Human Neutrophil Granule Exocytosis in Response to Mycobacterium smegmatis
Abstract
:1. Introduction
2. Results
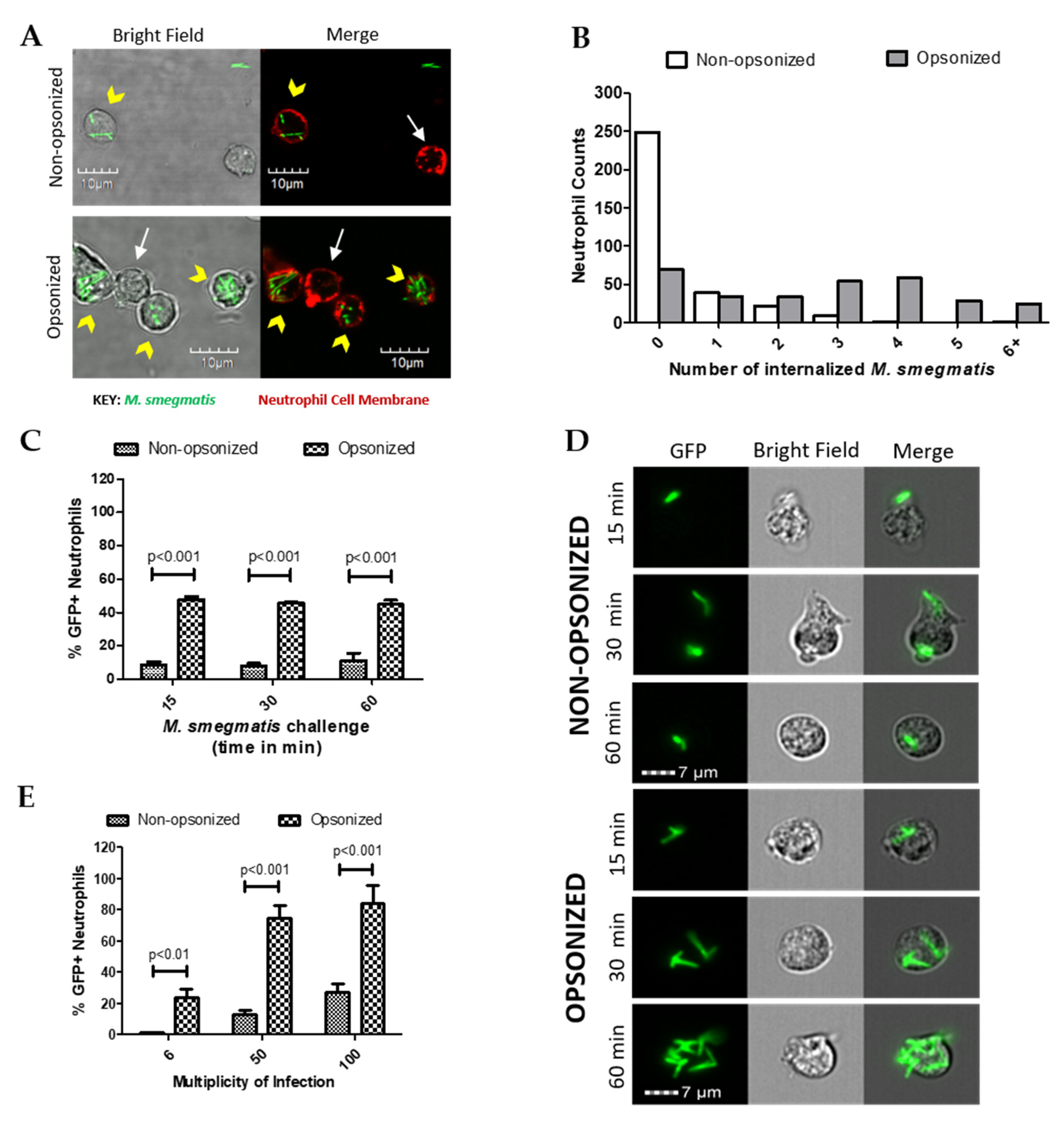
2.1. Opsonization Increases Neutrophil-M. smegmatis Interactions
2.2. Opsonized M. smegmatis Induces the Release of Gelatinase Granules
2.3. M. smegmatis Does Not Cause or Inhibit the Release of Secretory Vesicles, Specific, or Azurophilic Granules at Early Time Points
2.4. Supernatants from M. smegmatis-Treated Neutrophils Degrade Extracellular Matrix Proteins
2.5. M. smegmatis Pre-Treatment Enhances Gelatinase Granule Exocytosis by Secondary Stimuli
2.6. M. smegmatis Cell Wall Glycolipid Stimulation Causes Gelatinase Granule Release
2.7. M. smegmatis Induces Gelatinase and Specific Granule Exocytosis after Extended Timepoints
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shimizu, F.; Hatano, Y.; Okamoto, O.; Katagiri, K.; Fujiwara, S.; Sato, S.; Kato, A.; Uezato, H.; Asato, Y.; Takahashi, K. Mycobacterium smegmatis soft tissue infection. Int. J. Derm. 2012, 51, 1518–1520. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.; Chandra, J.; Mandal, R.N.; Dutta, R.; Jain, N.K. Fatal pulmonary infection caused by Mycobacterium smegmetis in an infant. Indian J. Pediatrics 1995, 62, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.H.; Senanayake, S.; Talaulikar, G.S. Peritoneal dialysis-related peritonitis due to Mycobacterium smegmatis. Perit. Dial. Int. 2011, 31, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Sevrin, A.; Reboli, A.C. Disseminated Mycobacterium smegmatis Infection Associated With an Implantable Cardioverter Defibrillator. Infect. Dis. Clin. Pr. 2009, 17, 349–351. [Google Scholar] [CrossRef]
- Skiest, D.J.; Levi, M.E. Catheter-related bacteremia due to Mycobacterium smegmatis. South Med. J. 1998, 91, 36–37. [Google Scholar] [CrossRef]
- Saffo, Z.; Ognjan, A. Mycobacterium smegmatis infection of a prosthetic total knee arthroplasty. IDCases 2016, 5, 80–82. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Lu, D.; Zhang, X.; Li, H.; Meng, S.; Pan, Y.S.; Boyd, A.S.; Ma, L.; Tang, Y.W. Mycobacterium smegmatis in skin biopsy specimens from patients with suppurative granulomatous inflammation. J. Clin. Microbiol. 2013, 51, 1028–1030. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, J.; Burkhardt, U.; Rusch-Gerdes, S.; Amthor, M.; Richter, E.; Zugehor, M.; Rosahl, W.; Ernst, M. Non-tubercular mycobacterial infection of the lungs due to Mycobacterium smegmatis. Pneumologie 2001, 55, 238–243. [Google Scholar] [CrossRef]
- Pierre-Audigier, C.; Jouanguy, E.; Lamhamedi, S.; Altare, F.; Rauzier, J.; Vincent, V.; Canioni, D.; Emile, J.F.; Fischer, A.; Blanche, S.; et al. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon gamma receptor deficiency. Clin. Infect. Dis. 1997, 24, 982–984. [Google Scholar] [CrossRef] [Green Version]
- Pennekamp, A.; Pfyffer, G.E.; Wuest, J.; George, C.A.; Ruef, C. Mycobacterium smegmatis infection in a healthy woman following a facelift: case report and review of the literature. Ann. Plast. Surg. 1997, 39, 80–83. [Google Scholar] [CrossRef]
- Newton, J.A., Jr.; Weiss, P.J.; Bowler, W.A.; Oldfield, E.C., 3rd. Soft-tissue infection due to Mycobacterium smegmatis: report of two cases. Clin. Infect. Dis. 1993, 16, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Plaus, W.J.; Hermann, G. The surgical management of superficial infections caused by atypical mycobacteria. Surgery 1991, 110, 99–103. [Google Scholar] [PubMed]
- Vonmoos, S.; Leuenberger, P.; Beer, V.; de Haller, R. [Pleuropulmonary infection caused by Mycobacterium smegmatis. Case description and literature review]. Schweiz Med. Wochenschr 1986, 116, 1852–1856. [Google Scholar] [PubMed]
- Alqurashi, M.M.; Alsaileek, A.; Aljizeeri, A.; Bamefleh, H.S.; Alenazi, T.H. Mycobacterium smegmatis causing a granulomatous cardiomediastinal mass. IDCases 2019, 18, e00608. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, R.E.; Besra, G.S.; Belisle, J.T.; Barry, C.E., 3rd. Identification of a gene involved in the biosynthesis of cyclopropanated mycolic acids in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 1995, 92, 6630–6634. [Google Scholar] [CrossRef] [Green Version]
- Voskuil, M.I.; Bartek, I.L.; Visconti, K.; Schoolnik, G.K. The response of mycobacterium tuberculosis to reactive oxygen and nitrogen species. Front. Microbiol. 2011, 2, 105. [Google Scholar] [CrossRef] [Green Version]
- Primm, T.P.; Lucero, C.A.; Falkinham, J.O., 3rd. Health impacts of environmental mycobacteria. Clin. Microbiol. Rev. 2004, 17, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Faldt, J.; Dahlgren, C.; Ridell, M.; Karlsson, A. Priming of human neutrophils by mycobacterial lipoarabinomannans: role of granule mobilisation. Microbes Infect 2001, 3, 1101–1109. [Google Scholar] [CrossRef]
- Nauseef, W.M. How human neutrophils kill and degrade microbes: an integrated view. Immunol. Rev. 2007, 219, 88–102. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borregaard, N.; Cowland, J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997, 89, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N.; Sørensen, O.E.; Theilgaard-Mönch, K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007, 28, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Bartlomiejczyk, M.A.; Swierzko, A.S.; Brzostek, A.; Dziadek, J.; Cedzynski, M. Interaction of lectin pathway of complement-activating pattern recognition molecules with mycobacteria. Clin. Exp. Immunol. 2014, 178, 310–319. [Google Scholar] [CrossRef]
- Cougoule, C.; Constant, P.; Etienne, G.; Daffe, M.; Maridonneau-Parini, I. Lack of fusion of azurophil granules with phagosomes during phagocytosis of Mycobacterium smegmatis by human neutrophils is not actively controlled by the bacterium. Infect. Immun. 2002, 70, 1591–1598. [Google Scholar] [CrossRef] [Green Version]
- N’Diaye, E.N.; Darzacq, X.; Astarie-Dequeker, C.; Daffe, M.; Calafat, J.; Maridonneau-Parini, I. Fusion of azurophil granules with phagosomes and activation of the tyrosine kinase Hck are specifically inhibited during phagocytosis of mycobacteria by human neutrophils. J. Immunol. 1998, 161, 4983–4991. [Google Scholar]
- Gebhard, S.; Humpel, A.; McLellan, A.D.; Cook, G.M. The alternative sigma factor SigF of Mycobacterium smegmatis is required for survival of heat shock, acidic pH and oxidative stress. Microbiology 2008, 154, 2786–2795. [Google Scholar] [CrossRef] [Green Version]
- Faldt, J.; Dahlgren, C.; Ridell, M. Difference in neutrophil cytokine production induced by pathogenic and non-pathogenic mycobacteria. APMIS 2002, 110, 593–600. [Google Scholar] [CrossRef]
- Jena, P.; Mohanty, S.; Mohanty, T.; Kallert, S.; Morgelin, M.; Lindstrom, T.; Borregaard, N.; Stenger, S.; Sonawane, A.; Sorensen, O.E. Azurophil granule proteins constitute the major mycobactericidal proteins in human neutrophils and enhance the killing of mycobacteria in macrophages. PLoS ONE 2012, 7, e50345. [Google Scholar] [CrossRef] [Green Version]
- Peyron, P.; Maridonneau-Parini, I.; Stegmann, T. Fusion of human neutrophil phagosomes with lysosomes in vitro: involvement of tyrosine kinases of the Src family and inhibition by mycobacteria. J. Biol. Chem. 2001, 276, 35512–35517. [Google Scholar] [CrossRef] [Green Version]
- Astarie-Dequeker, C.; Nigou, J.; Puzo, G.; Maridonneau-Parini, I. Lipoarabinomannans activate the protein tyrosine kinase Hck in human neutrophils. Infect. Immun. 2000, 68, 4827–4830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez Flores, E.; Tian, S.; Sizova, M.; Epstein, S.S.; Lamont, R.J.; Uriarte, S.M. Peptoanaerobacter stomatis Primes Human Neutrophils and Induces Granule Exocytosis. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, D.M.; Redford, P.S.; Wilkinson, R.J.; O’Garra, A.; Martineau, A.R. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 2012, 33, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Fietta, A.; Francioli, C.; Gialdroni Grassi, G. Mycobacterial lipoarabinomannan affects human polymorphonuclear and mononuclear phagocyte functions differently. Haematologica 2000, 85, 11–18. [Google Scholar] [PubMed]
- Bernut, A.; Nguyen-Chi, M.; Halloum, I.; Herrmann, J.L.; Lutfalla, G.; Kremer, L. Mycobacterium abscessus-Induced Granuloma Formation Is Strictly Dependent on TNF Signaling and Neutrophil Trafficking. Plos Pathog 2016, 12, e1005986. [Google Scholar] [CrossRef] [PubMed]
- Kroon, E.E.; Coussens, A.K.; Kinnear, C.; Orlova, M.; Moller, M.; Seeger, A.; Wilkinson, R.J.; Hoal, E.G.; Schurr, E. Neutrophils: Innate Effectors of TB Resistance? Front. Immunol. 2018, 9, 2637. [Google Scholar] [CrossRef] [Green Version]
- Malcolm, K.C.; Caceres, S.M.; Pohl, K.; Poch, K.R.; Bernut, A.; Kremer, L.; Bratton, D.L.; Herrmann, J.L.; Nick, J.A. Neutrophil killing of Mycobacterium abscessus by intra- and extracellular mechanisms. PLoS ONE 2018, 13, e0196120. [Google Scholar] [CrossRef]
- Castro, A.G.; Esaguy, N.; Macedo, P.M.; Aguas, A.P.; Silva, M.T. Live but not heat-killed mycobacteria cause rapid chemotaxis of large numbers of eosinophils in vivo and are ingested by the attracted granulocytes. Infect. Immun. 1991, 59, 3009–3014. [Google Scholar] [CrossRef] [Green Version]
- Delafont, V.; Samba-Louaka, A.; Cambau, E.; Bouchon, D.; Moulin, L.; Hechard, Y. Mycobacterium llatzerense, a waterborne Mycobacterium, that resists phagocytosis by Acanthamoeba castellanii. Sci. Rep. 2017, 7, 46270. [Google Scholar] [CrossRef] [Green Version]
- Dubois, V.; Laencina, L.; Bories, A.; Le Moigne, V.; Pawlik, A.; Herrmann, J.L.; Girard-Misguich, F. Identification of Virulence Markers of Mycobacterium abscessus for Intracellular Replication in Phagocytes. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Corleis, B.; Korbel, D.; Wilson, R.; Bylund, J.; Chee, R.; Schaible, U.E. Escape of Mycobacterium tuberculosis from oxidative killing by neutrophils. Cell. Microbiol. 2012, 14, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Coffey, M.J.; Phare, S.M.; Peters-Golden, M. Role of leukotrienes in killing of Mycobacterium bovis by neutrophils. Prostaglandins Leukot. Essent. Fat. Acids 2004, 71, 185–190. [Google Scholar] [CrossRef]
- Hartmann, P.; Becker, R.; Franzen, C.; Schell-Frederick, E.; Romer, J.; Jacobs, M.; Fatkenheuer, G.; Plum, G. Phagocytosis and killing of Mycobacterium avium complex by human neutrophils. J. Leukoc Biol. 2001, 69, 397–404. [Google Scholar] [PubMed]
- Blatchford, G.J.; Perry, R.E.; Thorson, A.G.; Christensen, M.A. Rectal prolapse: rational therapy without foreign material. Neth. J. Surg. 1989, 41, 126–128. [Google Scholar] [PubMed]
- Cundall, M.; Sun, Y.; Miranda, C.; Trudeau, J.B.; Barnes, S.; Wenzel, S.E. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. J. Allergy Clin. Immunol. 2003, 112, 1064–1071. [Google Scholar] [CrossRef]
- Sengelov, H.; Kjeldsen, L.; Borregaard, N. Control of exocytosis in early neutrophil activation. J. Immunol. 1993, 150, 1535–1543. [Google Scholar]
- Uriarte, S.M.; Powell, D.W.; Luerman, G.C.; Merchant, M.L.; Cummins, T.D.; Jog, N.R.; Ward, R.A.; McLeish, K.R. Comparison of proteins expressed on secretory vesicle membranes and plasma membranes of human neutrophils. J. Immunol. (Baltimore, Md. 1950) 2008, 180, 5575–5581. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.H.; Liu, H.; Ge, B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell. Mol. Immunol. 2017, 14, 963–975. [Google Scholar] [CrossRef]
- Augustyniak, D.; Roszkowiak, J.; Wisniewska, I.; Skala, J.; Gorczyca, D.; Drulis-Kawa, Z. Neuropeptides SP and CGRP Diminish the Moraxella catarrhalis Outer Membrane Vesicle- (OMV-) Triggered Inflammatory Response of Human A549 Epithelial Cells and Neutrophils. Mediat. Inflamm. 2018, 2018, 4847205. [Google Scholar] [CrossRef] [Green Version]
- Handing, J.W.; Ragland, S.A.; Bharathan, U.V.; Criss, A.K. The MtrCDE Efflux Pump Contributes to Survival of Neisseria gonorrhoeae From Human Neutrophils and Their Antimicrobial Components. Front. Microbiol. 2018, 9, 2688. [Google Scholar] [CrossRef]
- Armstrong, C.L.; Miralda, I.; Neff, A.C.; Tian, S.; Vashishta, A.; Perez, L.; Le, J.; Lamont, R.J.; Uriarte, S.M. Filifactor alocis Promotes Neutrophil Degranulation and Chemotactic Activity. Infect. Immun. 2016, 84, 3423–3433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmisson, J.S.; Tian, S.; Armstrong, C.L.; Vashishta, A.; Klaes, C.K.; Miralda, I.; Jimenez-Flores, E.; Le, J.; Wang, Q.; Lamont, R.J.; et al. Filifactor alocis modulates human neutrophil antimicrobial functional responses. Cell. Microbiol. 2018, 20, e12829. [Google Scholar] [CrossRef] [PubMed]
- Taheri, N.; Fahlgren, A.; Fallman, M. Yersinia pseudotuberculosis Blocks Neutrophil Degranulation. Infect. Immun. 2016, 84, 3369–3378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snall, J.; Linner, A.; Uhlmann, J.; Siemens, N.; Ibold, H.; Janos, M.; Linder, A.; Kreikemeyer, B.; Herwald, H.; Johansson, L.; et al. Differential neutrophil responses to bacterial stimuli: Streptococcal strains are potent inducers of heparin-binding protein and resistin-release. Sci. Rep. 2016, 6, 21288. [Google Scholar] [CrossRef]
- Soehnlein, O.; Oehmcke, S.; Ma, X.; Rothfuchs, A.G.; Frithiof, R.; van Rooijen, N.; Morgelin, M.; Herwald, H.; Lindbom, L. Neutrophil degranulation mediates severe lung damage triggered by streptococcal M1 protein. Eur. Respir. J. 2008, 32, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Arnett, E.; Vadia, S.; Nackerman, C.C.; Oghumu, S.; Satoskar, A.R.; McLeish, K.R.; Uriarte, S.M.; Seveau, S. The pore-forming toxin listeriolysin O is degraded by neutrophil metalloproteinase-8 and fails to mediate Listeria monocytogenes intracellular survival in neutrophils. J. Immunol. 2014, 192, 234–244. [Google Scholar] [CrossRef] [Green Version]
- SenGupta, S.; Hittle, L.E.; Ernst, R.K.; Uriarte, S.M.; Mitchell, T.C. A Pseudomonas aeruginosa hepta-acylated lipid A variant associated with cystic fibrosis selectively activates human neutrophils. J. Leukoc. Biol. 2016, 100, 1047–1059. [Google Scholar] [CrossRef]
- Johansson, A.; Claesson, R.; Hanstrom, L.; Sandstrom, G.; Kalfas, S. Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans. J. Periodontal. Res. 2000, 35, 85–92. [Google Scholar] [CrossRef]
- Claesson, R.; Johansson, A.; Belibasakis, G.; Hanstrom, L.; Kalfas, S. Release and activation of matrix metalloproteinase 8 from human neutrophils triggered by the leukotoxin of Actinobacillus actinomycetemcomitans. J. Periodontal. Res. 2002, 37, 353–359. [Google Scholar] [CrossRef]
- Lacy, P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Yin, C.; Heit, B. Armed for destruction: formation, function and trafficking of neutrophil granules. Cell Tissue Res. 2018, 371, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Uriarte, S.M.; Rane, M.J.; Luerman, G.C.; Barati, M.T.; Ward, R.A.; Nauseef, W.M.; McLeish, K.R. Granule exocytosis contributes to priming and activation of the human neutrophil respiratory burst. J. Immunol. 2011, 187, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinhauer, K.; Eschenbacher, I.; Radischat, N.; Detsch, C.; Niederweis, M.; Goroncy-Bermes, P. Rapid evaluation of the mycobactericidal efficacy of disinfectants in the quantitative carrier test EN 14563 by using fluorescent Mycobacterium terrae. Appl. Environ. Microbiol. 2010, 76, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnov, A.; Solga, M.D.; Lannigan, J.; Criss, A.K. An improved method for differentiating cell-bound from internalized particles by imaging flow cytometry. J. Immunol. Methods 2015. [Google Scholar] [CrossRef] [Green Version]
- Jog, N.R.; Rane, M.J.; Lominadze, G.; Luerman, G.C.; Ward, R.A.; McLeish, K.R. The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am. J. Physiol. Cell Physiol. 2007, 292, C1690–C1700. [Google Scholar] [CrossRef] [Green Version]
- McLeish, K.R.; Uriarte, S.M.; Tandon, S.; Creed, T.M.; Le, J.; Ward, R.A. Exocytosis of neutrophil granule subsets and activation of prolyl isomerase 1 are required for respiratory burst priming. J. Innate Immun. 2013, 5, 277–289. [Google Scholar] [CrossRef] [Green Version]
- Unemori, E.N.; Pickford, L.B.; Salles, A.L.; Piercy, C.E.; Grove, B.H.; Erikson, M.E.; Amento, E.P. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J. Clin. Investig. 1996, 98, 2739–2745. [Google Scholar] [CrossRef]





| None Associated | Internalized | Attached | Internalized and Attached | Total Neutrophil Counts | |
|---|---|---|---|---|---|
| Non-opsonized | 220 (69%) | 46 (14%) | 29 (9%) | 26 (8%) | 321 |
| Opsonized | 58 (19%) | 121 (40%) | 11 (4%) | 115 (38%) | 305 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miralda, I.; Klaes, C.K.; Graham, J.E.; Uriarte, S.M. Human Neutrophil Granule Exocytosis in Response to Mycobacterium smegmatis. Pathogens 2020, 9, 123. https://doi.org/10.3390/pathogens9020123
Miralda I, Klaes CK, Graham JE, Uriarte SM. Human Neutrophil Granule Exocytosis in Response to Mycobacterium smegmatis. Pathogens. 2020; 9(2):123. https://doi.org/10.3390/pathogens9020123
Chicago/Turabian StyleMiralda, Irina, Christopher K. Klaes, James E. Graham, and Silvia M. Uriarte. 2020. "Human Neutrophil Granule Exocytosis in Response to Mycobacterium smegmatis" Pathogens 9, no. 2: 123. https://doi.org/10.3390/pathogens9020123





