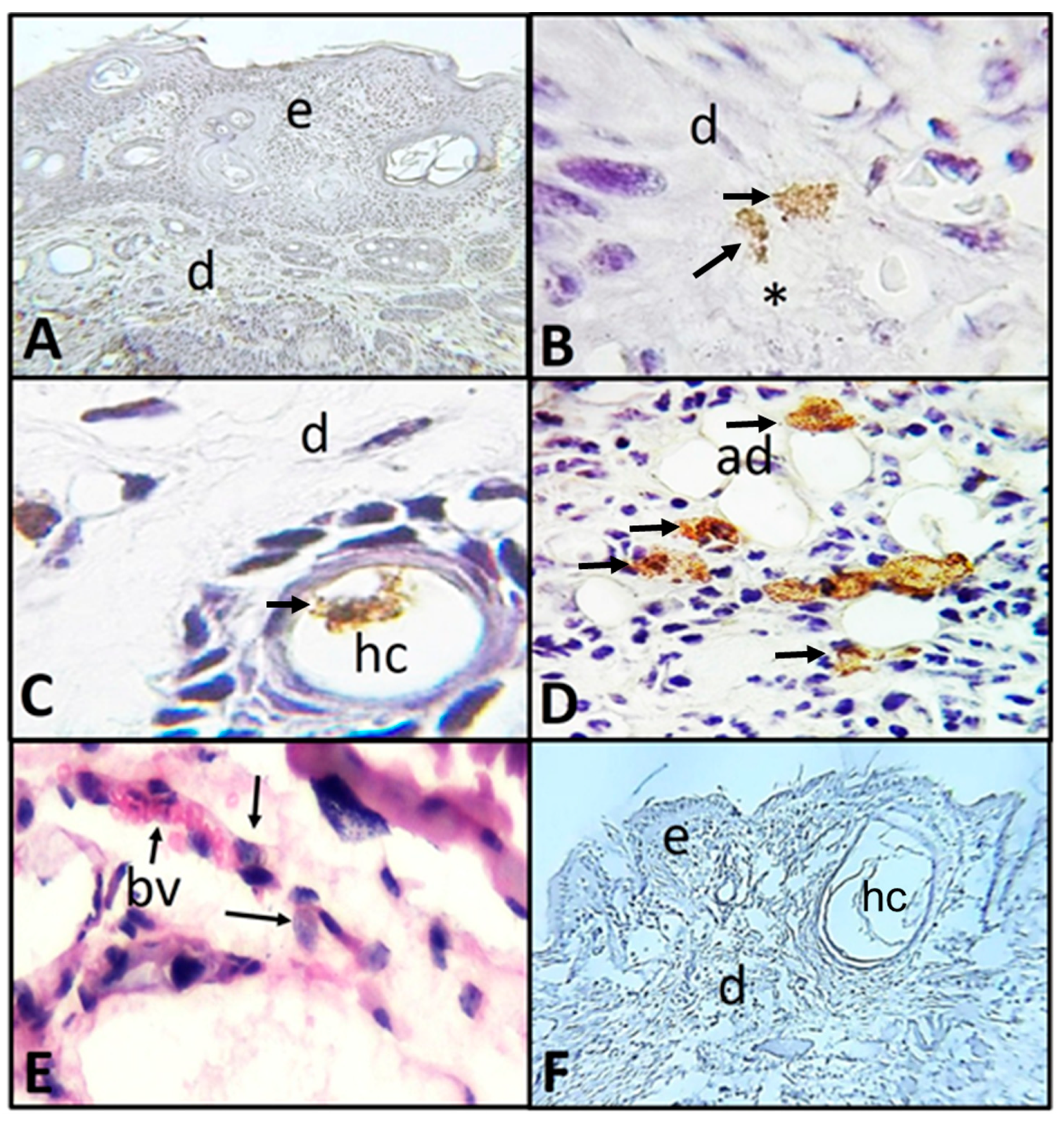
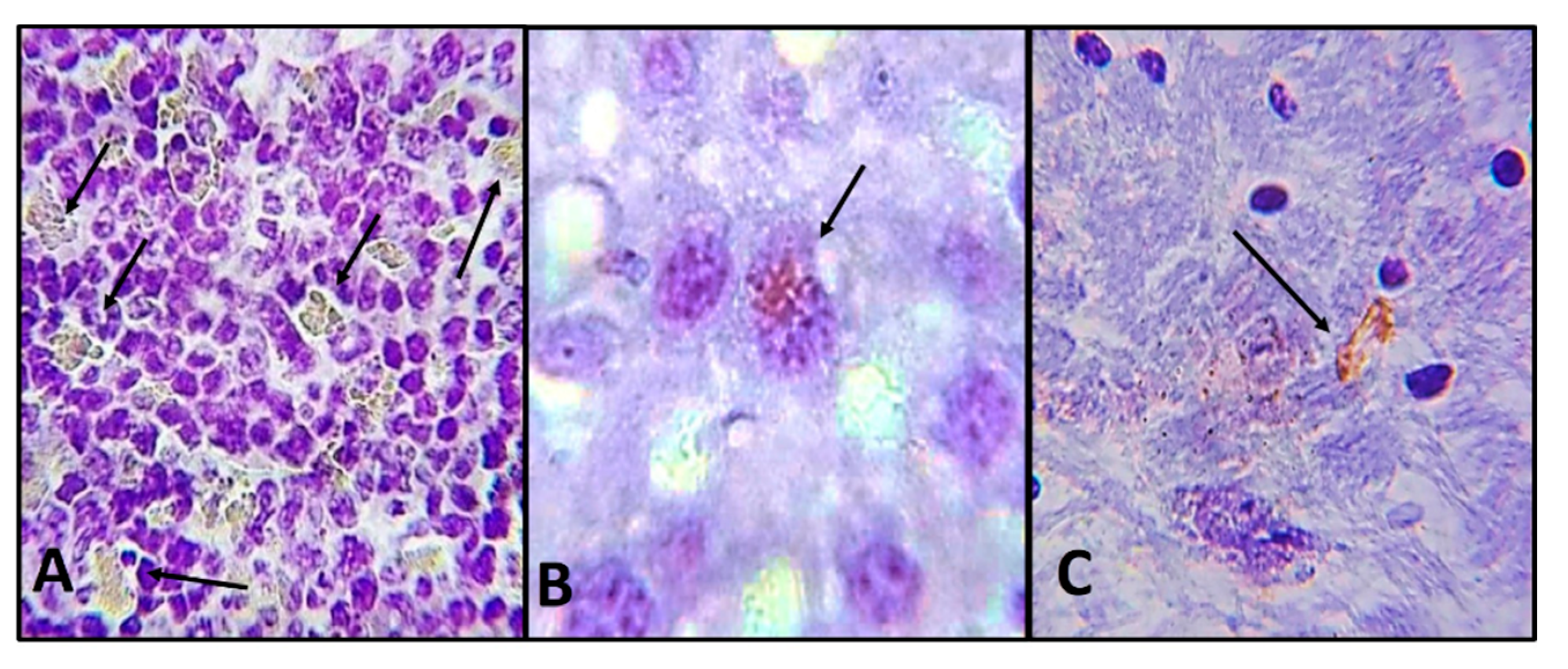
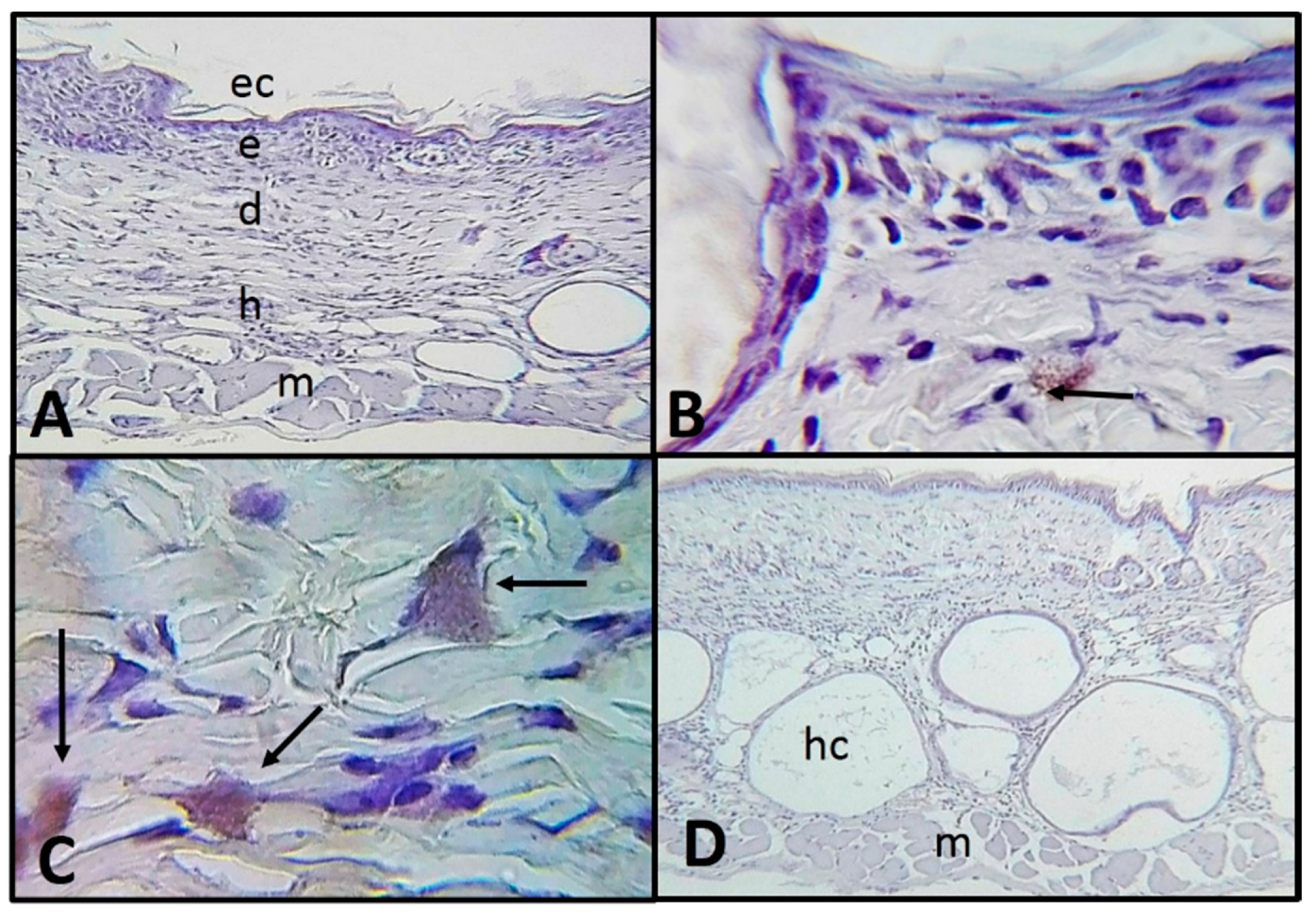
2.2. Histological and Immunohistochemistry Analysis of the Interaction of A. castellanii Trophozoites with Skin Chronically Irradiated with UV-B Light
2.3. In Vitro Determination of the Adhesion of A. castellanii Trophozoites to Type I Collagen and Its Collagenolytic Activity
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Mice Maintenance and Experimental Use, Ethical Considerations
5.2. Amoebic Cultivation
5.3. Reactivation of A. castellanii Virulence
5.4. SKH-1 Mice Chronic Irradiation with UV-B Light
5.5. Murine Model of A. castellanii Skin Infection
5.6. Immunohistochemistry Analysis of the Interaction between A. castellanii Trophozoites and Skin from SKH-1 Mice
5.7. Adhesion and Collagenolytic Activity of A. castellanii Trophozoites to Type I Collagen
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schuster, F.L. Cultivation of pathogenic and opportunistic free-living amebas. Clin. Microbiol. Rev. 2002, 15, 342–354. [Google Scholar] [CrossRef]
- Kalra, S.K.; Sharma, P.; Shyam, K.; Tejan, N.; Ghoshal, U. Acanthamoeba and its pathogenic role in Granulomatous Amebic Encephalitis. Exp. Parasitol. 2019, 208, 107788. [Google Scholar] [CrossRef] [PubMed]
- Visvesvara, G.; Moura, H.; Schuster, F. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia madrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.L.; Visvesvara, G.S. Amebae and ciliated protozoa as causal agents of waterborne zoonotic disease. Vet. Parasitol. 2004, 126, 91–120. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.J. Infection of the central nervous system due to Acanthamoeba. Rev. Infect. Dis. 1991, 13, 399–402. [Google Scholar] [CrossRef]
- Massilamany, C.; Marciano-Cabral, F.; Rocha-Azevedo, B.D.; Jamerson, M.; Gangaplara, A.; Steffen, D.; Zabad, R.; Illes, Z.; Sobel, R.A.; Reddy, J. SJL Mice Infected with Acanthamoeba Castellanii Develop Central Nervous System Autoimmunity Through the Generation of Cross-Reactive T Cells for Myelin Antigens. PLoS ONE 2014, 9, e98506. [Google Scholar] [CrossRef]
- Libbey, J.E.; Matthew, F.; Fujinami, R.S. Role of pathogens in multiple sclerosis. Int. Rev. Immunol. 2014, 33, 266–283. [Google Scholar] [CrossRef]
- Barsoum, R.S. Parasitic Infections in Organ Transplantation. Exp. Clin. Transpl. 2004, 2, 258–267. [Google Scholar]
- Kandukuri, S.; Morgan, M.; Zakowski, P.; Visvesvara, G.; Falk, J.; Roy, S.; Chaux, G.; Ghandehari, S.; Wu, J. Transplant patient with skin nodules. Am. J. Dermatopathol. 2015, 37, 254–256. [Google Scholar] [CrossRef]
- Winsent, F.; Dietert, J.; Tschen, J.; Swaby, M.; Bangert, C.A. A rare case of cutaneous acanthamoebiasis in a renal transplant patient. Dermatol. Online J. 2017, 23. [Google Scholar]
- Brondfield, M.N.; Reid, M.J.A.; Rutishauser, R.L.; Cope, J.R.; Tang, J.; Ritter, J.M.; Matanock, A.; Ali, I.; Doernberg, S.B.; Hilts-Horeczko, A.; et al. Disseminated Acanthamoeba infection in a heart transplant recipient treated successfully with a miltefosine-containing regimen: Case report and review of the literature. Transpl. Infect. Dis. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.J.; Visvesvara, G. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 1997, 7, 583–598. [Google Scholar] [CrossRef]
- Omaña-Molina, M.; González-Robles, A.; Salazar-Villatoro, L.I.; Cristóbal-Ramos, A.R.; González-Lázaro, M.; Salinas-Moreno, E.; Méndez-Cruz, R.; Sánchez-Cornejo, M.; De la Torre-González, E.; Martínez-Palomo, A. Acanthamoeba castellanii: Morphological analysis of the interaction with human cornea. Exp. Parasitol. 2010, 126, 73–78. [Google Scholar] [CrossRef]
- Gullett, J.; Mills, J.; Hadley, K.; Podemski, B.; Pitts, L.; Gelber, R. Disseminated granulomatous Acanthamoeba infection presenting as an unusual skin lesion. Am. J. Med. 1979, 67, 891–896. [Google Scholar] [CrossRef]
- Walia, R.; Montoya, J.G.; Visvesvera, G.S.; Booton, G.C.; Doyle, R.L. A case of successful treatment of cutaneous Acanthamoeba infection in a lung transplant recipient. Transpl. Infect. Dis. 2007, 9, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Rivera, F.; Bonilla, P.; Ramírez, E.; Calderón, A.; Gallegos, E.; Rodríguez, S.; Ortiz, R.; Hernández, D.; Rivera, V. Seasonal distribution of air-borne pathogenic and free-living amoebae in Mexico City and its suburbs. Water Air Soil Pollut. 1994, 74, 65–87. [Google Scholar] [CrossRef]
- Hunt, S.J.; Reed, S.L.; Mathews, W.C.; Torian, B. Cutaneous Acanthamoeba infection in the acquired immunodeficiency syndrome: Response to multidrug therapy. Cutis 1995, 56, 285–287. [Google Scholar]
- Van Hamme, C.; Dumont, M.; Lachapelle, J.M. Cutaneous acanthamoebiasis in a lung transplant patient. Ann. Dermatol. Venereol. 2001, 128, 1237–1240. [Google Scholar]
- Casper, T.; Basset, D.; Leclercq, C.; Fabre, J.; Peyron-Raison, N.; Reynes, J. Disseminated Acanthamoeba infection in a patient with AIDS: Response to 5-fluorocytosine therapy. Clin. Infect. Dis. 1999, 29, 944–945. [Google Scholar] [CrossRef]
- Galarza, C.; Ramos, W.; Gutiérrez, E.L.; Ronceros, G.; Terán, M.; Uribe, M.; Navincopa, M.; Ortega-Loayza, A.G. Cutaneous acanthamebiasis infection in immunocompetent and immunocompromised patients. Int. J. Dermatol. 2009, 48, 1324–1329. [Google Scholar] [CrossRef]
- Murakawa, G.J.; McCalmont, T.; Altman, J.; Telang, G.H.; Hoffman, M.D.; Kantor, G.R.; Berger, T.G. Disseminated acanthamebiasis in patients with AIDS. A report of five cases and a review of the literature. Arch. Dermatol. 1995, 131, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Slater, C.A.; Sickel, J.Z.; Visvesvara, G.S.; Pabico, R.C.; Gaspari, A.A. Brief report: Successful treatment of disseminated Acanthamoeba infection in an immunocompromised patient. N. Engl. J. Med. 1994, 331, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.P.; Galindo, R.L.; Kraus, E.S.; Ghanem, K.G. Disseminated acanthamebiasis in a renal transplant recipient with osteomyelitis and cutaneous lesions: Case report and literature review. Clin. Infect. Dis. 2002, 35, 43–49. [Google Scholar] [CrossRef]
- Friedland, L.R.; Raphael, S.A.; Deutsch, E.S.; Johal, J.; Martyn, L.J.; Visvesvara, G.S.; Lischner, H.W. Disseminated Acanthamoeba infection in a child with symptomatic human immunodeficiency virus infection. Pediatr. Infect. Dis. J. 1992, 11, 404–407. [Google Scholar] [CrossRef]
- Tan, B.; Weldon-Linne, C.M.; Rhone, D.P.; Penning, C.L.; Visvesvara, G.S. Acanthamoeba infection presenting as skin lesions in patients with at acquired immunodeficiency syndrome. Arch. Pathol. Lab. Med. 1993, 117, 1043–1046. [Google Scholar] [PubMed]
- Cabello-Vílchez, A.M. Balamuthia mandrillaris en el Perú, lesiones cutáneas, meningoencefalitis y métodos de cultivo. Infectio 2016, 20, 107–119. [Google Scholar] [CrossRef]
- Górnik, K.; Kuźna-Grygiel, W. Histological studies of selected organs of mice experimentally with Acanthamoeba spp. Folia Morphol. 2005, 64, 161–167. [Google Scholar]
- Omaña-Molina, M.; González-Robles, A.; Salazar-Villatoro, L.I.; Lorenzo-Morales, J.; Cristóbal-Ramos, A.R.; Hernández-Ramírez, V.I.; Talamás-Rohana, P.; Méndez, C.A.R.; Martínez-Palomo, A. Reevaluating the role of Acanthamoeba proteases in tissue invasion: Observation of cytopathogenic mechanisms on MDCK cell monolayers and hamster corneal cells. Biomed. Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Omaña-Molina, M.; Sánchez-Rocha, R.; Hernández-Martínez, D.; Romero-Grijalva, M.; Salinas-Lara, C.; Rodríguez-Sosa, M.; Juárez-Avelar, I.; Salazar-Villatoro, L.; González-Robles, A.; Méndez-Cruz, A.R.; et al. Type 2 diabetes mellitus BALB/c mice are more susceptible to granulomatous amoebic encephalitis: Immunohistochemical study. Exp. Parasitol. 2017, 183, 150–159. [Google Scholar] [CrossRef]
- Omaña-Molina, M.; Hernández-Martínez, D.; Sánchez-Rocha, R.; Cárdenas-Lemus, U.; Salinas-Lara, C.; Méndez-Cruz, A.R.; Colín-Barenque, L.; Aley-Medina, P.; Espinosa-Villanueva, J.; Moreno-Fierros, L.; et al. In vivo CNS infection model of Acanthamoeba genotype T4: The early stages of infection lack presence of host inflammatory response and are a slow and contact-dependent process. Parasitol. Res. 2017, 116, 725–733. [Google Scholar] [CrossRef]
- Mäkinen, M.; Stenbäck, F. Skin tumor development and keratin expression in different experimental models. Relation to inducing agent and target tissue structure. Exp. Toxic. Pathol. 1998, 50, 199–208. [Google Scholar] [CrossRef]
- Cano, G.A.; Gómez, G.F.J.; Álvarez, S.N.; Sánchez-Pedreño, G.P.; Vicente, O.V. Modelo de fotocarcinogénesis cutánea en ratones SKH-1 por radiación ultravioleta. Rev. Esp. Patol. 2010, 43, 191–195. [Google Scholar] [CrossRef]
- Benavides, F.; Oberyszyn, T.M.; VanBuskirk, A.M.; Reeve, V.E.; Kusewitt, D.F. The hairless mouse in skin research. J. Dermatol. Sci. 2009, 53, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Munive, A.M.; Rojas, A.M. Amebiasis intestinal y cutánea. Rev. Med. Costa Centroam. 2008, 65, 153–157. [Google Scholar]
- Torres, V.; Camacho, F.; Mihm, M.; Sober, A.; Sánchez, I.C. Dermatología Práctica, 1st ed.; Ibero-Latinoamericana Press: Mexico City, Mexico, 2005; pp. 283–284. [Google Scholar]
- González-Robles, A.; Omaña-Molina, M.; Salazar-Villatoro, L.; Flores-Maldonado, C.; Lorenzo-Morales, J.; Reyes-Batlle, M.; Arnalich-Montiel, F.; Martínez-Palomo, A. Acanthamoeba culbertsoni isolated from a clinical case with intraocular dissemination: Structure and in vitro analysis of the interaction with hamster cornea and MDCK epithelial cell monolayers. Exp. Parasitol. 2017, 183, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Carrada, B.T. Amebiasis cutánea: Parasitosis emergente y letal. Piel 2005, 20, 28–34. [Google Scholar] [CrossRef]
- Łanocha-Arendarczyk, N.; Kolasa-Wołosiuk, A.; Wojciechowska-Koszko, I.; Kot, K.; Roszkowska, P.; Krasnodębska-Szponder, B.; Paczkowska, E.; Machaliński, B.; Łuczkowska, K.; Wiszniewska, B.; et al. Changes in the immune system in experimental acanthamoebiasis in immunocompetent and immunosuppressed hosts. Parasit. Vectors 2018, 11, 517. [Google Scholar] [CrossRef]
- Jaison, P.L.; Cao, Z.; Panjwani, N. Binding of Acanthamoeba to [corrected] mannose-glycoproteins of corneal epithelium: Effect of injury. Curr. Eye Res. 1998, 17, 770–776. [Google Scholar] [CrossRef]
- Kennett, M.J.; Hook, R.R., Jr.; Franklin, C.L.; Riley, L.K. Acanthamoeba castellanii: Characterization of an adhesin molecule. Exp. Parasitol. 1999, 92, 161–169. [Google Scholar] [CrossRef]
- Cao, Z.; Jefferson, D.M.; Panjwani, N. Role of carbohydrate-mediated adherence in cytopathogenic mechanisms of Acanthamoeba. J. Biol. Chem. 1998, 273, 15838–15845. [Google Scholar] [CrossRef]
- He, Y.G.; Niederkorn, J.Y.; McCulley, J.P.; Stewart, G.L.; Meyer, D.R.; Silvany, R.; Dougherty, J. In Vivo and In Vitro Collogenolytic Activity of Acanthamoeba castellanii. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2235–2240. [Google Scholar]
- Rocha-Azevedo, B.D.; Jamerson, M.; Cabral, G.A.; Silva-Filho, F.C.; Marciano-Cabral, F. Acanthamoeba Interaction with Extracellular Matrix Glycoproteins: Biological and Biochemical Characterization and Role in Cytotoxicity and Invasiveness. J. Eukaryot. Microbiol. 2009, 56, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Garner, A. Pathology of corneal acanthamoebic infection. In The Cornea: Transactions of the World Congress on the Cornea III; Cavanagh, H.D., Ed.; Raven Press: New York, NY, USA, 1988; pp. 535–539. [Google Scholar]
- Paltiel, M.; Powell, E.; Lynch, J.; Baranowski, B.; Martins, C. Disseminated Cutaneous Acanthamebiasis: A Case Report and Review of the Literature. Cutis 2004, 73, 241–248. [Google Scholar]
- Magaña, M.L.; Fernández-Díez, J.; Magaña, M. Cutaneous amebiasis in pediatrics. Arch Dermatol. 2008, 144, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Godbold, G.D.; Mann, B.J. Involvement of the actin cytoskeleton and p21rho-family GTPases in the pathogenesis of the human protozoan parasite Entamoeba histolytica. Braz. J. Med. Biol. Res. 1998, 31, 1049–1058. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omaña-Molina, M.A.; González-Robles, A.; Salazar-Villatoro, L.; Bernal-Escobar, A.; Durán-Díaz, A.; Méndez-Cruz, A.R.; Martínez-Palomo, A. Silicone Hydrogel contact lenses surface promote Acanthamoeba castellanii trophozoites adherence: Qualitative and quantitative analysis. Eye Contact Lens. 2014, 40, 132–139. [Google Scholar] [CrossRef]
- Culbertson, C.G.; Smith, J.W.; Cohen, H.K.; Minner, J.R. Experimental infection of mice and monkeys by Acanthamoeba. Am. J. Pathol. 1959, 35, 185–197. [Google Scholar]
- García-Bores, A.M.; Bello, C.; Campos, Y.; Benitez, J.C.; Flores, S.; Canales, M.; Hernández, T.; Avila, J.G. Photoprotective activity of Yucca periculosa polyphenols. Bol. Latinoam. Caribe Plantas Med. Aromát. 2010, 9, 100–108. [Google Scholar]
- Rojas-Hernández, S.; Jarillo-Luna, A.; Rodríguez-Monroy, M.; Moreno-Fierros, L.; Campos-Rodríguez, R. Immunohistochemical characterization of the initial stages of Naegleria fowleri meningoencephalitis in mice. Parastol. Res. 2004, 94, 31–36. [Google Scholar]
- Muñoz, M.L.; Calderón, J.; Rojkind, M. The collagenase of Entamoeba histolytica. J. Exp. Med. 1982, 155, 42–51. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Jasso, M.; Hernández-Martínez, D.; Avila-Acevedo, J.G.; Benítez-Flores, J.d.C.; Gallegos-Hernández, I.A.; García-Bores, A.M.; Espinosa-González, A.M.; Villamar-Duque, T.E.; Castelan-Ramírez, I.; González-Valle, M.d.R.; et al. Morphological Description of the Early Events during the Invasion of Acanthamoeba castellanii Trophozoites in a Murine Model of Skin Irradiated under UV-B Light. Pathogens 2020, 9, 794. https://doi.org/10.3390/pathogens9100794
Hernández-Jasso M, Hernández-Martínez D, Avila-Acevedo JG, Benítez-Flores JdC, Gallegos-Hernández IA, García-Bores AM, Espinosa-González AM, Villamar-Duque TE, Castelan-Ramírez I, González-Valle MdR, et al. Morphological Description of the Early Events during the Invasion of Acanthamoeba castellanii Trophozoites in a Murine Model of Skin Irradiated under UV-B Light. Pathogens. 2020; 9(10):794. https://doi.org/10.3390/pathogens9100794
Chicago/Turabian StyleHernández-Jasso, Mariana, Dolores Hernández-Martínez, José Guillermo Avila-Acevedo, José del Carmen Benítez-Flores, Isis Amara Gallegos-Hernández, Ana María García-Bores, Adriana Montserrat Espinosa-González, Tomás Ernesto Villamar-Duque, Ismael Castelan-Ramírez, María del Rosario González-Valle, and et al. 2020. "Morphological Description of the Early Events during the Invasion of Acanthamoeba castellanii Trophozoites in a Murine Model of Skin Irradiated under UV-B Light" Pathogens 9, no. 10: 794. https://doi.org/10.3390/pathogens9100794
APA StyleHernández-Jasso, M., Hernández-Martínez, D., Avila-Acevedo, J. G., Benítez-Flores, J. d. C., Gallegos-Hernández, I. A., García-Bores, A. M., Espinosa-González, A. M., Villamar-Duque, T. E., Castelan-Ramírez, I., González-Valle, M. d. R., & Omaña-Molina, M. (2020). Morphological Description of the Early Events during the Invasion of Acanthamoeba castellanii Trophozoites in a Murine Model of Skin Irradiated under UV-B Light. Pathogens, 9(10), 794. https://doi.org/10.3390/pathogens9100794





