The Pathogenesis of Tuberculosis–The Koch Phenomenon Reinstated
Abstract
:1. Introduction
2. The Koch Phenomenon
3. X-rays of Developing TB
4. Delayed Type Hypersensitivity (DTH)–Both Protection and Perifocal Inflammation
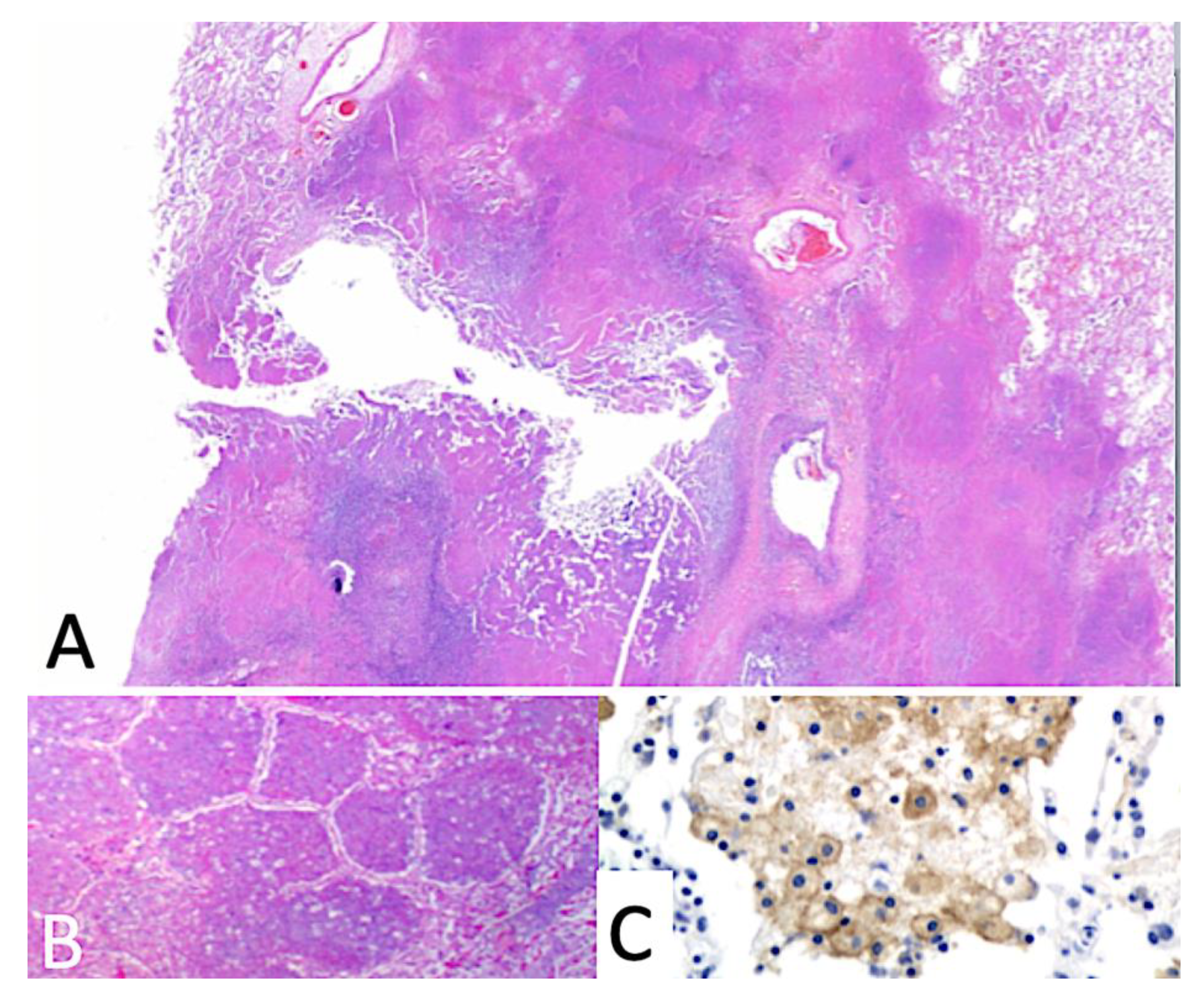
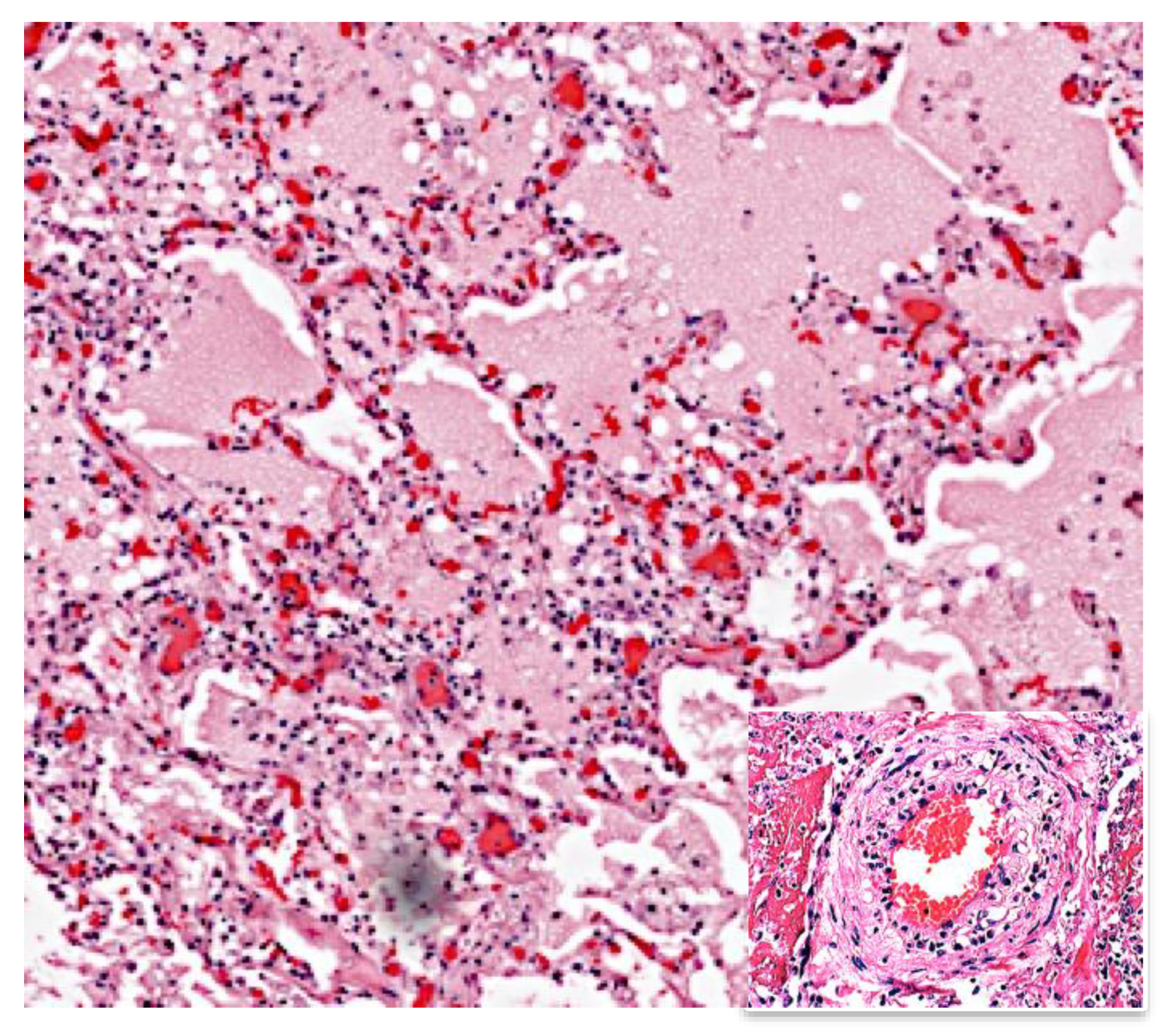
5. Early Lesion of Post-Primary TB (PPTB)
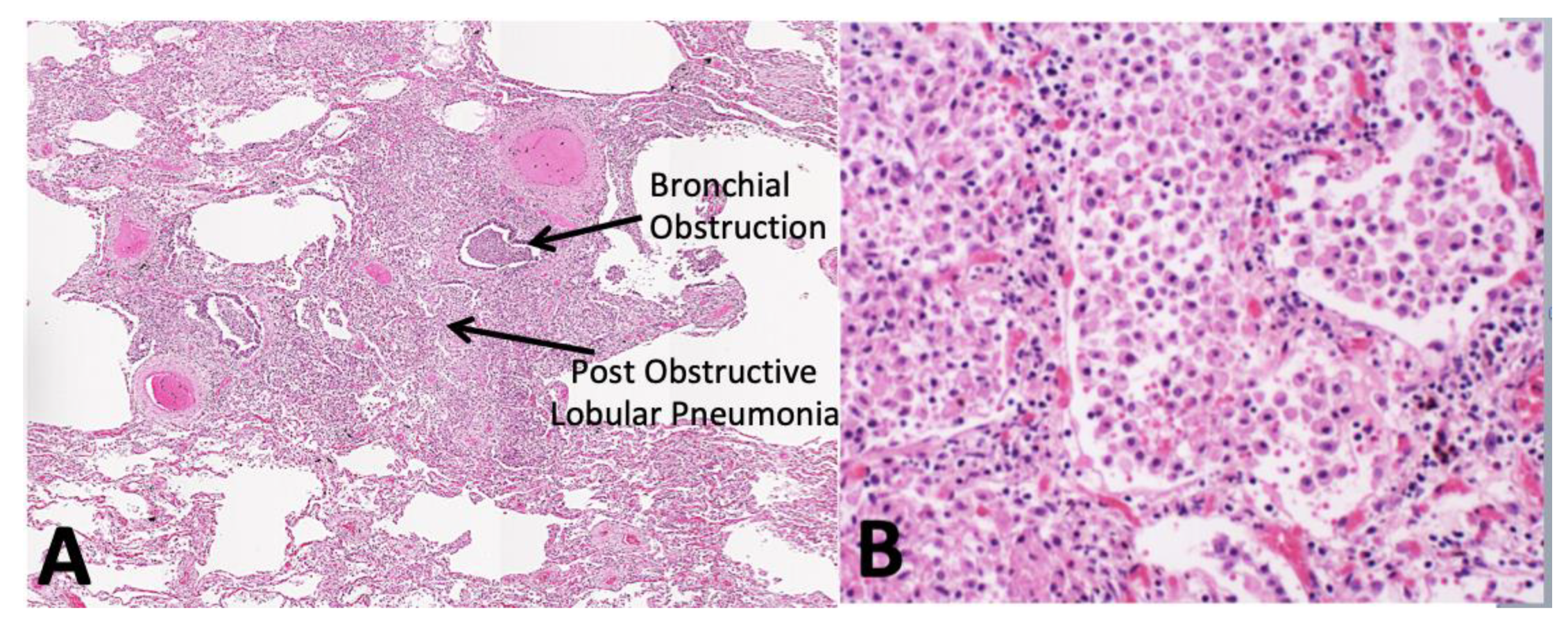
5.1. Role of Bronchial Obstruction in Cavitation
5.2. Synthesis of Secreted Mycobacterial Antigens in Alveolar Macrophages
5.3. TDM as an Invisibility Cloak for Intracellular MTB
5.4. Nature of Hypersensitive Tissue–Trm, PD1/PD-L1 and more
6. Later Lesions of PPTB
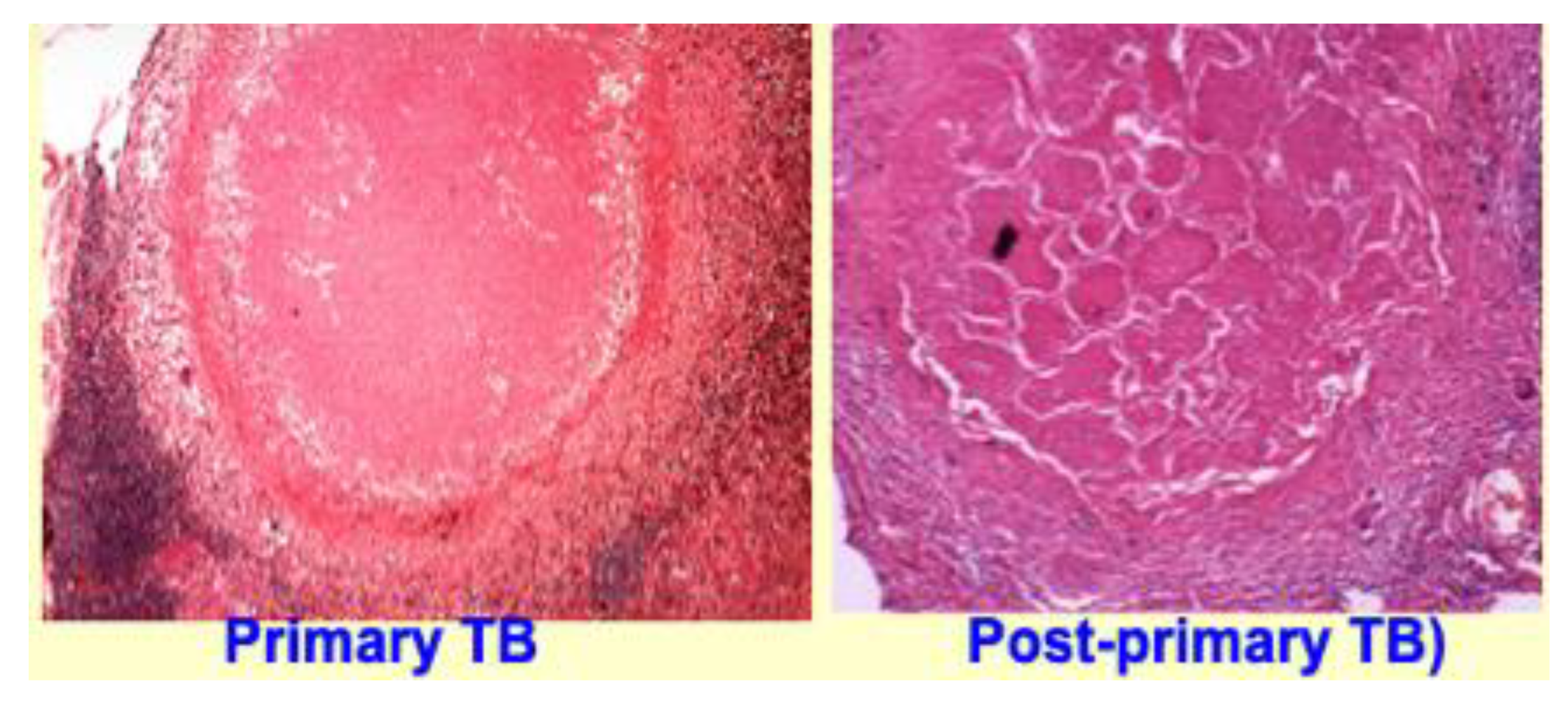
6.1. Primary and Post-Primary Granulomas
6.2. Non-Cultivable MTB and Their Resuscitation
7. Need for a Paradigm Shift
8. Animal Models
9. Immunity to Primary and Post-Primary TB
10. Potential of Advancing Technology
11. Summary
Funding
Conflicts of Interest
References
- Moliva, J.I.; Turner, J.; Torrelles, J.B. Immune responses to bacillus calmette–Guérin vaccination: Why do they fail to protect against mycobacterium tuberculosis? Front. Immunol. 2017, 8, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rich, A.R. The Pathogenesis of Tuberculosis, 2nd ed.; Charles C. Thomas: Springfield, IL, USA, 1951. [Google Scholar]
- Koch, R. A Further Communication on A Remedy for Tuberculosis. Br. Med. J. 1890, 2, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Koch, R. The First Communication Relating to a Method to Cure Tuberculosis; Birnbaum, M., Ed.; The Project Gutenberg: Salt Lake City, UT, USA, 1890. [Google Scholar]
- Medlar, E.M. A study of the process of caseation in tuberculosis. Am. J. Pathol. 1926, 2, 275–290.13. [Google Scholar] [PubMed]
- Hunter, R.L.; Actor, J. The pathogenesis of post-primary tuberculosis. A game changer for vaccine development. Tuberculosis 2019, 116, S114–S117. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L. The pathogenesis of tuberculosis: The early infiltrate of post-primary (adult pulmonary) tuberculosis: A distinct disease entity. Front. Immunol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hunter, R.L.; Jagannath, C.; Actor, J.K. Pathology of postprimary tuberculosis in humans and mice: Contradiction of long-held beliefs. Tuberculosis 2007, 87, 267–278. [Google Scholar] [CrossRef]
- Hunter, R.L. Pathology of post primary tuberculosis of the lung: An illustrated critical review. Tuberculosis 2011, 91, 497–509. [Google Scholar] [CrossRef] [Green Version]
- Pottenger, F.M. The Interpretation of clinical pulmonary tuberculosis in terms of allergy. Am. Rev. Tuberc. 1927, 43, 145–161. [Google Scholar]
- Pinner, M. Pulmonary Tuberculosis in the Adult. Its Fundamental Aspects; Charles C. Thomas: Springfield, IL, USA, 1945. [Google Scholar]
- Kayne, G.G.; Pagel, W.; O’Shaughenessy, L. Pulmonary Tuberculosis, Pathology, Diagnosis, Management and Prevention; Oxford University Press: London, UK, 1948. [Google Scholar]
- Canetti, G. Pathogenesis of tuberculosis in man. Ann. N. Y. Acad. Sci. 1968, 154, 13–18. [Google Scholar] [CrossRef]
- McHenry, M.L.; Williams, S.M.; Stein, C.M. Genetics and evolution of tuberculosis pathogenesis: New perspectives and approaches. Infect. Genet. Evol. 2020, 81, 104204. [Google Scholar] [CrossRef]
- Seddon, J.A.; Chiang, S.S.; Esmail, H.; Coussens, A.K. The wonder years: What can primary school children teach us about immunity to mycobacterium tuberculosis? Front. Immunol. 2018, 9, 2946. [Google Scholar] [CrossRef] [PubMed]
- Opie, E. Phhistogenesis and Latent Tuberculosus Infection. Am. Rev. Tuberc. 1922, 6, 525–546. [Google Scholar]
- Gutierrez, M.C.; Brisse, S.; Brosch, R.; Fabre, M.; Omaïs, B.; Marmiesse, M.; Supply, P.; Vincent, V. Ancient origin and gene mosaicism of the progenitor of mycobacterium tuberculosis. PLoS Pathog. 2005, 1, e5. [Google Scholar] [CrossRef] [PubMed]
- North, R.J.; Jung, Y.J. Immunity to tuberculosis. Annu. Rev. Immunol. 2004, 22, 599–623. [Google Scholar] [CrossRef] [PubMed]
- Abel, L.; Fellay, J.; Haas, D.W.; Schurr, E.; Srikrishna, G.; Urbanowski, M.E.; Chaturvedi, N.; Srinivasan, S.; Johnson, D.H.; Bishai, W.R. Genetics of human susceptibility to active and latent tuberculosis: Present knowledge and future perspectives. Lancet Infect. Dis. 2018, 18, e64–e75. [Google Scholar] [CrossRef]
- Anonymous. 2019 Update. In WHO Guidelines on Tuberculosis Infection Prevention and Control; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Dubos, R.; Dubos, G. The White Plague. Tuberculosis, Man, and Society; Rutgers Univesity Press: Brunswick, NJ, USA, 1987. [Google Scholar]
- Van Rie, A.; Warren, R.M.; Richardson, M.; Victor, T.C.; Gie, R.P.; Enarson, D.A.; Beyers, N.; Van Helden, P.D. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N. Engl. J. Med. 1999, 341, 1174–1179. [Google Scholar] [CrossRef]
- Youmans, G.P. Chapter 13. Relationship between delayed hypersensitivity and immunity in tuberculosis. In Tuberculosis; Youmans, G.P., Ed.; W.B. Saunders Co.: Philadelphia, PA, USA, 1979; pp. 302–326. [Google Scholar]
- Nunes-Alves, C.; Booty, M.G.; Carpenter, S.M.; Jayaraman, P.; Rothchild, A.C.; Behar, S.M. In search of a new paradigm for protective immunity to TB. Nat. Rev. Genet. 2014, 12, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Elkington, P.T.; Bateman, A.C.; Thomas, G.J.; Ottensmeier, C.H. Implications of tuberculosis reactivation after immune checkpoint inhibition. Am. J. Respir. Crit. Care Med. 2018, 198, 1451–1453. [Google Scholar] [CrossRef]
- Kumar, P. Adult pulmonary tuberculosis as a pathological manifestation of hyperactive antimycobacterial immune response. Clin. Transl. Med. 2016, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Reece, S.T.; Stride, N.; Ovendale, P.; Reed, S.G.; Campos-Neto, A. Skin test performed with highly purified mycobacterium tuberculosis recombinant protein triggers tuberculin shock in infected guinea pigs. Infect. Immun. 2005, 73, 3301–3306. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.L.; Tsenova, L.; Aman, M.H.; Bekker, L.G.; Freeman, S.; Mangaliso, B.; Schröder, U.; Jagirdar, J.; Rom, W.N.; Tovey, M.G. Mycobacterial antigens exacerbate disease manifestations in mycobacterium tuberculosis-infected Mice. Infect. Immun. 2002, 70, 2100–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, R.V.Z.; Esmail, A.; Bateman, M.E.; Dawson, R.; Goldin, J.; Van Rikxoort, E.; Douoguih, M.; Pau, M.G.; Sadoff, J.; McClain, J.B.; et al. Safety and immunogenicity of adenovirus 35 tuberculosis vaccine candidate in adults with active or previous tuberculosis. A randomized trial. Am. J. Respir. Crit. Care Med. 2017, 195, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Cardona, P.J. The progress of therapeutic vaccination with regard to tuberculosis. Front. Microbiol. 2016, 7, 1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.L.; Turner, O.C.; Basaraba, R.J.; Belisle, J.T.; Huygen, K.; Orme, I. Pulmonary necrosis resulting from DNA vaccination against tuberculosis. Infect. Immun. 2003, 71, 2192–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, O.B.C.G. vaccination complicated by Koch’s phenomenon and lupus vulgaris. Acta Tuberc. Scand. 1955, 30, 259–270. [Google Scholar] [PubMed]
- Lees, D.B. The Bradshaw Lecture on the Diagnosis and Treatment of Incipient Pulmonarhy Tuberculosis; HK Lewis: London, UK, 1913. [Google Scholar]
- Lincoln, E.M.; Grethmann, W. The potetial dangers of tuberculin tests. J. Pediatr. 1939, 15, 682–696. [Google Scholar] [CrossRef]
- Hunter, R.L.; Actor, J.K.; Hwang, S.-A.; Karev, V.; Jagannath, C. Pathogenesis of post primary tuberculosis: Immunity and hypersensitivity in the development of cavities. Ann. Clin. Lab. Sci. 2014, 44, 365–387. [Google Scholar]
- Levine, E.R. Chapter 7, Classification of reinfection pulmonary tuberculosis. In The Fundamentals of Pulmonary Tuberculosis and its Complications for Students, Teachers and Practicing Physicians. Sponsored by The American College of Physicians; Hayes, E., Ed.; Charles C. Thomas: Springfield, IL, USA, 1949; pp. 97–113. [Google Scholar]
- Dannenberg, A.; Collins, F. Progressive pulmonary tuberculosis is not due to increasing numbers of viable bacilli in rabbits, mice and guinea pigs, but is due to a continuous host response to mycobacterial products. Tuberculosis 2001, 81, 229–242. [Google Scholar] [CrossRef]
- Canetti, G. The Tubercle Bacillus in the Pulmonary Lesion of Man. Histobacteriology and Its Bearing on the Therapy of Pulmonary Tuberculosis; Springer Publishing Company Inc.: New York, NY, USA, 1955. [Google Scholar]
- Wolff, E. Perifocal infiltration in juvenile tuberculosis: With case reports. Cal. West Med. 1928, 29, 170–173. [Google Scholar]
- Dutta, N.; Karakousis, P. Latent tuberculosis infection: Myths, models, and molecular mechanisms. Microbiol. Mol. Biol. Rev. 2014, 78, 343–371. [Google Scholar] [CrossRef] [Green Version]
- Drain, P.K.; Bajema, K.L.; Dowdy, D.; Dheda, K.; Naidoo, K.; Schumacher, S.; Ma, S.; Meermeier, E.; Lewinsohn, D.M.; Sherman, D.R. Incipient and subclinical tuberculosis: A clinical review of early stages and progression of infection. Clin. Microbiol. Rev. 2018, 31, e00021-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, J.G.; Itoh, H. Tree-in-bud pattern of pulmonary tuberculosis on thin-section CT: Pathological implications. Korean J. Radiol. 2018, 19, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Im, J.G.; Itoh, H.; Shim, Y.S.; Lee, J.H.; Ahn, J.; Han, M.C.; Noma, S. Pulmonary tuberculosis: CT findings--early active disease and sequential change with antituberculous therapy. Radiology 1993, 186, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Skoura, E.; Zumla, A.; Bomanji, J.B. Imaging in tuberculosis. Int. J. Infect. Dis. 2015, 32, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Overend, W. The Radiology of the Chest Line. Pulmonary Tuberculosis; ForgottenBooks.com: London, UK, 1920; Volume 1. [Google Scholar]
- Dunham, H.K.; Skavlem, J.H. X-ray observations of the pathogenesis of pulmonary tuberculosis. Tuberculosis 1924, 5, 217–232. [Google Scholar] [CrossRef]
- Amberson, J.B. The Significance of Roentgenographic Mutations of The Lesions of Chronic Pulmonary Tuberculosis. Am. Rev. Tuberc. 1922, 6, 591–610. [Google Scholar]
- Opie, E.; McPhedran, F. The Contagion of Tuberculosis. Am. Rev. Tuberc. 1926, 14, 347–419. [Google Scholar]
- Medlar, E.M. The behavior of pulmonary tuberculous lesions; a pathological study. Am. Rev. Tuberc. 1955, 71, 1–244. [Google Scholar]
- Carswell, R. Pathological Anatomy. Illustrations of the Elementary Forms of Disease; Longman, Orme, Brown, Green and Longman: London, UK, 1838. [Google Scholar]
- Ophuls, W. Statistical survey of tuberculosis on the basis of a series of 3000 post-mortem examinations. In National Tuberculosis Association; Transactions of the Nineteenth Annual Meeting; Hamilton Printing Co Albany: New York, NY, USA, 1923; pp. 252–256. [Google Scholar]
- Opie, E.L. Pathology of the tuberculosis of childhood and its bearing on clinical work. BMJ 1927, 2, 1130–1135. [Google Scholar] [CrossRef] [Green Version]
- Kerley, P. Assmann’s focus. Br. J. Tuberc. 1935, 29, 19–25. [Google Scholar] [CrossRef]
- Amberson, J.B. A retrospect of tuberculosis: 1865–1965. Am. Rev. Respir. Dis. 1966, 93, 343–351. [Google Scholar] [PubMed]
- Ziemele, B.; Ranka, R.; Ozere, I. Pediatric and adolescent tuberculosis in Latvia, 2011–2014: Case detection, diagnosis and treatment. Int. J. Tuberc. Lung Dis. 2017, 21, 637–645. [Google Scholar] [CrossRef]
- Ankrah, A.O.A.; Glaudemans, A.; Maes, C.; Van de Wiele, R.; Dierckx, M.; Vorster, S.M.M. Tuberculosis. Semin. Nucl. Med. 2018, 48, 108–130. [Google Scholar] [CrossRef] [PubMed]
- Kerley, P. Discussion on the Early Diagniosis of Pulmonary Tuberculosis. Proc. R. Soc. Med. 1933, 27, 164–167. [Google Scholar]
- Kayne, G.G. Origin, diagnosis, and management of early bronchogenic tuberculosis. BMJ 1941, 2, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunham, K. The pathological anatomy of pulmonary tuberculosis as recorded by stereoscopic radiograms of the chest; and the value of this knowledge in the care of the tuberculous patient. Proc. R. Soc. Med. 1927, 21, 61–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunn, F.D. Tuberculosis. In Pathology, 4th ed.; Anderson, W.A.D., Ed.; C.V. Mosby Company: St. Louis, MO, USA, 1961; pp. 243–263. [Google Scholar]
- Kaufmann, S.H.E.; Evans, T.G.; Hanekom, W. Tuberculosis vaccines: Time for a global strategy. Sci. Transl. Med. 2015, 7, 276fs278. [Google Scholar] [CrossRef] [Green Version]
- Bothamley, G.; Grange, J. The Koch phenomenon and delayed hypersensitivity: 1891–1991. Tuberculosis 1991, 72, 7–11. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Galli, S.J.; Dvorak, A.M. Cellular and vascular manifestations of cell-mediated immunity. Hum. Pathol. 1986, 17, 122–137. [Google Scholar] [CrossRef]
- Geczy, C.L.; Meyer, P.A. Leukocyte procoagulant activity in man: An in vitro correlate of delayed-type hypersensitivity. J. Immunol. 1982, 128, 331–336. [Google Scholar]
- Doherty, M.L.; Bassett, H.F.; Quinn, P.J.; Davis, W.C.; Kelly, A.P.; Monaghan, M.L. A sequential study of the bovine tuberculin reaction. Immunology 1996, 87, 9–14. [Google Scholar] [PubMed]
- Kager, L.M.; Blok, D.C.; Lede, I.O.; Rahman, W.; Afroz, R.; Bresser, P.; Van Der Zee, J.; Ghose, A.; Visser, C.E.; De Jong, M.D.; et al. Pulmonary tuberculosis induces a systemic hypercoagulable state. J. Infect. 2015, 70, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Erokhin, V.V.; Mishin, V.Y.; Chukanov, V.I.; Guiller, D.V. Caseous Pneumonia, Pathologic Anatomy, Pathogenesis, Diagnosis, Clinical Course and Treatment, A Manual for Practitioners; Meditsina Publishers: Moscow, Russia, 2008. [Google Scholar]
- Surkova, L.K.; Dius’Mikeeva, M.I. Acutely progressive pulmonary tuberculosis: Morphological and bacteriological features. Probl. Tuberk. 2003, 3, 33–35. [Google Scholar]
- Wolberg, A.S.; Aleman, M.M.; Leiderman, K.; Machlus, K.R. Procoagulant activity in hemostasis and thrombosis. Anesthesia Analg. 2012, 114, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Dannenberg, A.M. Roles of cytotoxic delayed-type hypersensitivity and macrophage-activating cell-mediated immunity in the pathogenesis of tuberculosis. Immunobiology 1994, 191, 461–473. [Google Scholar] [CrossRef]
- Dannenberg, A. Pathogenesis of pulmonary tuberculosis in Koch Centennial Memorial. Am. Rev. Respir. Dis. 1982, 125, 25–30. [Google Scholar]
- Reuter, H.; Wood, R.; Schaaf, H.S.; Donald, P.R. Chapter 34 Overview of extrapulmonary tuberculosis in adults and children. In Tuberculosis. A Comprehensive Clinical Reference; Schaaf, H., Zumla, A.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 377–390. [Google Scholar]
- Laennec, R. A Treatise on Diseases of the Chest in Which They Are Described According to Their Anatomical Characters, and Their Diagnosis Established on a New Principle by Means of Acoustick Instruments; T&G Underwood: London, UK, 1821. [Google Scholar]
- Virchow, R. Cellular Pathology as Based Upon Physiological and Pathological Histology; John Churchill: London, UK, 1860. [Google Scholar]
- Osler, W.; McCrae, T. Chapter XXI, tuberculosis. In The Principles and Practice of Medicine, 9th ed.; D. Appleton and Company: New York, NY, USA; London, UK, 1921; pp. 184–255. [Google Scholar]
- Alcais, A.; Fieschi, C.; Abel, L.; Casanova, J.L. Tuberculosis in children and adults. J. Exp. Med. 2005, 202, 1617–1621. [Google Scholar] [CrossRef]
- Hunter, R.L. Tuberculosis as a three-act play: A new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis 2016, 97, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Dannenberg, A.M., Jr. Pathogenisis of Human Pulmonary Tuberculosis. Insights from the Rabbit Model; ASM Press: Washington, DC, USA, 2006. [Google Scholar]
- Pagel, W. Zur Histochemie der Lungentuberkulose, mit besonderer Berucksichtung der Fettsubstanzen und Lipoide. (Fat and lipoid content to tuberculous tissue. Histochemical investigation.). Virchows. Arch. Pathol. Anat. 1925, 256, 629–640. [Google Scholar] [CrossRef]
- Birkun, A.A. Disorders of fat metabolism in the lungs in tuberculosis. (pathomorphological and histochemical characteristics). Arkhiv. Patol. 1963, 25, 23–32. [Google Scholar]
- Hektoen, L.; Reisman, D. A Text-Book of Pathology for the Use of Students and Practitoners of Medicine and Surgery; W.B. Saunders & Company: London, UK; Philadelphia, PA, USA, 1901. [Google Scholar]
- Bennett, J.H. The Pathology and Treatment of Pulmonary Tuberculosis; Blanchard and Lea: Philadelphia, PA, USA, 1854. [Google Scholar]
- Tamura, A.; Hebisawa, A.; Fukushima, K.; Yotsumoto, H.; Mori, M. Lipoid pneumonia in lung cancer: Radiographic and pathological features. Jpn. J. Clin. Oncol. 1998, 28, 492–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betancourt, S.L.; Martínez-Jiménez, S.; Rossi, S.E.; Truong, M.T.; Carrillo, J.; Erasmus, J.J. Lipoid pneumonia: Spectrum of clinical and radiologic manifestations. Am. J. Roentgenol. 2010, 194, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L. On the pathogenesis of post primary tuberculosis: The role of bronchial obstruction in the pathogenesis of cavities. Tuberculosis 2011, 91, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G.; Cardona, P.J.; Kim, M.J.; Allain, S.; Altare, F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 2009, 10, 943–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, R.A.; Prez, R.M.D. Apical localization of pulmonary tuberculosis, chronic pulmonary histoplasmosis, and progressive massive fibrosis of the lung. Chest 1983, 83, 801–805. [Google Scholar] [CrossRef]
- Broch, B.L. Chapter 14, The role of the bronchial tree in the pathogenesis of pulmonary tuberculosis. In The Fundamentals of Pulmonary Tuberculosis and Its Complications for Students, Teachers and Practicing Physicians; Hayes, E., Ed.; Charles C. Thomas: Springfield, IL, USA, 1949; pp. 245–253. [Google Scholar]
- Ahonen, A.; Valavirta, K. Ultrastructure of cilia in pulmonary tuberculosis. Eur. J. Respir. Dis. Suppl. 1983, 128, 460–463. [Google Scholar]
- Altet, N.; Latorre, I.; Jiménez-Fuentes, M.Á.; Maldonado, J.; Molina, I.; González-Díaz, Y.; Milà, C.; García-García, E.; Muriel, B.; Villar-Hernández, R.; et al. Assessment of the influence of direct tobacco smoke on infection and active TB management. PLoS ONE 2017, 12, e0182998. [Google Scholar] [CrossRef] [Green Version]
- Pai, M.; Mohan, A.; Dheda, K.; Leung, C.C.; Yew, W.W.; Christopher, D.J.; Sharma, S.K. Lethal interaction: The colliding epidemics of tobacco and tuberculosis. Expert Rev. Anti-Infective Ther. 2007, 5, 385–391. [Google Scholar] [CrossRef]
- Leung, A.N. Pulmonary tuberculosis: The essentials. Radiology 1999, 210, 307–322. [Google Scholar] [CrossRef]
- Daley, C.L.; Gotway, M. Chapter 24 Imaging of tuberculosis in adults. In Tuberculosis. A Comprehensive Clinical Reference; Schaaf, H., Zumla, A.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 237–261. [Google Scholar]
- Behr, M.A.; Edelstein, P.H.; Ramakrishnan, L. Revisiting the timetable of tuberculosis. BMJ 2018, 362, k2738. [Google Scholar] [CrossRef] [Green Version]
- Cardona, P.-J. Reactivation or reinfection in adult tuberculosis: Is that the question? Int. J. Mycobacteriol. 2016, 5, 400–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, R.L.; Hwang, S.A.; Jagannath, C.; Actor, J.K. Cord factor as an invisibility cloak? A hypothesis for asymptomatic TB persistence. Tuberculosis 2016, 101, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Middlebrook, G. General discussion of Bloch’s paper. In CIBA Foundation Symposium on Experimental Tuberculosis; Wolstenholme, G., Ed.; Little Brown & Co.: Boston, MA, USA, 1995; p. 142. [Google Scholar]
- Middlebrook, G.; Dubos, R.J.; Pierce, C. Virulence and morphological characteristics of mammalian tubercle bacilli. J. Exp. Med. 1947, 86, 175–184. [Google Scholar] [CrossRef]
- Behling, C.A.; Bennett, B.; Takayama, K.; Hunter, R.L. Development of a trehalose 6,6′-dimycolate model which explains cord formation by Mycobacterium tuberculosis. Infect. Immun. 1993, 61, 2296–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kan-Sutton, C.; Jagannath, C.; Hunter, R.L.; Hunterjr, R. Trehalose 6,6′-dimycolate on the surface of Mycobacterium tuberculosis modulates surface marker expression for antigen presentation and costimulation in murine macrophages. Microbes Infect. 2009, 11, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Hunter, R.L.; Olsen, M.R.; Jagannath, C.; Actor, J.K. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann. Clin. Lab. Sci. 2006, 36, 371–386. [Google Scholar]
- Retzinger, G.S.; Meredith, S.C.; Hunter, R.L.; Takayama, K.; Kézdy, F.J. Identification of the physiologically active state of the mycobacterial glycolipid trehalose 6,6′-dimycolate and the role of fibrinogen in the biologic activities of trehalose 6,6′-dimycolate monolayers. J. Immunol. 1982, 129, 735–744. [Google Scholar]
- Syed, S.S.; Hunter, R.L. Studies on the toxic effects of quartz and a mycobacterial glycolipid, trehalose 6,6’-dimycolate. Ann Clin Lab Sci. 1997, 27, 375–383. [Google Scholar]
- Schabbing, R.W.; Garcia, A.; Hunter, R.L. Characterization of the trehalose 6,6′-dimycolate surface monolayer by scanning tunneling microscopy. Infect. Immun. 1994, 62, 754–756. [Google Scholar] [CrossRef] [Green Version]
- Behling, C.A.; Perez, R.L.; Kidd, M.R.; Staton, G.W.; Hunter, R.L. Induction of pulmonary granulomas, macrophage procoagulant activity, and tumor necrosis factor-alpha by trehalose glycolipids. Ann. Clin. Lab. Sci. 1993, 23, 256–266. [Google Scholar]
- Bloch, H. Virulence of mycobacteria. Bibl. Tuberc. 1955, 9, 49–61. [Google Scholar] [PubMed]
- Indrigo, J.; Hunter, R.L., Jr.; Actor, J.K. Influence of trehalose 6,6′-dimycolate (TDM) during mycobacterial infection of bone marrow macrophages. Microbiology 2002, 148, 1991–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indrigo, J.; Hunter, R.L., Jr.; Actor, J.K. Cord factor trehalose 6,6′-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology 2003, 149, 2049–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrella, J.L.; Kan-Sutton, C.; Gong, X.; Rajagoapalan, M.; Lewis, D.E.; Hunter, R.L.; Eissa, N.T.; Jagannath, C. A novel in vitro human macrophage model to study the persistence of mycobacterium tuberculosis using vitamin d3 and retinoic acid activated thp-1 macrophages. Front. Microbiol. 2011, 2, 67. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J. The Clinical Pathology of Pulmonary Tuberculosis in Adults. An Analysis of 500 Necropsies Personally Reformed or Observed. Am. Rev. Tuberc. 1922, 6, 975–993. [Google Scholar]
- Deli, F.; Romano, F.; Gualini, G.; Mariani, G.M.; Sala, I.; Veneziano, F.; Bertero, L.; Cassoni, P.; Aimetti, M. Resident memory T cells: Possible players in periodontal disease recurrence. J. Periodontal Res. 2020, 55, 324–330. [Google Scholar] [CrossRef]
- Ogongo, P.; Porterfield, J.Z.; Leslie, A. Lung tissue resident memory t-cells in the immune response to mycobacterium tuberculosis. Front. Immunol. 2019, 10, 992. [Google Scholar] [CrossRef] [Green Version]
- Bull, N.C.; Kaveh, D.A.; Garcia-Pelayo, M.; Stylianou, E.; McShane, H.; Hogarth, P. Induction and maintenance of a phenotypically heterogeneous lung tissue-resident CD4+ T cell population following BCG immunisation. Vaccine 2018, 36, 5625–5635. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Welsh, K.J.; Risin, S.A.; Actor, J.K.; Hunter, R.L. Immunopathology of postprimary tuberculosis: Increased t-regulatory cells and dec-205-positive foamy macrophages in cavitary lesions. Clin. Dev. Immunol. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.E.; Hunter, R.L.; Hwang, S.A. Morphoproteomic-guided host-directed therapy for tuberculosis. Front. Immunol. 2017, 8, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tezera, L.B.; Bielecka, M.K.; Ogongo, P.; Walker, N.F.; Ellis, M.; Garay-Baquero, D.J.; Thomas, K.; Reichmann, M.T.; Johnston, D.A.; Wilkinson, R.J.; et al. Anti-PD-1 immunotherapy leads to tuberculosis reactivation via dysregulation of TNF-α. eLife 2020, 9, e52668. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Schlesinger, L.S.; Friedman, A. Modeling granulomas in response to infection in the lung. PLoS ONE 2016, 11, e0148738. [Google Scholar] [CrossRef] [Green Version]
- Martini, K.; Loubet, A.; Bankier, A.; Bouam, S.; Morand, P.; Cassagnes, L.; Revel, M.-P.; Chassagnon, G. Nodular reverse halo sign in active pulmonary tuberculosis: A rare CT feature? Diagn. Interv. Imaging 2020, 101, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Armitige, L.; Wanger, A.; Hunter, R.L.; Hwang, S.A. Association of pellicle growth morphological characteristics and clinical presentation of Mycobacterium tuberculosis isolates. Tuberculosis 2016, 101, S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Kana, B.D.; Gordhan, B.G.; Downing, K.J.; Sung, N.; Vostroktunova, G.; Machowski, E.E.; Tsenova, L.; Young, M.; Kaprelyants, A.; Kaplan, G.; et al. The resuscitation-promoting factors ofMycobacterium tuberculosisare required for virulence and resuscitation from dormancy but are collectively dispensable for growthin vitro. Mol. Microbiol. 2008, 67, 672–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, T. The Structure of Scientific Revolutions; University of Chicago Press: Chicago, IL, USA, 1962. [Google Scholar]
- National Research Council. A New Biology for the 21st Century; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Woese, C.R. A new biology for a new century. Microbiol. Mol. Biol. Rev. 2004, 68, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Elkington, P.T.; Tebruegge, M.; Mansour, S. Tuberculosis: An infection-initiated autoimmune disease? Trends Immunol. 2016, 37, 815–818. [Google Scholar] [CrossRef] [Green Version]
- Behr, M.A.; Waters, W.R. Is tuberculosis a lymphatic disease with a pulmonary portal? Lancet Infect. Dis. 2014, 14, 250–255. [Google Scholar] [CrossRef]
- Young, D. Animal models of tuberculosis. Eur. J. Immunol. 2009, 39, 2011–2014. [Google Scholar] [CrossRef]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P. The Immune Response in Tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef] [PubMed]
- Pagel, W. Experimental studies on early pulmonary tuberculosis of the “adult type”. Br. J. Tuberc. 1936, 30, 204–218. [Google Scholar] [CrossRef]
- Lurie, M. The correlation between the histological changes and the fate of living tubercle bacilli in the organs of tubeculous rabbits. J. Exp. Med. 1932, 55, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L.; Actor, J.K.; Hwang, S.-A.; Khan, A.; Urbanowski, M.E.; Kaushal, D.; Jagannath, C. Pathogenesis and animal models of post-primary (bronchogenic) tuberculosis, a review. Pathogens 2018, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Urbanowski, M.E.; Ordonez, A.A.; Ruiz-Bedoya, C.A.; Jain, S.K.; Bishai, W.R. Cavitary tuberculosis: The gateway of disease transmission. Lancet Infect. Dis. 2020, 20, e117–e128. [Google Scholar] [CrossRef]
- Kubler, A.B.; Luna, C.; Larsson, N.C.; Ammerman, B.B.; Andrade, M.; Orandle, K.W.; Bock, Z.; Xu, U.; Bagci, D.J.; Molura, J.; et al. Mycobacterium tuberculosis dysregulates MMP/TIMP balance to drive rapid cavitation and unrestrained bacterial proliferation. J. Pathol. 2015, 235, 431–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.S.; Proulx, J.F.; Menzies, D.; Behr, M.A. Progression to tuberculosis disease increases with multiple exposures. Eur. Respir. J. 2016, 48, 1682–1689. [Google Scholar] [CrossRef] [Green Version]
- Hunter, R.L.; Olsen, M.; Jagannath, C.; Actor, J.K. Trehalose 6,6′-dimycolate and lipid in the pathogenesis of caseating granulomas of tuberculosis in mice. Am. J. Pathol. 2006, 168, 1249–1261. [Google Scholar] [CrossRef] [Green Version]
- Riaz, S.M.; Bjune, G.A.; Wiker, H.G.; Sviland, L.; Mustafa, T. Mycobacterial antigens accumulation in foamy macrophages in murine pulmonary tuberculosis lesions: Association with necrosis and making of cavities. Scand. J. Immunol. 2020, 91, e12866. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, T.; Phyu, S.; Nilsen, R.; Jonsson, R.; Bjune, G.A. A mouse model for slowly progressive primary tuberculosis. Scand. J. Immunol. 1999, 50, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, T.; Leversen, N.A.; Sviland, L.; Wiker, H.G. Differential in vivo expression of mycobacterial antigens in Mycobacterium tuberculosis infected lungs and lymph node tissues. BMC Infect. Dis. 2014, 14, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloch, H.; Noll, H. Studies on the virulence of tubercle bacilli. The effect of cord factor on murine Tuberculosis. Br. J. Exp. Pathol. 1955, 36, 8–17. [Google Scholar] [PubMed]
- Flynn, J.L.; Scanga, C.A.; Tanaka, K.E.; Chan, J. Effects of aminoguanidine on latent murine tuberculosis. J. Immunol. 1998, 160, 1796–1803. [Google Scholar] [PubMed]
- Scanga, C.A.; Flynn, J.L. Modeling tuberculosis in nonhuman primates. Cold Spring Harb. Perspect. Med. 2014, 4, a018564. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.L.; Flynn, J.L. The end of the binary era: Revisiting the spectrum of tuberculosis. J. Immunol. 2018, 201, 2541–2548. [Google Scholar] [CrossRef] [Green Version]
- Leong, F.J.; Dartois, V.; Dick, T. A Color Atlas of Comparative Pathology of Pulmonary Tuberculosis; CRC Press: New York, NY, USA, 2011. [Google Scholar]
- Helke, K.L.; Mankowski, J.L.; Manabe, Y.C. Animal models of cavitation in pulmonary tuberculosis. Tuberculosis 2006, 86, 337–348. [Google Scholar] [CrossRef]
- Barclay, W.R.; Anacker, R.L.; Brehmer, W.; Leif, W.; Ribi, E. Aerosol-induced tuberculosis in subhuman primates and the course of the disease after intravenous BCG vaccination. Infect. Immun. 1970, 2, 574–582. [Google Scholar] [CrossRef] [Green Version]
- Nattusamy, L.; Madan, K.; Bhalla, A.S.; Guleria, R. Reversed halo sign in active pulmonary tuberculosis. BMJ Case Rep. 2014. [Google Scholar] [CrossRef] [Green Version]
- Comas, I.; Chakravartti, J.; Small, P.M.; Galagan, J.E.; Niemann, S.; Kremer, K.; Ernst, J.D.; Gagneux, S. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat. Genet. 2010, 42, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Ernst, J.D. Mechanisms of M. tuberculosis immune evasion as challenges to TB vaccine design. Cell Host Microbe 2018, 24, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Coscollà, M.; Copin, R.; Sutherland, J.S.; Gehre, F.; De Jong, B.C.; Owolabi, O.; Mbayo, G.; Giardina, F.; Ernst, J.D.; Gagneux, S.M. tuberculosis T cell epitope analysis reveals paucity of antigenic variation and identifies rare variable TB antigens. Cell Host Microbe 2015, 18, 538–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra, E.R.; Francisco-Cruz, A.; Wistuba, I. State-of-the-art of profiling immune contexture in the era of multiplexed staining and digital analysis to study paraffin tumor tissues. Cancers 2019, 11, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunter, R.L. The Pathogenesis of Tuberculosis–The Koch Phenomenon Reinstated. Pathogens 2020, 9, 813. https://doi.org/10.3390/pathogens9100813
Hunter RL. The Pathogenesis of Tuberculosis–The Koch Phenomenon Reinstated. Pathogens. 2020; 9(10):813. https://doi.org/10.3390/pathogens9100813
Chicago/Turabian StyleHunter, Robert L. 2020. "The Pathogenesis of Tuberculosis–The Koch Phenomenon Reinstated" Pathogens 9, no. 10: 813. https://doi.org/10.3390/pathogens9100813
APA StyleHunter, R. L. (2020). The Pathogenesis of Tuberculosis–The Koch Phenomenon Reinstated. Pathogens, 9(10), 813. https://doi.org/10.3390/pathogens9100813



