Urinary N-acetyltyramine-O,β-glucuronide in Persons with Onchocerciasis-Associated Epilepsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Mass Spectrometry Analysis
2.3. Statistical Analysis
2.4. Ethical Considerations and Informed Consent
3. Results
3.1. Urinary NATOG Concentration in Ivermectin-Naive men without Epilepsy
3.2. Urinary NATOG Concentration in Ivermectin-Naive Persons with Epilepsy
3.3. Urinary NATOG Concentration of PWE, Four Months after Ivermectin or without Ivermectin Treatment
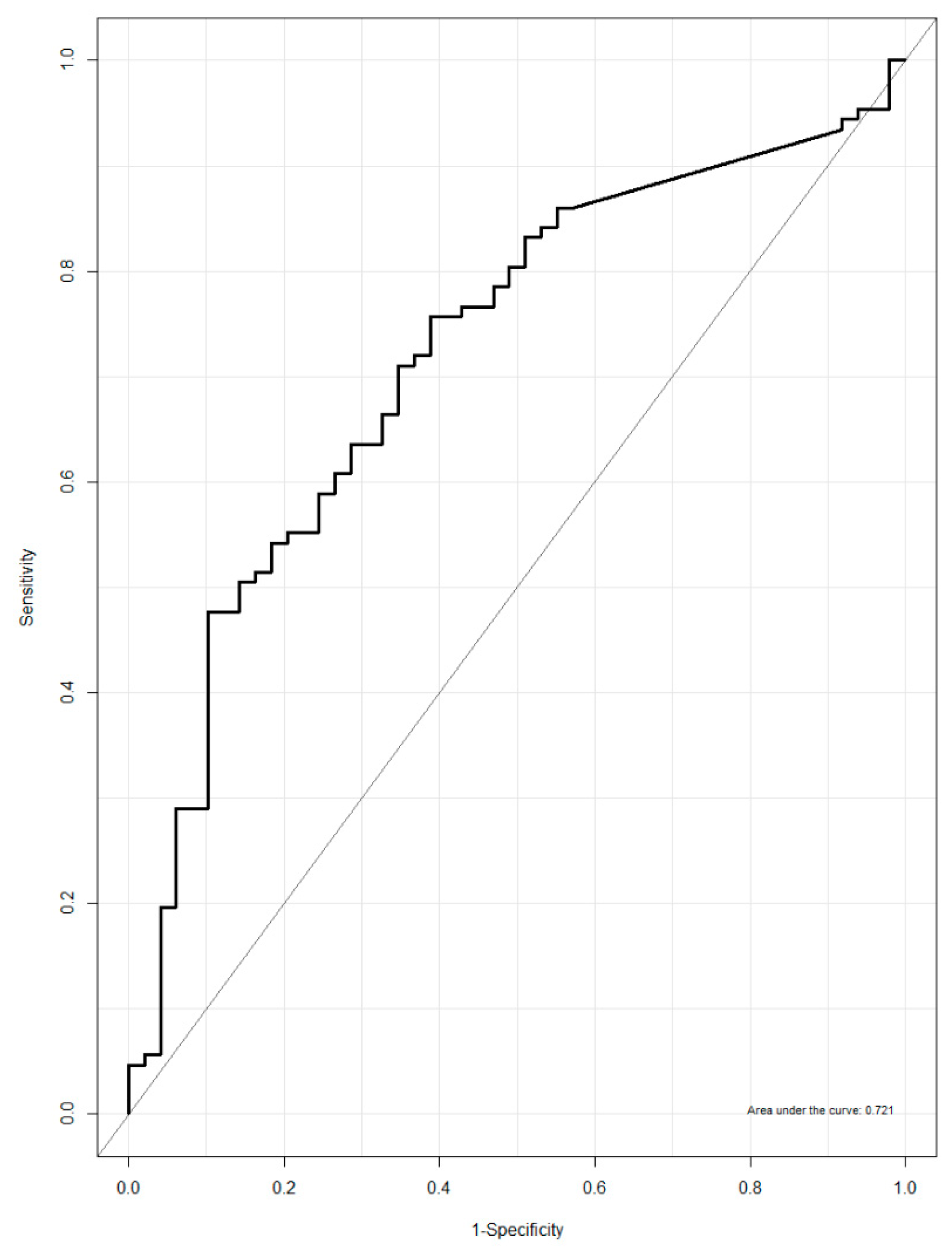
3.4. Urinary NATOG as a Biomarker for Active O. volvulus Infection
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colebunders, R.; Nelson Siewe, F.J.; Hotterbeekx, A. Onchocerciasis-Associated Epilepsy, an Additional Reason for Strengthening Onchocerciasis Elimination Programs. Trends Parasitol. 2018, 34, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Chesnais, C.B.; Nana-Djeunga, H.C.; Njamnshi, A.K.; Lenou-Nanga, C.G.; Boulle, C.; Bissek, A.Z.; Kamgno, J.; Colebunders, R.; Boussinesq, M. The temporal relationship between onchocerciasis and epilepsy: A population-based cohort study. Lancet Infect. Dis. 2018. [Google Scholar] [CrossRef]
- Colebunders, R.; Irani, J.; Post, R. Nodding syndrome—We can now prevent it. Int. J. Infect. Dis. 2016, 44, 61–63. [Google Scholar] [CrossRef][Green Version]
- Tekle, A.H.; Zoure, H.G.; Noma, M.; Boussinesq, M.; Coffeng, L.E.; Stolk, W.A.; Remme, J.H. Progress towards onchocerciasis elimination in the participating countries of the African Programme for Onchocerciasis Control: Epidemiological evaluation results. Infect. Dis. Poverty 2016, 5, 66. [Google Scholar] [CrossRef]
- WHO. Onchocerciasis and Its Control; Technical Report Series No.852; World Health Organisation: Geneva, Switzerland, 1995. [Google Scholar]
- Remme, J.H. Research for control: The onchocerciasis experience. Trop. Med. Int. Health 2004, 9, 243–254. [Google Scholar] [CrossRef]
- Weil, G.J.; Steel, C.; Liftis, F.; Li, B.W.; Mearns, G.; Lobos, E.; Nutman, T.B. A rapid-format antibody card test for diagnosis of onchocerciasis. J. Infect. Dis. 2000, 182, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Lipner, E.M.; Dembele, N.; Souleymane, S.; Alley, W.S.; Prevots, D.R.; Toe, L.; Boatin, B.; Weil, G.J.; Nutman, T.B. Field applicability of a rapid-format anti-Ov-16 antibody test for the assessment of onchocerciasis control measures in regions of endemicity. J. Infect. Dis. 2006, 194, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Globisch, D.; Moreno, A.Y.; Hixon, M.S.; Nunes, A.A.; Denery, J.R.; Specht, S.; Hoerauf, A.; Janda, K.D. Onchocerca volvulus-neurotransmitter tyramine is a biomarker for river blindness. Proc. Natl. Acad. Sci. USA 2013, 110, 4218–4223. [Google Scholar] [CrossRef] [PubMed]
- Globisch, D.; Eubanks, L.M.; Shirey, R.J.; Pfarr, K.M.; Wanji, S.; Debrah, A.Y.; Hoerauf, A.; Janda, K.D. Validation of onchocerciasis biomarker N-acetyltyramine-O-glucuronide (NATOG). Bioorg. Med. Chem. Lett. 2017, 27, 3436–3440. [Google Scholar] [CrossRef] [PubMed]
- Bennuru, S.; Cotton, J.A.; Ribeiro, J.M.; Grote, A.; Harsha, B.; Holroyd, N.; Mhashilkar, A.; Molina, D.M.; Randall, A.Z.; Shandling, A.D.; et al. Stage-Specific Transcriptome and Proteome Analyses of the Filarial Parasite Onchocerca volvulus and Its Wolbachia Endosymbiont. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Lagatie, O.; Njumbe Ediage, E.; Batsa Debrah, L.; Diels, L.; Nolten, C.; Vinken, P.; Debrah, A.; Dillen, L.; Silber, S.; Stuyver, L.J. Evaluation of the diagnostic potential of urinary N-Acetyltyramine-O,beta-glucuronide (NATOG) as diagnostic biomarker for Onchocerca volvulus infection. Parasit. Vectors 2016, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, E.; Mandro, M.; Mukendi, D.; Suykerbuyk, P.; Dolo, H.; Wonya'Rossi, D.; Ngave, F.; Ensoy-Musoro, C.; Laudisoit, A.; Hotterbeekx, A.; et al. High prevalence of epilepsy in onchocerciasis endemic health areas in Democratic Republic of the Congo. Infect. Dis. Poverty 2018, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Mandro, M.; Siewe Fodjo, J.N.; Mukendi, D.; Dusabimana, A.; Menon, S.; Haesendonckx, S.; Lokonda, R.; Nakato, S.; Nyisi, F.; Abhafule, G.; et al. Ivermectin as an adjuvant to anti-epileptic treatment in persons with onchocerciasis-associated epilepsy: A randomized proof-of-concept clinical trial. PLoS Negl. Trop. Dis. 2020, 14, e0007966. [Google Scholar] [CrossRef]
- Colebunders, R.; Mandro, M.; Mukendi, D.; Dolo, H.; Suykerbuyk, P.; Van Oijen, M. Ivermectin Treatment in Patients With Onchocerciasis-Associated Epilepsy: Protocol of a Randomized Clinical Trial. JMIR Res. Protoc. 2017, 6, 137. [Google Scholar] [CrossRef]
- Sen, P.K. Estimates of the Regression Coefficient Based on Kendall's Tau. J. Am. Stat. Assoc. 1968, 63, 1379–1389. [Google Scholar] [CrossRef]
- Lewis, C.B.; Adams, N. Phenobarbital. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Wolstenholme, A.J.; Rogers, A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology 2005, 131, 85–95. [Google Scholar] [CrossRef]
- Shirey, R.J.; Globisch, D.; Eubanks, L.M.; Hixon, M.S.; Janda, K.D. Noninvasive Urine Biomarker Lateral Flow Immunoassay for Monitoring Active Onchocerciasis. ACS Infect. Dis. 2018, 4, 1423–1431. [Google Scholar] [CrossRef] [PubMed]

| O. volvulus-infected a with Mild Epilepsy (n = 81) | O. volvulus-infected a with Severe Epilepsy (n = 45) | P-Value b | |
|---|---|---|---|
| Male: n (%) | 40 (49%) | 27 (60%) | 0.269 |
| Age in years: median (IQR) | 24 (18–32) | 22 (16–28) | 0.578 |
| Seizures per month: median (IQR) | 1 (0–2) | 4 (4–10) | <0.001 |
| Skin mf density: median (IQR) | 5 (0–40) | 19.5 (2–84) | 0.041 |
| Positive skin snip: n (%) | 54 (67%) | 37 (82%) | 0.029 |
| NATOG (µM): median (IQR) | 2.17 (1–7.59) | 7.62 (2.52–15.5) | 0.008 |
| Effect | OR | 95% CI | P-Value | |
|---|---|---|---|---|
| Log-transformed urinary NATOG before ivermectin treatment | 3.116 | 1.970 | 4.930 | <0.001 |
| Log-transformed urinary NATOG after ivermectin treatment | 1.343 | 1.023 | 1.764 | 0.034 |
| Age | 1.003 | 0.980 | 1.026 | 0.823 |
| Female vs. male | 1.124 | 0.609 | 2.074 | 0.708 |
| Number of seizures in last 2 months prior to ivermectin treatment | 1.036 | 0.989 | 1.085 | 0.132 |
| Effect | Estimate | 95% CI | P-Value | |
|---|---|---|---|---|
| Log-transformed urinary NATOG before ivermectin treatment | 2.344 | 1.895 | 2.900 | <0.0001 |
| Log-transformed urinary NATOG after ivermectin treatment | 1.560 | 1.169 | 2.081 | 0.003 |
| Age | 1.003 | 0.984 | 1.023 | 0.726 |
| Female vs. male | 1.185 | 1.066 | 1.318 | 0.521 |
| Number of seizures last 2 months before ivermectin | 1.025 | 0.974 | 1.079 | 0.344 |
| O. volvulus-Infected PWE a (n = 134) | |||
|---|---|---|---|
| Received Ivermectin (n = 92) | Did Not Receive Ivermectin (n = 42) | P-Value b | |
| Male: n (%) | 45 (49%) | 23 (55%) | 0.374 |
| Age in years: median (IQR) | 24 (18–32) | 22 (17–29) | 0.272 |
| OV16 RDT-positive: n (%) | 58 (63%) | 27 (64%) | 0.825 |
| Skin mf density at baseline: median (IQR) | 8.75 (0–77.5) | 12.5 (0.5–55.5) | 0.536 |
| Positive skin snip at baseline*: n (%) | 63 (70.8%) | 39 (76.3%) | 0.665 |
| Skin mf density at follow-up: median (IQR) | 0 (0–1.5) | 0 (0–20) | 0.081 |
| Positive skin snip at follow-up: n (%) | 28 (31.5%) | 19 (47.5%) | 0.113 |
| Mf % reduction: median (IQR) | 100% (92.3–100.0) | 83.1% (66.7–100.00) | 0.014 |
| NATOG (µM) at baseline: median (IQR) | 3.7 (1.2–8.5) | 3.6 (1.4–9.0) | 0.983 |
| NATOG (µM) at follow-up: median (IQR) | 1.6 (0–3.22) | 1.7 (0–5.19) | 0.654 |
| NATOG (µM) % reduction: median (IQR) | 75.1% (18.6–100.0) | 64.9% (8.0–83.1) | 0.101 |
| Seizures per month at baseline: median (IQR) | 2.0 (0.0–2.5) | 4.0 (2.0–4.0) | <0.001 |
| Classified by NATOG as: | O. volvulus Infection | Total | |
|---|---|---|---|
| No active Infection | Active Infection | ||
| Negative (n) | 47 | 90 | 137 |
| Positive (n) | 2 | 17 | 19 |
| Total | 49 | 107 | 156 |
| Study | Country | Population (n) | NATOG concentration (µM) | Remarks | |||
|---|---|---|---|---|---|---|---|
| Average ± SEM | Median | Min | Max | ||||
| Globisch et al. (2013) | Ghana + Cameroon | O. volvulus positive (81) | 36.9 ± 4 | na | na | na | O. volvulus infection diagnosed by nodule palpation or skin snips, but mf densities not reported |
| Ghana + Cameroon | Uninfected control (16) | 7.0 ± 2.7 | na | na | na | ||
| North America | Non-endemic control (17) | 1.1 ± 0.2 | na | na | na | ||
| Guatemala | O. volvulus positive (20) | 8.4 ± 1.6 | na | na | na | ||
| Ghana + Cameroon | Lymphatic filariasis (23) | 4.2 ± 0.7 | na | na | na | ||
| Ghana | O. volvulus positive, 20 months after doxycyclin (24) | 9.5 ± 1.7 | na | na | na | ||
| Ghana | O. volvulus positive, 20 months after placebo (14) | 33.5 ± 10.7 | na | na | na | ||
| Globisch et al. (2017) | Ghana + Cameroon | O. volvulus positive (145) | 42.8 ± 3.7 | 29.3 | 0.9 | 276 | O. volvulus infection diagnosed by nodule palpation or skin snips, but mf densities not reported |
| Ghana + Cameroon | Uninfected control (118) | 6.4 ± 0.7 | 3.6 | 0.2 | 39.6 | ||
| Ghana + Cameroon | L. loa infection (100) | 14.7 ± 2.5 | 6.8 | 0.4 | 175.6 | ||
| Ghana + Cameroon | M. perstans infection (25) | 13.6 ± 2.5 | 11.4 | 0.3 | 46.4 | ||
| Ghana + Cameroon | L. loa + M. perstans infection (3) | 6.0 ± 2.7 | 6.5 | 1 | 10.4 | ||
| Ghana + Cameroon | O. volvulus + L. loa infection (21) | 16.6 ± 2.8 | 13.8 | 0.8 | 41.1 | ||
| Ghana + Cameroon | O. volvulus + M. perstans infection (29) | 29.2 ± 4.8 | 17.1 | 2.2 | 92.8 | ||
| Ghana + Cameroon | O. volvulus + L. loa + M. perstans infection (8) | 100.5 ± 33.5 | 66.4 | 4.7 | 246.5 | ||
| Lagatie et al. (2016) | Ghana | O. volvulus positive (98) | 1.06 ± 0.16 | na | na | na | O. volvulus infection diagnosed by nodule palpation, skin snips and OV16 RDT; 82% previously received ivermectin; in 89% no mf in skin snips |
| Ghana | Endemic control (50) | 0.95 ± 0.8 | na | na | na | ||
| Ghana | Lympathic filariasis (51) | 0.99 ± 0.17 | na | na | na | ||
| Europe | Non-endemic control (18) | 0.66 ± 0.18 | na | na | na | ||
| Current study | DRC | All active O. volvulus infected (117) | 9.7 ± 1.4 | 5.3 | 0 | 103 | O. volvulus infection diagnosed by nodule palpation, skin snips and OV16 RDT |
| DRC | O. volvulus uninfected (55) | 3.2 ± 0.9 | 1.3 | 0 | 34.5 | ||
| DRC | O. volvulus negative no epilepsy (19) | 3 ± 1.5 | 0.71 | 0 | 28.6 | ||
| DRC | O. volvulus infected no epilepsy (20) | 3.7 ± 0.9 | 2.23 | 0 | 116.3 | ||
| DRC | O. volvulus infected with mild epilepsy (81) | 6.1 ± 1.2 | 2.17 | 0 | 58.9 | ||
| DRC | O. volvulus infected with severe epilepsy (45) | 12 ± 3 | 7.62 | 0 | 103 | ||
| DRC | O. volvulus infected with epilepsy, before ivermectin (134) | 8.2 ± 1.3 | 3.67 | 0 | 103 | ||
| DRC | O. volvulus infected with epilepsy, after ivermectin (92) | 3 ± 0.6 | 1.55 | 0 | 33.8 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotterbeekx, A.; Dusabimana, A.; Mandro, M.; Abhafule, G.M.; Deogratias, W.; Siewe Fodjo, J.N.; Abrams, S.; Colebunders, R. Urinary N-acetyltyramine-O,β-glucuronide in Persons with Onchocerciasis-Associated Epilepsy. Pathogens 2020, 9, 191. https://doi.org/10.3390/pathogens9030191
Hotterbeekx A, Dusabimana A, Mandro M, Abhafule GM, Deogratias W, Siewe Fodjo JN, Abrams S, Colebunders R. Urinary N-acetyltyramine-O,β-glucuronide in Persons with Onchocerciasis-Associated Epilepsy. Pathogens. 2020; 9(3):191. https://doi.org/10.3390/pathogens9030191
Chicago/Turabian StyleHotterbeekx, An, Alfred Dusabimana, Michel Mandro, Germain M Abhafule, Wonya’Rossy Deogratias, Joseph N. Siewe Fodjo, Steven Abrams, and Robert Colebunders. 2020. "Urinary N-acetyltyramine-O,β-glucuronide in Persons with Onchocerciasis-Associated Epilepsy" Pathogens 9, no. 3: 191. https://doi.org/10.3390/pathogens9030191
APA StyleHotterbeekx, A., Dusabimana, A., Mandro, M., Abhafule, G. M., Deogratias, W., Siewe Fodjo, J. N., Abrams, S., & Colebunders, R. (2020). Urinary N-acetyltyramine-O,β-glucuronide in Persons with Onchocerciasis-Associated Epilepsy. Pathogens, 9(3), 191. https://doi.org/10.3390/pathogens9030191






