Gut Symbiotic Microbial Communities in the IUCN Critically Endangered Pinna nobilis Suffering from Mass Mortalities, Revealed by 16S rRNA Amplicon NGS
Abstract
:1. Introduction
2. Materials and Methods
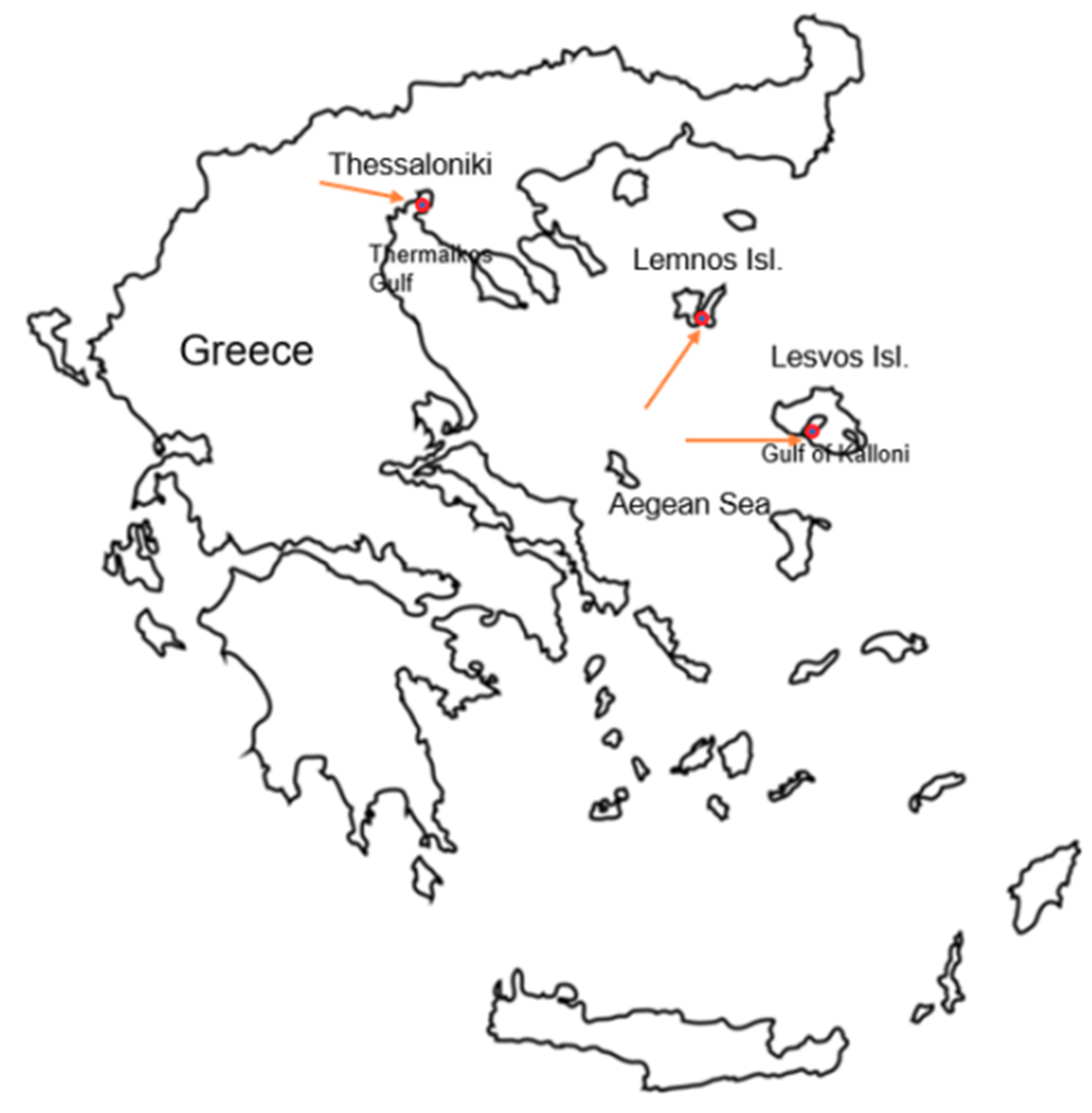
Assessment of Gut Microbiome in P. nobilis from Greek Mass Mortality Events
3. Results
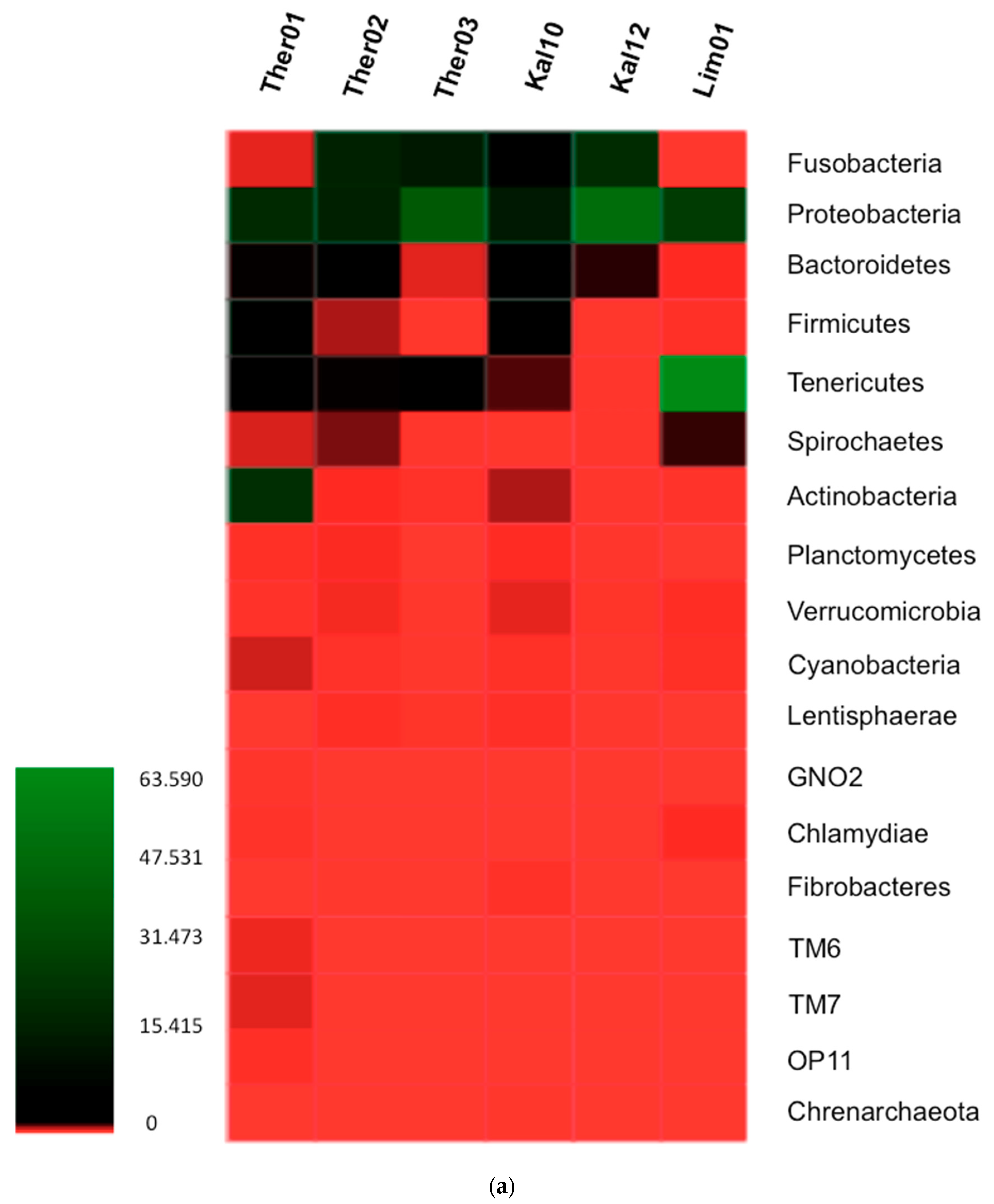
3.1. Gut Microbiota Phylum Analysis in Pinna nobilis
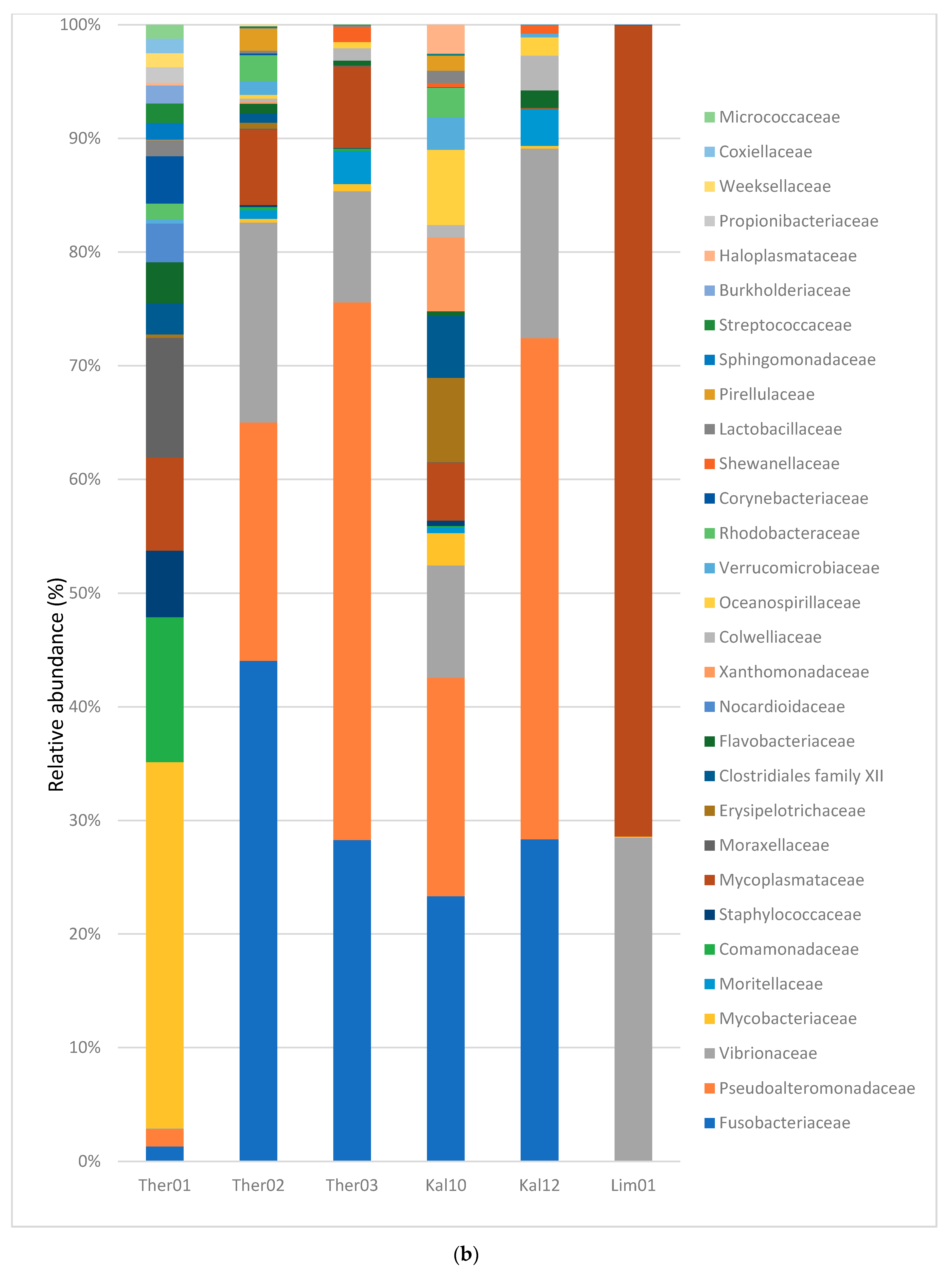
3.2. Gut Microbiota Families Analysis in Pinna nobilis
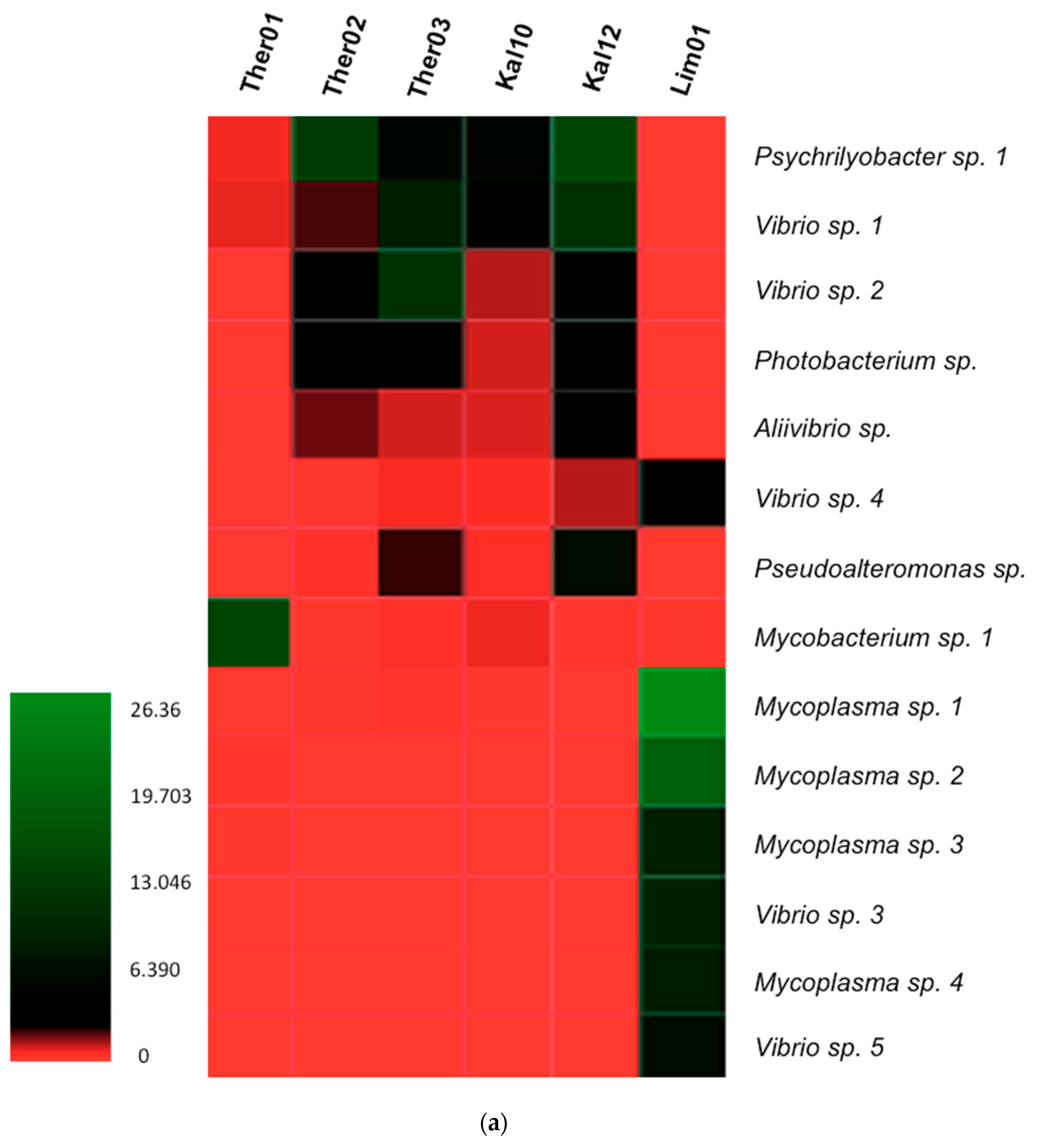
3.3. Gut Microbiota Genus Analysis in Pinna nobilis
3.4. Blastn Search of Bacterial Genera
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sibley, C.D.; Peirano, G.; Church, D.L. Molecular methods for pathogen and microbial community detection and characterization: Current and potential application in diagnostic microbiology. Infect. Genet. Evol. 2012, 12, 505–521. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Theis, K.R. Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol. 2015, 13, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.; Cunha, A.; Castilho, F.; Romalde, J.L.; Pereira, M.J. Microbial contamination and purification of bivalve shellfish: Crucial aspects in monitoring and future perspectives—A mini-review. Food Control. 2011, 22, 805–816. [Google Scholar] [CrossRef]
- Vezzulli, L.; Stagnaro, L.; Grande, C.; Tassistro, G.; Canesi, L.; Pruzzo, C. Comparative 16SrDNA Gene-Based Microbiota Profiles of the Pacific Oyster (Crassostrea gigas) and the Mediterranean Mussel (Mytilus galloprovincialis) from a Shellfish Farm (Ligurian Sea, Italy). Microb. Ecol. 2018, 75, 495–504. [Google Scholar] [CrossRef]
- Prado, S.; Romalde, J.L.; Barja, J.L. Review of probiotics for use in bivalve hatcheries. Vet. Microbiol. 2010, 145, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Ringø, E.; Zhou, Z.; Vecino, J.L.G.; Wadsworth, S.; Romero, J.; Krogdahl, A.; Olsen, R.E.; Dimitroglou, A.; Foey, A.; Davies, S.; et al. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquac. Nutr. 2016, 22, 219–282. [Google Scholar] [CrossRef] [Green Version]
- Flint, H.J. The impact of nutrition on the human microbiome. Nutr. Rev. 2012, 70. [Google Scholar] [CrossRef]
- Biesalski, H.K. Nutrition meets the microbiome: Micronutrients and the microbiota. Ann. N. Y. Acad. Sci. 2016, 1372, 53–64. [Google Scholar] [CrossRef]
- Matozzo, V.; Marin, M.G. Bivalve immune responses and climate changes: Is there a relationship? Invertebr. Surviv. J. 2011, 8, 70–77. [Google Scholar]
- Garnier, M.; Labreuche, Y.; Garcia, C.; Robert, M.; Nicolas, J.L. Evidence for the involvement of pathogenic bacteria in summer mortalities of the pacific oyster Crassostrea gigas. Microb. Ecol. 2007, 53, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Lokmer, A.; Mathias Wegner, K. Hemolymph microbiome of Pacific oysters in response to temperature, temperature stress and infection. ISME J. 2015, 9, 670–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, M.L.; Ward, J.E. Microbial Ecology of the Bivalvia, with an Emphasis on the Family Ostreidae. J. Shellfish Res. 2018, 37, 793–806. [Google Scholar] [CrossRef]
- Llewellyn, M.S.; Leadbeater, S.; Garcia, C.; Sylvain, F.E.; Custodio, M.; Ang, K.P.; Powell, F.; Carvalho, G.R.; Creer, S.; Elliot, J.; et al. Parasitism perturbs the mucosal microbiome of Atlantic Salmon. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bayer, T.; Arif, C.; Ferrier-Pagès, C.; Zoccola, D.; Aranda, M.; Voolstra, C.R. Bacteria of the genus Endozoicomonas dominate the microbiome of the Mediterranean gorgonian coral Eunicella cavolini. Mar. Ecol. Prog. Ser. 2013, 479, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Blanquer, A.; Uriz, M.J.; Cebrian, E.; Galand, P.E. Snapshot of a bacterial microbiome shift during the early symptoms of a massive sponge die-off in the western Mediterranean. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisterhans, G.; Raymond, N.; Girault, E.; Lambert, C.; Bourrasseau, L.; de Montaudouin, X.; Garabetian, F.; Jude-Lemeilleur, F. Structure of Manila Clam (Ruditapes philippinarum) Microbiota at the Organ Scale in Contrasting Sets of Individuals. Microb. Ecol. 2016, 71, 194–206. [Google Scholar] [CrossRef]
- Li, Y.F.; Yang, N.; Liang, X.; Yoshida, A.; Osatomi, K.; Power, D.; Batista, F.M.; Yang, J.L. Elevated seawater temperatures decrease microbial diversity in the gut of Mytilus coruscus. Front. Physiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.F.; Chen, Y.W.; Xu, J.K.; Ding, W.Y.; Shao, A.Q.; Zhu, Y.T.; Wang, C.; Liang, X.; Yang, J.L. Temperature elevation and Vibrio cyclitrophicus infection reduce the diversity of haemolymph microbiome of the mussel Mytilus coruscus. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Milan, M.; Smits, M.; Dalla Rovere, G.; Iori, S.; Zampieri, A.; Carraro, L.; Martino, C.; Papetti, C.; Ianni, A.; Ferri, N.; et al. Host-microbiota interactions shed light on mortality events in the striped venus clam Chamelea gallina. Mol. Ecol. 2019, 28, 4486–4499. [Google Scholar] [CrossRef]
- Green, T.J.; Siboni, N.; King, W.L.; Labbate, M.; Seymour, J.R.; Raftos, D. Simulated Marine Heat Wave Alters Abundance and Structure of Vibrio Populations Associated with the Pacific Oyster Resulting in a Mass Mortality Event. Microb. Ecol. 2019, 77, 736–747. [Google Scholar] [CrossRef]
- Theodorou, J.A.; James, R.; Tagalis, D.; Tzovenis, I.; Hellio, C.; Katselis, G. Density and size structure of the endangered fan mussel Pinna nobilis (Linnaeus 1758), in the shallow water zone of Maliakos Gulf, Greece. Acta Adriat. 2017, 58, 63–76. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tsirintanis, K.; Tsaparis, D.; Doukas, D.; Sini, M.; Athanassopoulou, F.; Κolygas, M.; Tontis, D.; Koutsoubas, D.; Bakopoulos, V. The cryptogenic parasite Haplosporidium pinnae invades the Aegean Sea and causes the collapse of Pinna nobilis populations. Aquat. Invasions 2019, 14, 150–164. [Google Scholar] [CrossRef]
- Zotou, M.; Gkrantounis, P.; Karadimou, E.; Tsirintanis, K.; Sini, M.; Poursanidis, D.; Azzolin, M.; Dailianis, T.; Kytinou, E.; Issaris, Y.; et al. Pinna nobilis in the Greek seas (NE Mediterranean): On the brink of extinction? Mediterr. Mar. Sci. 2020, 21, 575–591. [Google Scholar] [CrossRef]
- Rabaoui, L.; Tlig-Zouari, S.; Katsanevakis, S.; Ben Hassine, O.K. Modelling population density of Pinna nobilis (Bivalvia) on the eastern and southeastern coast of Tunisia. J. Molluscan Stud. 2010, 76, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Sanna, D.; Cossu, P.; Dedola, G.L.; Scarpa, F.; Maltagliati, F.; Castelli, A.; Franzoi, P.; Lai, T.; Cristo, B.; Curini-Galletti, M.; et al. Mitochondrial DNA Reveals Genetic Structuring of Pinna nobilis across the Mediterranean Sea. PLoS ONE 2013, 8, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanna, D.; Debola, G.L.; Scarpa, F.; Lai, T.; Cossu, P.; Curini-Galletti, M.; Francalacci, P.; Casu, M. New mitochondrial and nuclear primers for the Mediterranean marine bivalve Pinna nobilis. Mediterr. Mar. Sci. 2014, 15, 416. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Luis, M.; Borg, J.A.; Morell, C.; Banach-Esteve, G.; Deudero, S. Influence of boat anchoring on Pinna nobilis: A field experiment using mimic units. Mar. Freshw. Res. 2015, 66, 786–794. [Google Scholar] [CrossRef] [Green Version]
- Kersting, D.; Benabdi, M.; Čižmek, H.; Grau, A.; Jimenez, C.; Katsanevakis, S.; Öztürk, B.; Tuncer, S.; Tunesi, L.; Vázquez-Luis, M.; et al. Pinna nobilis. The IUCN Red List of Threatened Species 2019: e.T160075998A160081499. 2019. Available online: https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T160075998A160081499.en (accessed on 20 October 2020).
- Darriba, S. First haplosporidan parasite reported infecting a member of the Superfamily Pinnoidea (Pinna nobilis) during a mortality event in Alicante (Spain, Western Mediterranean). J. Invertebr. Pathol. 2017, 148, 14–19. [Google Scholar] [CrossRef]
- Vázquez-Luis, M.; Álvarez, E.; Barrajón, A.; García-March, J.R.; Grau, A.; Hendriks, I.E.; Jiménez, S.; Kersting, D.; Moreno, D.; Pérez, M.; et al. S.O.S. Pinna nobilis: A mass mortality event in western Mediterranean Sea. Front. Mar. Sci. 2017, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Catanese, G.; Grau, A.; Valencia, J.M.; Garcia-March, J.R.; Vázquez-Luis, M.; Alvarez, E.; Deudero, S.; Darriba, S.; Carballal, M.J.; Villalba, A. Haplosporidium pinnae sp. nov., a haplosporidan parasite associated with mass mortalities of the fan mussel, Pinna nobilis, in the Western Mediterranean Sea. J. Invertebr. Pathol. 2018, 157, 9–24. [Google Scholar] [CrossRef]
- Panarese, R.; Tedesco, P.; Chimienti, G.; Latrofa, M.S.; Quaglio, F.; Passantino, G.; Buonavoglia, C.; Gustinelli, A.; Tursi, A.; Otranto, D. Haplosporidium pinnae associated with mass mortality in endangered Pinna nobilis (Linnaeus 1758) fan mussels. J. Invertebr. Pathol. 2019, 164, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Cabanellas-Reboredo, M.; Vázquez-Luis, M.; Mourre, B.; Álvarez, E.; Deudero, S.; Amores, Á.; Addis, P.; Ballesteros, E.; Barrajón, A.; Coppa, S.; et al. Tracking a mass mortality outbreak of pen shell Pinna nobilis populations: A collaborative effort of scientists and citizens. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattos, A.; Giantsis, I.A.; Karagiannis, D.; Michaelidis, B. First detection of the invasive Haplosporidian and Mycobacteria parasites hosting the endangered bivalve Pinna nobilis in Thermaikos Gulf, North Greece. Mar. Environ. Res. 2020, 155, 104889. [Google Scholar] [CrossRef] [PubMed]
- García-March, J.R.; Tena, J.; Henandis, S.; Vázquez-Luis, M.; López, D.; Téllez, C.; Prado, P.; Navas, J.I.; Bernal, J.; Catanese, G.; et al. Can we save a marine species affected by a highly infective, highly lethal, waterborne disease from extinction? Biol. Conserv. 2020, 243, 108498. [Google Scholar] [CrossRef]
- Čižmek, H.; Čolić, B.; Gračan, R.; Grau, A.; Catanese, G. An emergency situation for pen shells in the Mediterranean: The Adriatic Sea, one of the last Pinna nobilis shelters, is now affected by a mass mortality event. J. Invertebr. Pathol. 2020, 173, 107388. [Google Scholar] [CrossRef]
- Carella, F.; Aceto, S.; Pollaro, F.; Miccio, A.; Iaria, C.; Carrasco, N.; Prado, P.; De Vico, G. A mycobacterial disease is associated with the silent mass mortality of the pen shell Pinna nobilis along the Tyrrhenian coastline of Italy. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Prado, P.; Carrasco, N.; Catanese, G.; Grau, A.; Cabanes, P.; Carella, F.; García-March, J.R.; Tena, J.; Roque, A.; Bertomeu, E.; et al. Presence of Vibrio mediterranei associated to major mortality in stabled individuals of Pinna nobilis L. Aquaculture 2020, 519, 734899. [Google Scholar] [CrossRef]
- Carella, F.; Elisabetta, A.; Simone, F.; Fulvio, S.; Daniela, M.; Prado, P.; Rossella, P.; Marino, F.; Eleonora, F.; Tobia, P.; et al. In the Wake of the Ongoing Mass Mortality Events: Co-occurrence of Mycobacterium, Haplosporidium and Other Pathogens in Pinna nobilis Collected in Italy and Spain (Mediterranean Sea). Front. Mar. Sci. 2020, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Sawabe, T.; Ogura, Y.; Matsumura, Y.; Feng, G.; Rohul Amin, A.K.M.; Mino, S.; Nakagawa, S.; Sawabe, T.; Kumar, R.; Fukui, Y.; et al. Updating the Vibrio clades defined by multilocus sequence phylogeny: Proposal of eight new clades, and the description of Vibrio tritonius sp. nov. Front. Microbiol. 2013, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Poli, A.; Romano, I.; Mastascusa, V.; Buono, L.; Orlando, P.; Nicolaus, B.; Leone, L.; Hong, K.W.; Chan, K.G.; Goh, K.M.; et al. Vibrio coralliirubri sp. nov., a new species isolated from mucus of red coral (Corallium rubrum) collected at Procida island, Italy. Antonie van Leeuwenhoek 2018, 111, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Hsiao, I.S.; Hsu, C.H.; Chen, J.C. Change in water temperature on the immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus. Fish Shellfish Immunol. 2004, 17, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, D.; de Decker, S.; Haffner, P.; Cobret, L.; Robert, M.; Garcia, C. A large-scale epidemiological study to identify bacteria pathogenic to Pacific Oyster Crassostrea gigas and correlation between virulence and metalloprotease-like activity. Microb. Ecol. 2010, 59, 787–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa Solomieu, V.; Renault, T.; Travers, M.A. Mass mortality in bivalves and the intricate case of the Pacific oyster, Crassostrea gigas. J. Invertebr. Pathol. 2015, 131, 2–10. [Google Scholar] [CrossRef]
- Mandas, D.; Salati, F.; Polinas, M.; Sanna, M.A.; Zobba, R.; Burrai, G.P.; Alberti, A.; Antuofermo, E. Histopathological and Molecular Study of Pacific Oyster Tissues Provides Insights into V. aestuarianus Infection Related to Oyster Mortality. Pathogens 2020, 9, 492. [Google Scholar] [CrossRef]
- Tubiash, H.S.; Chanley, P.E.; Leifson, E. Bacillary necrosis, a disease of larval and juvenile bivalve mollusks. I. Etiology and epizootiology. J. Bacteriol. 1965, 90, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Luna-González, A.; Maeda-Martínez, A.N.; Sainz, J.C.; Ascencio-Valle, F. Comparative susceptibility of veliger larvae of four bivalve mollusks to a Vibrio alginolyticus strain. Dis. Aquat. Organ. 2002, 49, 221–226. [Google Scholar] [CrossRef]
- Gomez-Leon, J.; Villamil, L.; Lemos, M.L.; Novoa, B.; Figueras, A. Isolation of Vibrio alginolyticus and Vibrio splendidus from Aquacultured Carpet Shell Clam (Ruditapes decussatus) Larvae Associated with Mass Mortalities. Appl. Environ. Microbiol. 2005, 71, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Sugumar, G.; Nakai, T.; Hirata, Y.; Matsubara, D.; Muroga, K. Vibrio splendidus biovar II as the causative agent of bacillary necrosis of Japanese oyster Crassostrea gigas larvae. Dis. Aquat. Organ. 1998, 33, 111–118. [Google Scholar] [CrossRef]
- Le Roux, F.; Gay, M.; Lambert, C.; Waechter, M.; Poubalanne, S.; Chollet, B.; Nicolas, J.L.; Berthe, F. Comparative analysis of Vibrio splendidus-related strains isolated during Crassostrea gigas mortality events. Aquat. Living Resour. 2002, 15, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Qiu, L.; Yu, Z.; Zi, J.; Yue, F.; Wang, L.; Zhang, H.; Teng, W.; Liu, X.; Song, L. Identification and characterisation of pathogenic Vibrio splendidus from Yesso scallop (Patinopecten yessoensis) cultured in a low temperature environment. J. Invertebr. Pathol. 2013, 114, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.; Miranda, C.D.; Opazo, R.; Romero, J. Characterization and pathogenicity of Vibrio splendidus strains associated with massive mortalities of commercial hatchery-reared larvae of scallop Argopecten purpuratus (Lamarck, 1819). J. Invertebr. Pathol. 2015, 124, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Domeneghetti, S.; Varotto, L.; Civettini, M.; Rosani, U.; Stauder, M.; Pretto, T.; Pezzati, E.; Arcangeli, G.; Turolla, E.; Pallavicini, A.; et al. Mortality occurrence and pathogen detection in Crassostrea gigas and Mytilus galloprovincialis close-growing in shallow waters (Goro lagoon, Italy). Fish Shellfish Immunol. 2014, 41, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Oden, E.; Burioli, E.A.V.; Trancart, S.; Pitel, P.H.; Houssin, M. Multilocus sequence analysis of Vibrio splendidus related-strains isolated from blue mussel Mytilus sp. during mortality events. Aquaculture 2016, 464, 420–427. [Google Scholar] [CrossRef]
- Hada, H.S.; West, P.A.; Lee, J.V. Vibrio tubiashii sp. nov., a pathogen of bivalve mollusks. Int. J. Syst. Bacteriol. 1984, 34, 1–4. [Google Scholar] [CrossRef]
- Prado, S.; Dubert, J.; Barja, J.L. Characterization of pathogenic vibrios isolated from bivalve hatcheries in Galicia, NW Atlantic coast of Spain. Description of Vibrio tubiashii subsp. europaensis subsp. nov. Syst. Appl. Microbiol. 2015, 38, 26–29. [Google Scholar] [CrossRef]
- Paillard, C.; Maes, P.; Oubella, R. Brown ring disease in clams. Annu. Rev. Fish Dis. 1994, 4, 219–240. [Google Scholar] [CrossRef]
- Paillard, C.; Le Roux, F.; Borrego, J.J. Bacterial disease in marine bivalves, a review of recent studies: Trends and evolution. Aquat. Living Resour. 2004, 17, 477–498. [Google Scholar] [CrossRef] [Green Version]
- Allam, B.; Paillard, C.; Ford, S.E. Pathogenicity of Vibrio tapetis, the etiological agent of brown ring disease in clams. Dis. Aquat. Organ. 2002, 48, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Paillard, C.; Allam, B.; Oubella, R. Effect of temperature on defense parameters in Manila clam Ruditapes philippinarum challenged with Vibrio tapetis. Dis. Aquat. Organ. 2004, 59, 249–262. [Google Scholar] [CrossRef]
- Bruto, M.; James, A.; Petton, B.; Labreuche, Y.; Chenivesse, S.; Alunno-Bruscia, M.; Polz, M.F.; Le Roux, F. Vibrio crassostreae, a benign oyster colonizer turned into a pathogen after plasmid acquisition. ISME J. 2017, 11, 1043–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Haim, Y.; Thompson, F.L.; Thompson, C.C.; Cnockaert, M.C.; Hoste, B.; Swings, J.; Rosenberg, E. Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int. J. Syst. Evol. Microbiol. 2003, 53, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.P.; Watson, M.A.; Needleman, D.S.; Church, K.M.; Häse, C.C. Mortalities of Eastern And Pacific oyster larvae caused by the pathogens Vibrio coralliilyticus and Vibrio tubiashii. Appl. Environ. Microbiol. 2015, 81, 292–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balbi, T.; Auguste, M.; Cortese, K.; Montagna, M.; Borello, A.; Pruzzo, C.; Vezzulli, L.; Canesi, L. Responses of Mytilus galloprovincialis to challenge with the emerging marine pathogen Vibrio coralliilyticus. Fish Shellfish Immunol. 2019, 84, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Jun, J.W.; Giri, S.S.; Chi, C.; Yun, S.; Kim, S.G.; Kim, S.W.; Han, S.J.; Kwon, J.; Oh, W.T.; et al. Identification and genome analysis of Vibrio coralliilyticus causing mortality of pacific oyster (Crassostrea gigas) larvae. Pathogens 2020, 9, 206. [Google Scholar] [CrossRef] [Green Version]
- Pujalte, M.J.; Garay, E. Proposal of Vibrio mediterranei sp. nov.: A New Marine Member of the Genus Vibrio. Int. J. Syst. Bact. 1986, 36, 278–281. [Google Scholar] [CrossRef]
- Thompson, F.L.; Hoste, B.; Thompson, C.C.; Huys, G.; Swings, J. The coral bleaching Vibrio shiloi Kushmaro et al. 2001 is a later synonym of Vibrio mediterranei Pujalte and Garay 1986. Syst. Appl. Microbiol. 2001, 24, 516–519. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Q.; He, Y.; Tao, Z.; Xu, M.; Luo, Q.; Chen, J.; Chen, H. Isolation and identification of Vibrio mediterranei 117-T6 as a pathogen associated with yellow spot disease of Pyropia (Bangiales, Rhodophyta). Aquaculture 2020, 526, 735372. [Google Scholar] [CrossRef]
- Thompson, F.L.; Thompson, C.C.; Li, Y.; Gomez-Gil, B.; Vandenberghe, J.; Hoste, B.; Swings, J. Vibrio kanaloae sp. nov., Vibrio pomeroyi sp. nov. and Vibrio chagasii sp. nov., from sea water and marine animals. Int. J. Syst. Evol. Microbiol. 2003, 53, 753–759. [Google Scholar] [CrossRef]
- Le Roux, F.; Goubet, A.; Thompson, F.L.; Faury, N.; Gay, M.; Swings, J.; Saulnier, D. Vibrio gigantis sp. nov., isolated from the haemolymph of cultured oysters (Crassostrea gigas). Int. J. Syst. Evol. Microbiol. 2005, 55, 2251–2255. [Google Scholar] [CrossRef]
- Fabbro, C.; Celussi, M.; Russell, H.; del Negro, P. Phenotypic and genetic diversity of coexisting Listonella anguillarum, Vibrio harveyi and Vibrio chagassi recovered from skin haemorrhages of diseased sand smelt, Atherina boyeri, in the Gulf of Trieste (NE Adriatic Sea). Lett. Appl. Microbiol. 2012, 54, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Lasa, A.; Avendaño-Herrera, R.; Estrada, J.M.; Romalde, J.L. Isolation and identification of Vibrio toranzoniae associated with diseased red conger eel (Genypterus chilensis) farmed in Chile. Vet. Microbiol. 2015, 179, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gil, B.; Roque, A.; Rotllant, G.; Peinado, L.; Romalde, J.L.; Doce, A.; Cabanillas-Beltrán, H.; Chimetto, L.A.; Thompson, F.L. Photobacterium swingsii sp. nov., isolated from marine organisms. Int. J. Syst. Evol. Microbiol. 2011, 61, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.J.; Lemos, M.L.; Osorio, C.R. Photobacterium damselae subsp. Damselae, a bacterium pathogenic for marine animals and humans. Front. Microbiol. 2013, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Romalde, J.L.; Diéguez, A.L.; Lasa, A.; Balboa, S. New Vibrio species associated to molluscan microbiota: A review. Front. Microbiol. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Eggermont, M.; Bossier, P.; Pande, G.S.J.; Delahaut, V.; Rayhan, A.M.; Gupta, N.; Islam, S.S.; Yumo, E.; Nevejan, N.; Sorgeloos, P.; et al. Isolation of Vibrionaceae from wild blue mussel (Mytilus edulis) adults and their impact on blue mussel larviculture. FEMS Microbiol. Ecol. 2017, 93, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Gil, B.; Roque, A.; Rotllant, G.; Romalde, J.L.; Doce, A.; Eggermont, M.; Defoirdt, T. Photobacterium sanguinicancri sp. nov. isolated from marine animals. Antonie van Leeuwenhoek 2016, 109, 817–825. [Google Scholar] [CrossRef]
- Urbanczyk, H.; Ast, J.C.; Dunlap, P.V. Phylogeny, genomics, and symbiosis of Photobacterium. FEMS Microbiol. Rev. 2011, 35, 324–342. [Google Scholar] [CrossRef]
- Urbanczyk, H.; Ast, J.C.; Higgins, M.J.; Carson, J.; Dunlap, P.V. Reclassification of Vibrio fischeri, Vibrio logei, Vibrio salmonicida and Vibrio wodanis as Aliivibrio fischeri gen. nov., comb. nov., Aliivibrio logei comb. nov., Aliivibrio salmonicida comb. nov. and Aliivibrio wodanis comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 2823–2829. [Google Scholar] [CrossRef] [Green Version]
- Stabili, L.; Giangrande, A.; Pizzolante, G.; Caruso, G.; Alifano, P. Characterization of Vibrios Diversity in the Mucus of the Polychaete Myxicola infundibulum (Annellida, Polichaeta). Microb. Ecol. 2014, 67, 186–194. [Google Scholar] [CrossRef]
- Fidopiastis, P.M.; Von Boletzky, S.; Ruby, E.G. A new niche for Vibrio logei, the predominant light organ symbiont of squids in the genus Sepiola. J. Bacteriol. 1998, 180, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebob, T.J.; Mboto, C.I.; Asikong, B.E.E.; Ukwuoma, I.C. Vibrio cholerae Incursion in Africa, the Journey So Far. J. Sci. Res. Rep. 2019, 25, 1–12. [Google Scholar] [CrossRef]
- Olafsen, J.A.; Mikkelsen, H.V.; Glaever, H.M.; Hansen, G.H. Indigenous bacteria in hemolymph and tissues of marine bivalves at low temperatures. Appl. Environ. Microbiol. 1993, 59, 1848–1854. [Google Scholar] [CrossRef] [Green Version]
- Offret, C.; Desriac, F.; Le Chevalier, P.; Mounier, J.; Jégou, C.; Fleury, Y. Spotlight on antimicrobial metabolites from the marine bacteria Pseudoalteromonas: Chemodiversity and ecological significance. Mar. Drugs 2016, 14, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longeon, A.; Peduzzi, J.; Barthélemy, M.; Corre, S.; Nicolas, J.L.; Guyot, M. Purification and partial identification of novel antimicrobial protein from marine bacterium Pseudoalteromonas species strain X153. Mar. Biotechnol. 2004, 6, 633–641. [Google Scholar] [CrossRef]
- Kesarcodi-Watson, A.; Miner, P.; Nicolas, J.L.; Robert, R. Protective effect of four potential probiotics against pathogen-challenge of the larvae of three bivalves: Pacific oyster (Crassostrea gigas), flat oyster (Ostrea edulis) and scallop (Pecten maximus). Aquaculture 2012, 344–349, 29–34. [Google Scholar] [CrossRef]
- Ma, Y.X.; Liu, J.C.; Li, M.; Tao, W.; Yu, Z.C.; Liu, Y. Bin The use of Pseudoalteromonas sp. F15 in larviculture of the Yesso scallop, Patinopecten yessoensis. Aquac. Res. 2019, 50, 1844–1850. [Google Scholar] [CrossRef]
- Sandaa, R.A.; Brunvold, L.; Magnesen, T.; Bergh, Ø. Monitoring the opportunistic bacteria Pseudoalteromonas sp. LT-13 in a great scallop, Pecten maximus hatchery. Aquaculture 2008, 276, 14–21. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Z.; Shi, Y.; Ding, H.H. Microbiological analysis and microbiota in oyster: A review. Invertebr. Surviv. J. 2016, 13, 374–388. [Google Scholar] [CrossRef]
- King, W.L.; Siboni, N.; Williams, N.L.R.; Kahlke, T.; Nguyen, K.V.; Jenkins, C.; Dove, M.; O’Connor, W.; Seymour, J.R.; Labbate, M. Variability in the composition of pacific oyster microbiomes across oyster families exhibiting different levels of susceptibility to OsHV-1 μvar disease. Front. Microbiol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Clerissi, C.; de Lorgeril, J.; Petton, B.; Lucasson, A.; Escoubas, J.M.; Gueguen, Y.; Dégremont, L.; Mitta, G.; Toulza, E. Microbiota Composition and Evenness Predict Survival Rate of Oysters Confronted to Pacific Oyster Mortality Syndrome. Front. Microbiol. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, T.W.; Baer, J.G.; Ward, J.E. Direct Comparison of Fecal and Gut Microbiota in the Blue Mussel (Mytilus edulis) Discourages Fecal Sampling as a Proxy for Resident Gut Community. Microb. Ecol. 2020, 1–13. [Google Scholar] [CrossRef]
- Razin, S.; Yogev, D.; Naot, Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 1998, 62, 1094–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harshbarger, J.C.; Chang, S.C.; Otto, S.V. Chlamydiae (with Phages), Mycoplasmas, and Rickettsiae in Chesapeake Bay Bivalves. Science 1977, 196, 666–668. [Google Scholar] [CrossRef]
- Azevedo, C. Occurrence of an unusual branchial mycoplasma-like infection in cockle Cerastoderma edule (Moliusca, Bivalvia). Dis. Aquat. Organ. 1993, 16, 55–59. [Google Scholar] [CrossRef]
- King, G.M.; Judd, C.; Kuske, C.R.; Smith, C. Analysis of Stomach and Gut Microbiomes of the Eastern Oyster (Crassostrea virginica) from Coastal Louisiana, USA. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Lokmer, A.; Kuenzel, S.; Baines, J.F.; Wegner, K.M. The role of tissue-specific microbiota in initial establishment success of Pacific oysters. Environ. Microbiol. 2016, 18, 970–987. [Google Scholar] [CrossRef] [Green Version]
- Cleary, D.F.R.; Becking, L.E.; Polónia, A.R.M.; Freitas, R.M.; Gomes, N.C.M. Composition and predicted functional ecology of mussel-associated bacteria in Indonesian marine lakes. Antonie van Leeuwenhoek 2015, 107, 821–834. [Google Scholar] [CrossRef]
- Pavlinec, Ž.; Zupičić, I.G.; Oraić, D.; Petani, B.; Mustać, B.; Mihaljević, Ž.; Beck, R.; Zrnčić, S. Assessment of predominant bacteria in noble pen shell (Pinna nobilis) collected in the Eastern Adriatic Sea. Environ. Monit. Assess. 2020, 192. [Google Scholar] [CrossRef]
- Davidovich, N.; Morick, D.; Carella, F. Mycobacteriosis in aquatic invertebrates: A review of its emergence. Microorganisms 2020, 8, 1249. [Google Scholar] [CrossRef]
- Grimm, C.; Huntsberger, C.; Markey, K.; Inglis, S.; Smolowitz, R. Identification of a Mycobacterium sp. as the causative agent of orange nodular lesions in the Atlantic sea scallop Placopecten magellanicus. Dis. Aquat. Organ. 2016, 118, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, F.; Sanna, D.; Azzena, I.; Mugetti, D.; Cerruti, F.; Hosseini, S.; Cossu, P.; Pinna, S.; Grech, D.; Cabana, D.; et al. Multiple non-species-specific pathogens possibly triggered the mass mortality in pinna nobilis. Life 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Šarić, T.; Župan, I.; Aceto, S.; Villari, G.; Palić, D.; De Vico, G.; Carella, F. Epidemiology of noble pen shell (Pinna nobilis l. 1758) mass mortality events in adriatic sea is characterised with rapid spreading and acute disease progression. Pathogens 2020, 9, 776. [Google Scholar] [CrossRef] [PubMed]



| Specimen id | H. pinnae | Mycobacterium spp. |
|---|---|---|
| Kal10 | + | |
| Kal12 | + | + |
| Lim01 | + | + |
| Ther01 | + | |
| Ther02 | + | + |
| Ther03 | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lattos, A.; Giantsis, I.A.; Karagiannis, D.; Theodorou, J.A.; Michaelidis, B. Gut Symbiotic Microbial Communities in the IUCN Critically Endangered Pinna nobilis Suffering from Mass Mortalities, Revealed by 16S rRNA Amplicon NGS. Pathogens 2020, 9, 1002. https://doi.org/10.3390/pathogens9121002
Lattos A, Giantsis IA, Karagiannis D, Theodorou JA, Michaelidis B. Gut Symbiotic Microbial Communities in the IUCN Critically Endangered Pinna nobilis Suffering from Mass Mortalities, Revealed by 16S rRNA Amplicon NGS. Pathogens. 2020; 9(12):1002. https://doi.org/10.3390/pathogens9121002
Chicago/Turabian StyleLattos, Athanasios, Ioannis A. Giantsis, Dimitrios Karagiannis, John A. Theodorou, and Basile Michaelidis. 2020. "Gut Symbiotic Microbial Communities in the IUCN Critically Endangered Pinna nobilis Suffering from Mass Mortalities, Revealed by 16S rRNA Amplicon NGS" Pathogens 9, no. 12: 1002. https://doi.org/10.3390/pathogens9121002
APA StyleLattos, A., Giantsis, I. A., Karagiannis, D., Theodorou, J. A., & Michaelidis, B. (2020). Gut Symbiotic Microbial Communities in the IUCN Critically Endangered Pinna nobilis Suffering from Mass Mortalities, Revealed by 16S rRNA Amplicon NGS. Pathogens, 9(12), 1002. https://doi.org/10.3390/pathogens9121002






