Hemolysin-Producing Strains among Diarrheagenic Escherichia coli Isolated from Children under 2 Years Old with Diarrheal Disease
Abstract
:1. Introduction
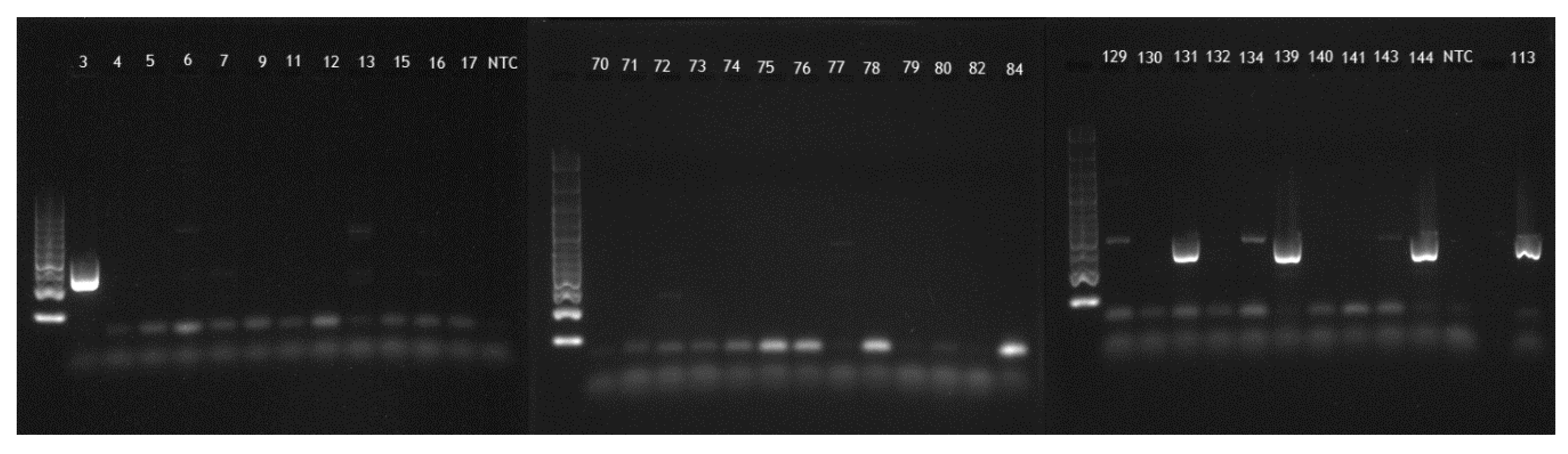
2. Results
3. Discussion
4. Materials and Methods
4.1. PCR
4.2. Hemolytic Activity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guarino, A.; Ashkenazi, S.; Gendrel, D.; Vecchio, A.L.; Shamir, R.; Szajewska, H. European society for pediatric gastroenterology, hepatology, and nutrition/European society for pediatric infectious diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 132–152. [Google Scholar] [CrossRef]
- Salam, M.; Lindberg, G.; Dite, P.; Khalif, I.; Salazar-Lindo, E.; Ramakrishna, B.S.; Goh, K.; Thomson, A.; Khan, A.G.; Krabshuis, D.J.; et al. Acute diarrhea in adults and children: A global perspective. J. Clin. Gastroenterol. 2012, 47, 12–20. [Google Scholar]
- Ogilvie, I.M.; Khoury, H.; Goetghebeur, M.M.; El Khoury, A.C.; Giaquinto, C. Burden of community-acquired and nosocomial rotavirus gastroenteritis in the pediatric population of Western Europe: A scoping review. BMC Infect. Dis. 2012, 12, 62. [Google Scholar] [CrossRef]
- Zollner-Schwetz, I.; Krause, R. Therapy of acute gastroenteritis: Role of antibiotics. Clin. Microbiol. Infect. 2015, 21, 744–749. [Google Scholar] [CrossRef] [Green Version]
- Bruzzese, E.; Giannattasio, A.; Guarino, A. Antibiotic treatment of acute gastroenteritis in children. F1000Research 2018, 7, 193. [Google Scholar] [CrossRef]
- Shane, A.L.; Mody, R.K.; Crump, J.A.; Tarr, P.I.; Steiner, T.S.; Kotloff, K.; Langley, J.M.; Wanke, C.; Warren, C.A.; Cheng, A.C.; et al. 2017 infectious diseases society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin. Infect. Dis. 2017, 65, e45–e80. [Google Scholar] [CrossRef] [Green Version]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; De Silva, N.R.; Gargouri, N.; et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [Green Version]
- Feng, P.; Weagant, S.D.; Karen, J. BAM Chapter 4A: Diarrheagenic Escherichia Coli; United States Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Debroy, C.; Fratamico, P.M.; Roberts, E. Molecular serogrouping of Escherichia coli. Anim. Health Res. Rev. 2018, 19, 1–16. [Google Scholar] [CrossRef]
- Shridhar, P.B.; Patel, I.R.; Gangiredla, J.; Noll, L.W.; Shi, X.; Bai, J.; Nagaraja, T.G. DNA microarray-based genomic characterization of the pathotypes of Escherichia coli O26, O45, O103, O111, and O145 isolated from feces of feedlot cattle. J. Food Prot. 2019, 82, 395–404. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Mangione-Smith, R.; Hicks, L.A. How to prescribe fewer unnecessary antibiotics: Talking points that work with patients and their families. Am. Fam. Physician 2016, 94, 200–202. [Google Scholar]
- Vecchio, A.L.; Liguoro, I.; Bruzzese, D.; Scotto, R.; Parola, L.; Gargantini, G.; Guarino, A. Adherence to guidelines for management of children hospitalized for acute diarrhea. Pediatr. Infect. Dis. J. 2014, 33, 1103–1108. [Google Scholar] [CrossRef]
- Bellido-Blasco, J.; Arnedo-Pena, A. Epidemiology of infectious diarrhea. Encycl. Environ. Health 2011, 659–671. [Google Scholar] [CrossRef]
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.; Zimsen, S.R.; Albertson, S.B.; Stanaway, J.D.; Deshpande, A.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Ai, J.; Liu, W.; Ji, H.; Zhang, X.; Peng, Z.; Wu, Y.; Shi, Y.; Shen, W.; Bao, C. Epidemiology of infectious diarrhoea and the relationship with etiological and meteorological factors in Jiangsu Province, China. Sci. Rep. 2019, 9, 19571–19579. [Google Scholar] [CrossRef]
- Radlovic, N.; Lekovic, Z.; Vuletic, B.; Radlovic, V.; Simić, D.M. Acute diarrhea in children. Srp. Arh. Celok. Lek. 2015, 143, 755–762. [Google Scholar] [CrossRef]
- Tam, C.C.; O’Brien, S.J.; Tompkins, D.S.; Bolton, F.J.; Berry, L.; Dodds, J.; Choudhury, D.; Halstead, F.; Iturriza-Gómara, M.; Mather, K.; et al. Changes in causes of acute gastroenteritis in the United Kingdom over 15 Years: Microbiologic findings from 2 prospective, population-based studies of infectious intestinal disease. Clin. Infect. Dis. 2012, 54, 1275–1286. [Google Scholar] [CrossRef]
- Olesen, B.; Neimann, J.; Böttiger, B.; Ethelberg, S.; Schiellerup, P.; Jensen, C.; Helms, M.; Scheutz, F.; Olsen, K.E.P.; Krogfelt, K.A.; et al. Etiology of diarrhea in young children in Denmark: A case-control study. J. Clin. Microbiol. 2005, 43, 3636–3641. [Google Scholar] [CrossRef] [Green Version]
- Lorrot, M.; Bon, F.; El Hajje, M.J.; Aho, S.; Wolfer, M.; Giraudon, H.; Kaplon, J.; Marc, E.; Raymond, J.; Lebon, P.; et al. Epidemiology and clinical features of gastroenteritis in hospitalised children: Prospective survey during a 2-year period in a Parisian hospital, France. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 30, 361–368. [Google Scholar] [CrossRef]
- Yu, J.; Jing, H.; Lai, S.; Xu, W.; Li, M.; Wu, J.; Liu, W.; Yuan, Z.; Chen, Y.; Zhao, S.; et al. Etiology of diarrhea among children under the age five in China: Results from a five-year surveillance. J. Infect. 2015, 71, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Dumitrescu, A.; Carp, S.; Ilie, M.M.; Dumea, E.; Rugina, S.; Halichidis, S.; Cambrea, S.C. Etiology of acute diarrhea in patients requiring hospitalization in Clinical Infectious Diseases Hospital—Constanța. BMC Infect. Dis. 2014, 14, O27. [Google Scholar] [CrossRef] [Green Version]
- Elias, N.; Pop, D. Etiology and complications of acute gastroenteritis in hospitalized children. Rom. J. Pediatr. 2019, 68, 171–175. [Google Scholar] [CrossRef]
- Robins-Browne, R.M.; Holt, K.E.; Ingle, D.J.; Hocking, D.M.; Yang, J.; Tauschek, M. Are Escherichia coli pathotypes still relevant in the era of whole-genome sequencing? Front. Cell. Infect. Microbiol. 2016, 6, 141. [Google Scholar] [CrossRef] [Green Version]
- Fratamico, P.M.; Debroy, C.; Liu, Y.; Needleman, D.S.; Baranzoni, G.M.; Feng, P. Advances in molecular serotyping and subtyping of Escherichia coli. Front. Microbiol. 2016, 7, 644. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control. External Quality Assurance Scheme for Typing of Verocytotoxin-Producing E. coli (VTEC); ECDC: Stockholm, Sweden, 2012.
- E. Coli Antisera SSI Diagnostica. Available online: https://www.ssidiagnostica.com/antisera/e-coli-antisera/ (accessed on 20 October 2020).
- Rasheed, M.U.; Jamil, K.; Thajuddin, N.; Pasupuleti, M.; Ahamed, P.; Muthukumaresan, K.P. Distribution of the stx1, stx2 and hlyA genes: Antibiotic profiling in Shiga-toxigenic E. coli strains isolated from food sources. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 348–361. [Google Scholar]
- Li, D.; Shen, M.; Xu, Y.; Liu, C.; Wang, W.; Wu, J.; Luo, X.; Jia, X.; Ma, Y. Virulence gene profiles and molecular genetic characteristics of diarrheagenic Escherichia coli from a hospital in western China. Gut Pathog. 2018, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, M.; White, P.; Jongsma, H.E.; Schofield, P.; Armstrong, D. Antibiotic prescribing and patient satisfaction in primary care in England: Cross-sectional analysis of national patient survey data and prescribing data. Br. J. Gen. Pract. 2015, 66, e40–e46. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Wong, J.G.; Lye, D.C.; Chen, M.I.; Loh, V.W.; Leo, Y.-S.; Lee, L.K.; Chow, A.L. Medical and psychosocial factors associated with antibiotic prescribing in primary care: Survey questionnaire and factor analysis. Br. J. Gen. Pract. 2017, 67, e168–e177. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control. Survey of Healthcare Workers’ Knowledge, Attitudes and Behaviors on Antibiotics, Antibiotic Use and Antibiotic Resistance in the EU/EEA; ECDC: Stockholm, Sweden, 2019.
- European Public Health Alliance. In the Red Zone—Antimicrobial Resis-Tance: Lessons from Romania; European Public Health Alliance: Brussels, Belgium, 2017. [Google Scholar]
- Usein, C.-R.; Tatu-Chitoiu, D.; Ciontea, S.; Condei, M.; Damian, M. Escherichia coli pathotypes associated with diarrhea in Romanian children younger than 5 years of age. Jpn. J. Infect. Dis. 2009, 62, 289–293. [Google Scholar]
- Cambrea, S.C. Antibiotic susceptibility of Escherichia coli strains isolated in a pediatric population from South Eastern Romania. J. Pediatr. Infect. Dis. 2015, 9, 157–162. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2011. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); ECDC: Stockholm, Sweden, 2012.
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe 2018; ECDC: Stockholm, Sweden, 2019.
- Hristea, A.; Ion, M.; Maxim, D.; Banic, L.; Nica, M.; Buzea, M.; Streinu-Cercel, A.; Olaru, I.D. Class 1 integrons in drug-resistant E. coli and K. pneumoniae from blood stream infections. Rev. Romana Med. Lab. 2012, 20, 8. [Google Scholar]
- Sepp, E.; Andreson, R.; Balode, A.; Bilozor, A.; Brauer, A.; Egorova, S.; Huik, K.; Ivanova, M.; Kaftyreva, L.; Kõljalg, S.; et al. Phenotypic and molecular epidemiology of ESBL-, AmpC-, and Carbapenemase-producing Escherichia coli in northern and eastern Europe. Front. Microbiol. 2019, 10, 2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, Y.; Iovleva, A.; Bonomo, R.A. The ecology of extended-spectrum β-lactamases (ESBLs) in the developed world. J. Travel Med. 2017, 24, S44–S51. [Google Scholar] [CrossRef] [PubMed]
- Coque, T.M.; Baquero, F.; Cantón, R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Eurosurveillance 2008, 13, 13. [Google Scholar]
- Local Prevalence of Extended-Spectrum Beta-Lactamase (ESBL) Producing Enterobacteriaceae Intestinal Carriers at Admission and Co-Expression of ESBL and OXA-48 Carbapenemase in Klebsiella Pneumoniae: A Prevalence Survey in a Spanish University Hospital—Abstract—Europe PMC. Available online: https://europepmc.org/article/pmc/pmc6429960 (accessed on 19 October 2020).
- Borcan, E.; Ghiţă, C.M.; Chifiriuc, M.C.; Măruţescu, L.; Isar, C.; Lazar, V. Antibiotic resistance of Gram negative bacilli strains isolated from the intensive care unit in Fundeni Clinical Institute, Bucharest, Romania. Roum. Arch. Microbiol. Immunol. 2010, 68, 228–234. [Google Scholar]
- Miftode, E.; Dorneanu, O.; Badescu, A.; Ghibu, L.; Leca, D.; Vremera, T.; Mereuţă, A. Emergence of a new group CTX-M enzyme in Romania and risk factors for extended spectrum beta-lactamase producing E. coli infections. Rev. Med. Chir. Soc. Med. Nat. Iasi 2012, 116, 477–480. [Google Scholar]
- Maciuca, I.E.; Williams, N.J.; Tuchilus, C.; Dorneanu, O.; Guguianu, E.; Carp-Carare, C.; Rimbu, C.; Timofte, D. High prevalence of Escherichia coli-producing CTX-M-15 extended-spectrum beta-lactamases in poultry and human clinical isolates in Romania. Microb. Drug Resist. 2015, 21, 651–662. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 474 M100 -S27; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2017. [Google Scholar]
- Magalhães, C.A.; Rossato, S.S.; Barbosa, A.S.; Dos Santos, T.O.; Elias, W.P.; Sircili, M.P.; Piazza, R.M. The ability of haemolysins expressed by atypical enteropathogenic Escherichia coli to bind to extracellular matrix components. Mem. Inst. Oswaldo Cruz 2011, 106, 146–152. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mare, A.; Man, A.; Toma, F.; Ciurea, C.N.; Coșeriu, R.L.; Vintilă, C.; Maier, A.C. Hemolysin-Producing Strains among Diarrheagenic Escherichia coli Isolated from Children under 2 Years Old with Diarrheal Disease. Pathogens 2020, 9, 1022. https://doi.org/10.3390/pathogens9121022
Mare A, Man A, Toma F, Ciurea CN, Coșeriu RL, Vintilă C, Maier AC. Hemolysin-Producing Strains among Diarrheagenic Escherichia coli Isolated from Children under 2 Years Old with Diarrheal Disease. Pathogens. 2020; 9(12):1022. https://doi.org/10.3390/pathogens9121022
Chicago/Turabian StyleMare, Anca, Adrian Man, Felicia Toma, Cristina Nicoleta Ciurea, Răzvan Lucian Coșeriu, Camelia Vintilă, and Adrian Cornel Maier. 2020. "Hemolysin-Producing Strains among Diarrheagenic Escherichia coli Isolated from Children under 2 Years Old with Diarrheal Disease" Pathogens 9, no. 12: 1022. https://doi.org/10.3390/pathogens9121022






