Detection of Crenosoma spp., Angiostrongylus vasorum and Aelurostrongylus abstrusus in Gastropods in Eastern Austria
Abstract
:1. Introduction
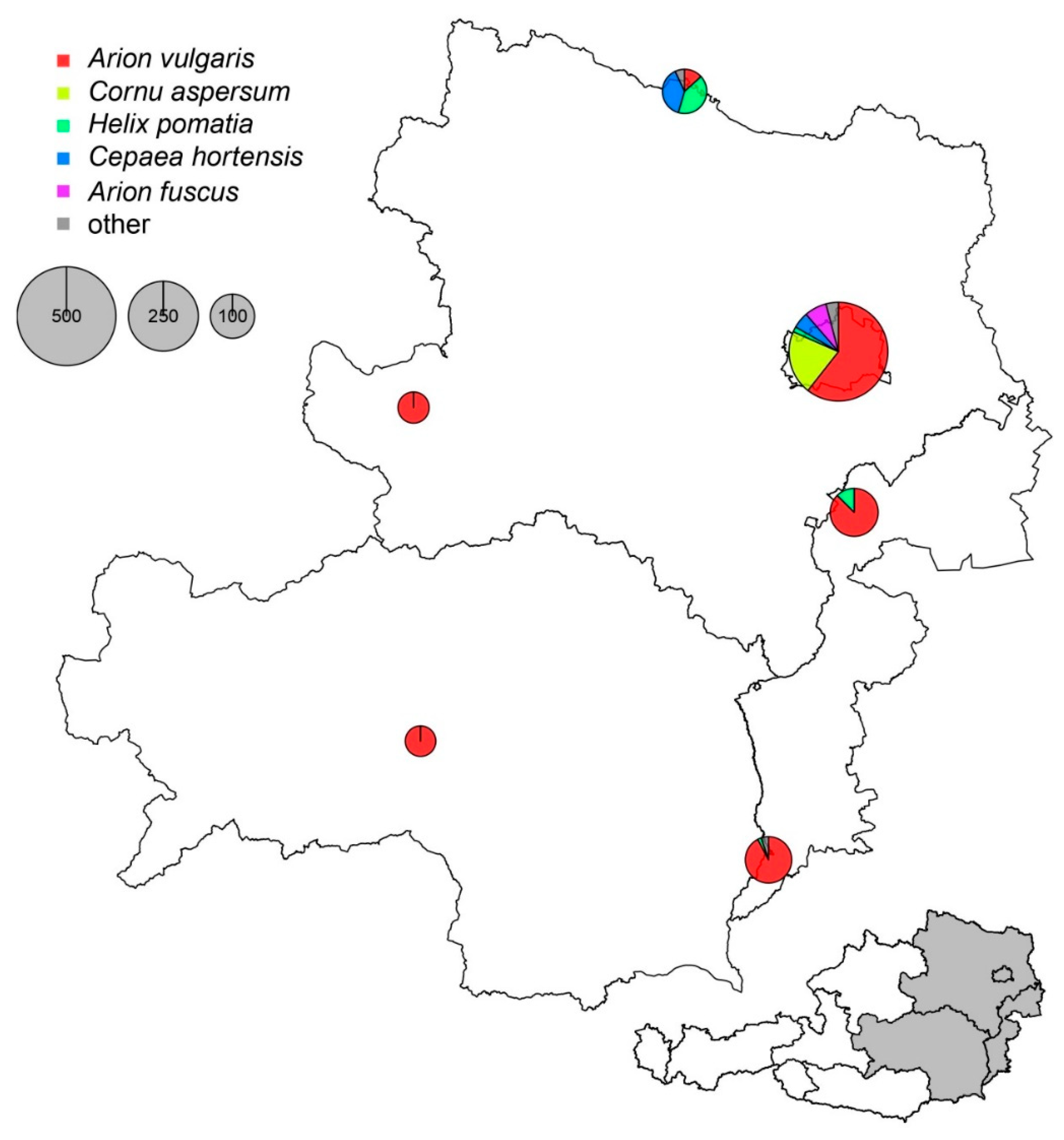
2. Results
3. Discussion
4. Materials and Methods
4.1. Snail Collection and Digestion
4.2. Molecular Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Traversa, D.; Di Cesare, A.; Conboy, G. Canine and feline cardiopulmonary parasitic nematodes in Europe: Emerging and underestimated. Parasites Vectors 2010, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, A.; Capelli, G.; Joachim, A.; Hinney, B.; Losson, B.; Kirkova, Z.; René-Martellet, M.; Papadopoulos, E.; Farkas, R.; Napoli, E.; et al. Lungworms and gastrointestinal parasites of domestic cats: A European perspective. Int. J. Parasitol. 2017, 47, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, A.; Traversa, D.; Manzocchi, S.; Meloni, S.; Grillotti, E.; Auriemma, E.; Pampurini, F.; Garofani, C.; Ibba, F.; Venco, L. Elusive Angiostrongylus vasorum infections. Parasites Vectors 2015, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisi, P.E.; Aste, G.; Traversa, D.; Di Cesare, A.; Febo, E.; Vignoli, M.; Santori, D.; Luciani, A.; Boari, A. Single and mixed feline lungworm infections: Clinical, radiographic and therapeutic features of 26 cases (2013–2015). J. Feline Med. Surg. 2016, 19, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Elsheikha, H.M.; Holmes, S.A.; Wright, I.; Morgan, E.R.; Lacher, D.W. Recent advances in the epidemiology, clinical and diagnostic features, and control of canine cardio-pulmonary angiostrongylosis. Vet. Res. 2014, 45, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maksimov, P.; Hermosilla, C.; Taubert, A.; Staubach, C.; Sauter-Louis, C.; Conraths, F.J.; Globokar, M.; Pantchev, N. GIS-supported epidemiological analysis on canine Angiostrongylus vasorum and Crenosoma vulpis infections in Germany. Parasites Vectors 2017, 10, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traversa, D.; Di Cesare, A. Diagnosis and management of lungworm infections in cats: Cornerstones, dilemmas and new avenues. J. Feline Med. Surg. 2016, 18, 7–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traversa, D.; Morelli, S.; Cassini, R.; Crisi, P.E.; Russi, I.; Grillotti, E.; Manzocchi, S.; Simonato, G.; Beraldo, P.; Viglietti, A.; et al. Occurrence of canine and feline extra-intestinal nematodes in key endemic regions of Italy. Acta Trop. 2019, 193, 227–235. [Google Scholar] [CrossRef]
- Taylor, C.S.; Gato, R.G.; Learmount, J.; Aziz, N.A.; Montgomery, C.; Rose, H.; Coulthwaite, C.L.; McGarry, J.W.; Forman, D.W.; Allen, S.; et al. Increased prevalence and geographic spread of the cardiopulmonary nematode Angiostrongylus vasorum in fox populations in Great Britain. Parasitology 2015, 142, 1190–1195. [Google Scholar] [CrossRef]
- Tiškina, V.; Lindqvist, E.-L.; Blomqvist, A.-C.; Orav, M.; Stensvold, C.R.; Jokelainen, P. Autochthonous Angiostrongylus vasorum in Finland. Vet. Rec. Open 2019, 6, e000314. [Google Scholar] [CrossRef] [Green Version]
- Traversa, D.; Torbidone, A.; Malatesta, D.; Guglielmini, C. Occurrence of fatal canine Angiostrongylus vasorum infection in Italy. Vet. Parasitol. 2008, 152, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Di Cesare, A.; Meloni, S.; Di Regalbono, A.F.; Milillo, P.; Pampurini, F.; Venco, L. Canine angiostrongylosis in Italy: Occurrence of Angiostrongylus vasorum in dogs with compatible clinical pictures. Parasitol. Res. 2013, 112, 2473–2480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bihr, T.; Conboy, G.A. Lungworm (Crenosoma vulpis) infection in dogs on Prince Edward Island. Can. Vet. J. 1999, 40, 555–559. [Google Scholar] [PubMed]
- De Liberato, C.; Berrilli, F.; Odorizi, L.; Scarcella, R.; Barni, M.; Amoruso, C.; Scarito, A.; Di Filippo, M.M.; Carvelli, A.; Iacoponi, F.; et al. Parasites in stray dogs from Italy: Prevalence, risk factors and management concerns. Acta Parasitol. 2018, 63, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Taubert, A.; Pantchev, N.; Vrhovec, M.G.; Bauer, C.; Hermosilla, C. Lungworm infections (Angiostrongylus vasorum, Crenosoma vulpis, Aelurostrongylus abstrusus) in dogs and cats in Germany and Denmark in 2003–2007. Vet. Parasitol. 2009, 159, 175–180. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Schnyder, M.; Traversa, D.; Di Cesare, A.; Wright, I.; Lacher, D.W. Updates on feline aelurostrongylosis and research priorities for the next decade. Parasites Vectors 2016, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Traversa, D.; Di Cesare, A. Feline lungworms: What a dilemma. Trends Parasitol. 2013, 29, 423–430. [Google Scholar] [CrossRef]
- Crisi, P.E.; Traversa, D.; Di Cesare, A.; Luciani, A.; Civitella, C.; Santori, D.; Boari, A. Irreversible pulmonary hypertension associated with Troglostrongylus brevior infection in a kitten. Res. Vet. Sci. 2015, 102, 223–227. [Google Scholar] [CrossRef]
- Gavrilović, P.; Jovanović, M.; Gavrilović, A.; Nešić, S. Fatal aelurostrongylosis in a kitten in Serbia. Acta Parasitol. 2017, 62, 488–491. [Google Scholar] [CrossRef]
- Crisi, P.E.; Di Cesare, A.; Boari, A. Feline Troglostrongylosis: Current Epizootiology, Clinical Features, and Therapeutic Options. Front. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Lange, M.K.; Vélez, J.; Hirzmann, J.; Gutiérrez-Arboleda, J.; Taubert, A.; Hermosilla, C.; Chaparro-Gutiérrez, J.J. The invasive giant African snail Lissachatina fulica as natural intermediate host of Aelurostrongylus abstrusus, Angiostrongylus vasorum, Troglostrongylus brevior, and Crenosoma vulpis in Colombia. PLoS Negl. Trop. Dis. 2019, 13, e0007277. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Tabares, F.; Groß, K.M.; Hirzmann, J.; Hoos, C.; Lange, M.K.; Taubert, A.; Hermosilla, C. Occurrence of canine and feline lungworms in Arion vulgaris in a park of Vienna: First report of autochthonous Angiostrongylus vasorum, Aelurostrongylus abstrusus and Troglostrongylus brevior in Austria. Parasitol. Res. 2020, 119, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Dimzas, D.; Morelli, S.; Traversa, D.; Di Cesare, A.; Van Bourgonie, Y.R.; Breugelmans, K.; Backeljau, T.; Di Regalbono, A.F.; Diakou, A. Intermediate gastropod hosts of major feline cardiopulmonary nematodes in an area of wildcat and domestic cat sympatry in Greece. Parasites Vectors 2020, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.A.A.; Daly, E.; Allen, S.; Rowson, B.; Greig, C.; Forman, D.W.; Morgan, E.R. Distribution of Angiostrongylus vasorum and its gastropod intermediate hosts along the rural-urban gradient in two cities in the United Kingdom, using real time PCR. Parasites Vectors 2016, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Lange, M.K.; Penagos-Tabares, F.; Hirzmann, J.; Failing, K.; Schaper, R.; Van Bourgonie, Y.; Backeljau, T.; Hermosilla, C.; Taubert, A. Prevalence of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Crenosoma vulpis larvae in native slug populations in Germany. Vet. Parasitol. 2018, 254, 120–130. [Google Scholar] [CrossRef]
- Valente, R.; Diaz, J.I.; Salomón, O.D.; Navone, G.T. Natural infection of the feline lungworm Aelurostrongylus abstrusus in the invasive snail Achatina fulica from Argentina. Vet. Parasitol. 2017, 235, 17–19. [Google Scholar] [CrossRef]
- Morelli, S.; Traversa, D.; Colombo, M.; Raue, K.; Strube, C.; Pollmeier, M.; Di Cesare, A. The effect of the hibernation on the larval development of Troglostrongylus brevior in the land snail Cornu aspersum. Vet. Parasitol. 2020, 282, 109123. [Google Scholar] [CrossRef]
- Conboy, G.; Guselle, N.; Schaper, R. Spontaneous Shedding of Metastrongyloid Third-Stage Larvae by Experimentally Infected Limax maximus. Parasitol. Res. 2017, 116, 41–54. [Google Scholar] [CrossRef]
- Ferdushy, T.; Kapel, C.M.; Webster, P.; Al-Sabi, M.; Grønvold, J. The occurrence of Angiostrongylus vasorum in terrestrial slugs from forests and parks in the Copenhagen area, Denmark. J. Helminthol. 2009, 83, 379–383. [Google Scholar] [CrossRef]
- Jeżewski, W.; Buńkowska-Gawlik, K.; Hildebrand, J.; Perec-Matysiak, A.; Laskowski, Z. Intermediate and paratenic hosts in the life cycle of Aelurostrongylus abstrusus in natural environment. Vet. Parasitol. 2013, 198, 401–405. [Google Scholar] [CrossRef]
- Helm, J.; Roberts, L.; Jefferies, R.; Shaw, S.E.; Morgan, E.R. Epidemiological survey of Angiostrongylus vasorum in dogs and slugs around a new endemic focus in Scotland. Vet. Rec. 2015, 177, 46. [Google Scholar] [CrossRef] [PubMed]
- Hicklenton, L.; Betson, M. Molecular detection of Angiostrongylus vasorum in gastropods in Surrey, UK. Parasitol. Res. 2019, 118, 1051–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaksch, K.; Eschner, A.; Rintelen, T.V.; Haring, E. DNA analysis of molluscs from a museum wet collection: A comparison of different extraction methods. BMC Res. Notes 2016, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, K.; Löwenstein, M.; Duscher, G.; Leschnik, M.; Joachim, A. Angiostrongylus vasorum, der “Französische Herzwurm”: Auch ein Problem in Österreich? Wien Tierarztl Monatsschr 2010, 97, 171. [Google Scholar]
- Reifinger, M.; Greszl, J. Pulmonale Angiostrongylose mit systemischer Ausbreitung und zentralnervaler Manifestation bei einem Hund. Zentralblatt für Veterinärmedizin B 1994, 41, 391–398. [Google Scholar] [CrossRef]
- Pipia, A.P.; Varcasia, A.; Tosciri, G.; Seú, S.; Manunta, M.L.; Mura, M.C.; Sanna, G.; Tamponi, C.; Brianti, E.; Scala, A. New insights onto cardiopulmonary nematodes of dogs in Sardinia, Italy. Parasitol. Res. 2014, 113, 1505–1509. [Google Scholar] [CrossRef]
- Schnyder, M.; Bilbrough, G.; Hafner, C.; Schaper, R. Angiostrongylus vasorum, “The French Heartworm”: A Serological Survey in Dogs from France Introduced by a Brief Historical Review. Parasitol. Res. 2017, 116, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Hajnalová, M.; Svobodová, V.; Schnyder, M.; Schaper, R.; Svoboda, M. Faecal detection of the lungworms Crenosoma vulpis and Angiostrongylus vasorum and serological detection of A. vasorum in dogs from the Czech Republic. Acta Vet. Brno 2017, 86, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Hinaidy, H.K. Die Parasitenfauna des Rotfuchses, Vulpes vulpes (L.), in Österreich. Zentralblatt für Veterinärmedizin Reihe B 1971, 18, 21–32. [Google Scholar] [CrossRef]
- Lassnig, H.; Prosl, H.; Hinterdorfer, F. Zur Parasitenfauna des Rotfuchses (Vulpes vulpes) in der Steiermark. Wien Tierarztl Monatsschr 1998, 85, 116–122. [Google Scholar]
- Hinney, B.; Gottwald, M.; Moser, J.; Reicher, B.; Schäfer, B.J.; Schaper, R.; Joachim, A.; Künzel, F. Examination of anonymous canine faecal samples provides data on endoparasite prevalence rates in dogs for comparative studies. Vet. Parasitol. 2017, 245, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Zottler, E.-M.; Bieri, M.; Basso, W.; Schnyder, M. Intestinal parasites and lungworms in stray, shelter and privately owned cats of Switzerland. Parasitol. Int. 2019, 69, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Kitchener, A.; Driscoll, C.; Nussberger, B. Felis Silvestris. The IUCN Red List of Threatened Species 2015: e.T60354712A50652361. Available online: https://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T60354712A50652361.en (accessed on 8 October 2020).
- National Park Thayatal. Available online: https://www.wildkatze-in-oesterreich.at/de/pages/wildkatze-in-oesterreich-20.aspx (accessed on 8 October 2020).
- Reischütz, P.L. Weichtiere (Mollusca). In Neobiota in Österreich; Essl, F., Rabitsch, W., Eds.; Umweltbundesamt: Vienna, Austria, 2002; pp. 239–250. [Google Scholar]
- Duscher, G.; Pleydell, D.; Prosl, H.; Joachim, A. Echinococcus multilocularis in Austrian Foxes from 1991 until 2004. J. Vet. Med. Ser. B 2006, 53, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.R.; Shaw, S.E.; Brennan, S.F.; De Waal, T.; Jones, B.R.; Mulcahy, G. Angiostrongylus vasorum: A real heartbreaker. Trends Parasitol. 2005, 21, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Hodžić, A.; Alić, A.; Klebić, I.; Kadrić, M.; Brianti, E.; Duscher, G.G. Red fox ( Vulpes vulpes ) as a potential reservoir host of cardiorespiratory parasites in Bosnia and Herzegovina. Vet. Parasitol. 2016, 223, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ash, L.R. Diagnostic Morphology of the Third-Stage Larvae of Angiostrongylus cantonensis, Angiostrongylus vasorum, Aelurostrongylus abstrusus, and Anafilaroides rostratus (Nematoda: Metastrongyloidea). J. Parasitol. 1970, 56, 249. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, A.; Crisi, P.E.; Bartolini, R.; Iorio, R.; Talone, T.; Filippi, L.; Traversa, D. Larval development of Angiostrongylus vasorum in the land snail Helix aspersa. Parasitol. Res. 2015, 114, 3649–3655. [Google Scholar] [CrossRef]
- Di Cesare, A.; Crisi, P.E.; Di Giulio, E.; Veronesi, F.; Frangipane di Regalbono, A.; Talone, T.; Traversa, D. Larval development of Aelurostrongylus abstrusus in Helix aspersa. Parasitol. Res. 2013, 112, 3101–3108. [Google Scholar] [CrossRef]
- Colella, V.; Mutafchiev, Y.; Cavalera, M.A.; Giannelli, A.; Lia, R.P.; Dantas-Torres, F.; Otranto, D. Development of Crenosoma vulpis in the common garden snail Cornu aspersum: Implications for epidemiological studies. Parasites Vectors 2016, 9, 208. [Google Scholar] [CrossRef] [Green Version]
- Gasser, R.B.; Chilton, N.B.; Hoste, H.; Beveridge, I. Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucleic Acids Res. 1993, 21, 2525–2526. [Google Scholar] [CrossRef] [Green Version]
- Annoscia, G.; Latrofa, M.S.; Campbell, B.E.; Giannelli, A.; Ramos, R.A.N.; Dantas-Torres, F.; Brianti, E.; Otranto, D. Simultaneous detection of the feline lungworms Troglostrongylus brevior and Aelurostrongylus abstrusus by a newly developed duplex-PCR. Vet. Parasitol. 2014, 199, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Latrofa, M.S.; Lia, R.P.; Giannelli, A.; Colella, V.; Santoro, M.; D’Alessio, N.; Campbell, B.E.; Parisi, A.; Dantas-Torres, F.; Mutafchiev, Y.; et al. Crenosoma vulpis in wild and domestic carnivores from Italy: A morphological and molecular study. Parasitol. Res. 2015, 114, 3611–3617. [Google Scholar] [CrossRef] [PubMed]


| Species | n | % | Province |
|---|---|---|---|
| Arion vulgaris | 882 | 66.8% | V, LA, ST, B |
| Cornu aspersum | 150 | 11.4% | V, LA |
| Helix pomatia | 96 | 7.3% | V, LA, ST, B |
| Cepaea hortensis | 93 | 7% | V, LA |
| Arion fuscus | 52 | 3.9% | V |
| Limax maximus | 26 | 2% | V, LA |
| Arianta arbustorum | 11 | 0.8% | LA |
| Cepaea nemoralis | 3 | 0.2% | ST |
| Limax cinereoniger | 3 | 0.2% | ST |
| Causacotachea vindobonensis | 2 | 0.1% | ST |
| Arion fasciatus | 1 | <0.1% | LA |
| Macrogastra ventricosa | 1 | <0.1% | V |
| Species | Host | Collection Site (Province) | Max. % Identity to GenBank Entries | GenBank ID |
|---|---|---|---|---|
| Angiostrongylus vasorum | Arion vulgaris | Danube Island (V) | 99.8% (EU627597) UK, dog | MT757393 |
| Aelurostrongylus abstrusus | Arion vulgaris | Friedensbrücke (V) | 100% (JX519458), cat | MT758698 |
| Aelurostrongylus abstrusus | Arion vulgaris | Friedensbrücke (V) | 100% (JX519458), cat | nd |
| Crenosoma striatum | Arion vulgaris | Gerasdorf (LA) | 100% (KR868716), Germany, hedgehog | MT757394 |
| Crenosoma vulpis | Limax maximus | Danube Island (V) | 100% (KR920039), Italy, fox, dog | MT758699 |
| Crenosoma vulpis | Arion vulgaris | Donaustadt (V) | 100% (KF836608), Germany, red fox | nd |
| Crenosoma vulpis | Arion vulgaris | Donaustadt (V) | 100% (KF836608), Germany, red fox | nd |
| Crenosoma vulpis | Arion vulgaris | Friedensbrücke (V) | 99.6% (KR920039), Italy, fox, dog | nd |
| Crenosoma vulpis | Arion vulgaris | Friedensbrücke (V) | 100% (KF836608), Germany, red fox | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuehrer, H.-P.; Morelli, S.; Bleicher, J.; Brauchart, T.; Edler, M.; Eisschiel, N.; Hering, T.; Lercher, S.; Mohab, K.; Reinelt, S.; et al. Detection of Crenosoma spp., Angiostrongylus vasorum and Aelurostrongylus abstrusus in Gastropods in Eastern Austria. Pathogens 2020, 9, 1046. https://doi.org/10.3390/pathogens9121046
Fuehrer H-P, Morelli S, Bleicher J, Brauchart T, Edler M, Eisschiel N, Hering T, Lercher S, Mohab K, Reinelt S, et al. Detection of Crenosoma spp., Angiostrongylus vasorum and Aelurostrongylus abstrusus in Gastropods in Eastern Austria. Pathogens. 2020; 9(12):1046. https://doi.org/10.3390/pathogens9121046
Chicago/Turabian StyleFuehrer, Hans-Peter, Simone Morelli, Julian Bleicher, Thomas Brauchart, Mirjam Edler, Nicole Eisschiel, Tatjana Hering, Sigrun Lercher, Karoline Mohab, Simon Reinelt, and et al. 2020. "Detection of Crenosoma spp., Angiostrongylus vasorum and Aelurostrongylus abstrusus in Gastropods in Eastern Austria" Pathogens 9, no. 12: 1046. https://doi.org/10.3390/pathogens9121046






