Ecology of Neglected Rodent-Borne American Orthohantaviruses
Abstract
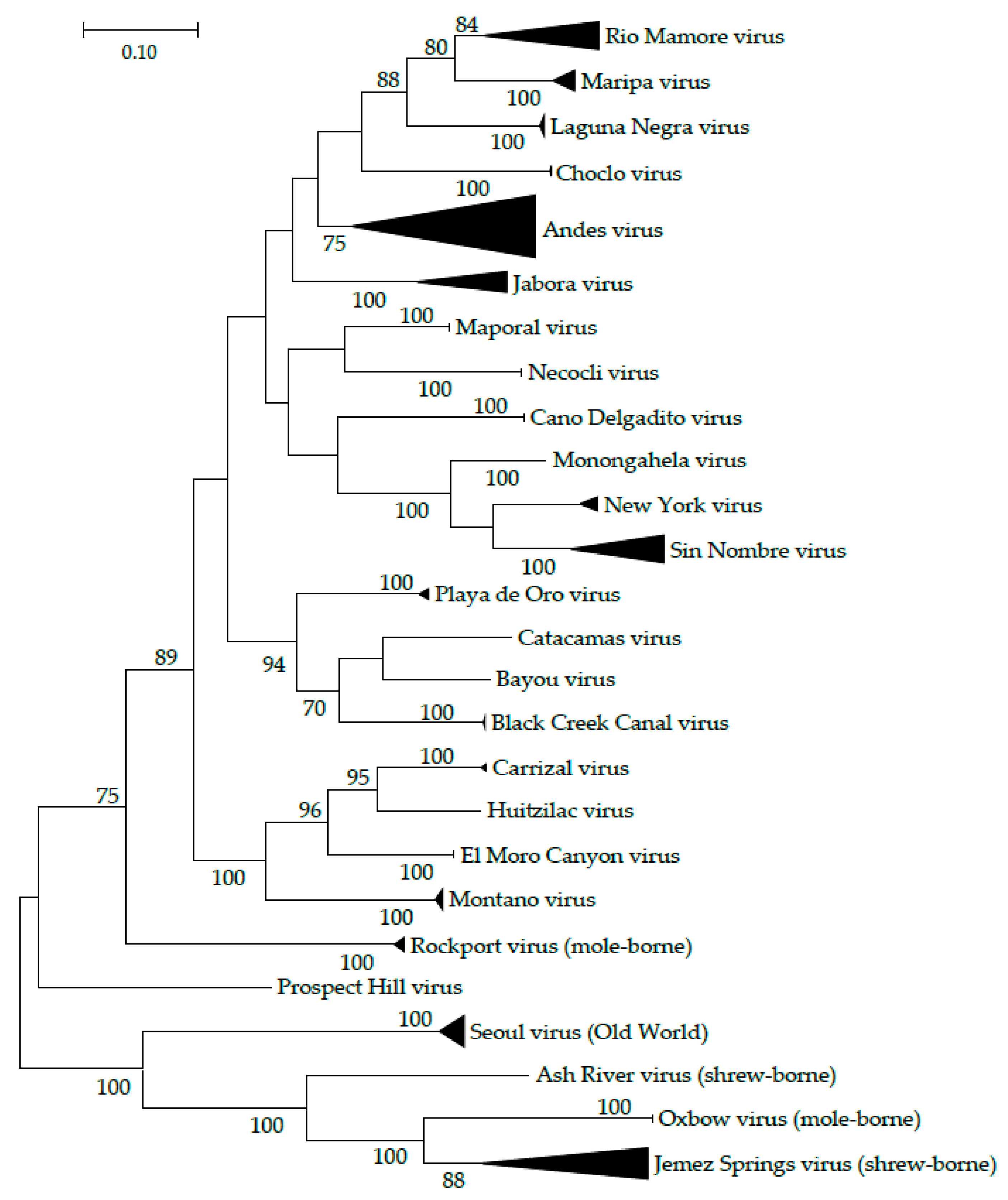
:1. Introduction
2. Host Diversity
3. Orthohantavirus Communities
4. Transmission among Rodents
5. Risk of Spillover to Humans
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Virus Strain/Genotype | Rodent Host | Genome Strands Sequenced | Virus Isolation | ||
|---|---|---|---|---|---|
| S | M | L | |||
| Andes virus | Oligoryzomys longicaudatus | X | X | X | |
| Oligoryzomys chacoensis | X | X | |||
| Oligoryzomys flavescens | X | X | |||
| Abrothrix longipilis | X | ||||
| Loxodontomys micopus | X | ||||
| Rattus rattus | X | ||||
| Araraquara virus | Bolomys lasiurus | X | X | ||
| Oxymycterus judex | X | X | |||
| Akodon montensis | X | ||||
| Juquitiba virus | Oligoryzomys fornesi | X | X | ||
| Oxymycterus nasutus | X | X | |||
| Oligoryzomys nigripes | X | X | |||
| Maciel virus | Bolomys obscurus | X | |||
| Pergamino virus | Akodon azarae | X | X | ||
| Oxymycterus rufus | X | ||||
| Tunari virus | Unknown | ||||
| Castelo dos Sonhos virus | Oligoryzomys uriaritensis | X | |||
| Lechiguanas virus | Oligoryzomys flavescens | X | |||
| Oligoryzomys nigripes | X | X | |||
| Bermejo virus | Oligoryzomys chacoensis | X | |||
| Oran virus | Oligoryzomys longicaudatus | X | |||
| Bayou virus | Oryzomys palustris | X | X | X | |
| Catacamas virus | Oryzomys couesi | X | X | X | |
| Playa de Oro virus | Oryzomys couesi | X | X | ||
| Sigmodon mascotensis | X | X | |||
| Black Creek Canal virus | Sigmodon hispidus | X | X | X | X |
| Muleshoe virus | Sigmodon hispidus | X | X | ||
| Caño Delgadito virus | Sigmodon alstoni | X | X | X | |
| Choclo virus | Oligoryzomys fulvescens (costaricensis) | X | X | ||
| Jabora virus | Akodon montensis | X | |||
| Carrizal virus | Reithrodontomys sumichrasti | X | X | X | |
| El Moro Canyon virus | Reithrodontomys megalotis | X | X | ||
| Reithrodontomys sumichrasti | X | X | |||
| Neotoma mexicana | X | ||||
| Rio Segundo virus | Reithrodontomys mexicanus | X | |||
| Huitzilac virus | Reithrodontomys megalotis | X | X | X | |
| Laguna Negra virus | Calomys laucha | X | X | X | |
| Maripa virus | Oligoryzomys fulvescens | X | X | ||
| Zygodontomys brevicauda | X | X | |||
| Rio Mamoré virus | Oligoryzomys microtis | X | X | X | |
| Anajatuba virus | Oligoryzomys fornesi | X | |||
| Rio Mearim virus | Holochilus sciureus | X | |||
| Maporal virus | Oligoryzomys fulvescens (delicatus) | X | X | X | |
| Montano virus | Peromyscus beatae | X | X | X | |
| Necocli virus | Zygodontomys brevicauda (cherriei) | X | X | ||
| Calabazo virus | Zygodontomys brevicauda (cherriei) | X | X | ||
| Prospect Hill virus | Microtus pennsylvanicus | X | |||
| Isla Vista virus | Microtus californicus | X | X | ||
| Peromyscus californicus | X | X | |||
| Bloodland Lake virus | Microtus ochrogaster | X | |||
| New York virus | Peromyscus leucopus | X | X | X | |
| Monongahela virus | Peromyscus maniculatus nubiterrae | X | X | ||
| Peromyscus leucopus | X | X | |||
| Blue River virus | Peromyscus leucopus | X | |||
| Sin Nombre virus | Peromyscus maniculatus | X | X | X | |
| Peromyscus californicus | X | X | |||
| Peromyscus attwateri | X | X | |||
| Peromyscus eremicus | X | ||||
| Peromyscus laceianus | X | X | |||
| Reithrodontomys fulvescens | X | X | |||
| Limestone Canyon virus | Peromyscus boylii | X | X | ||
| Peromyscus hylocetes | X | X | |||
| Peromyscus leucopus | X | X | |||
| Peromyscus levipes | X | X | |||
| Peromyscus melanotis | X | X | |||
| Peromyscus ochraventer | X | X | |||
| Peromyscus spicilegus | X | X | |||
References
- Anthony, S.J.; Epstein, J.H.; A Murray, K.; Navarrete-Macias, I.; Zambrana-Torrelio, C.; Solovyov, A.; Ojeda-Flores, R.; Arrigo, N.C.; Islam, A.; Khan, S.A.; et al. A strategy to estimate unknown viral diversity in mammals. mBio 2013, 4, e00598-13. [Google Scholar] [CrossRef] [Green Version]
- Parrish, C.R.; Holmes, E.C.; Morens, D.M.; Park, E.-C.; Burke, D.S.; Calisher, C.H.; Laughlin, C.A.; Saif, L.J.; Daszak, P. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 2008, 72, 457–470. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.G.; Wiethoelter, A.; Haseeb, M.A. The impact of human population pressure on flying fox niches and the potential consequences for Hendra virus spillover. Sci. Rep. 2017, 7, 8226. [Google Scholar] [CrossRef]
- Hahn, M.B.; Gurley, E.S.; Epstein, J.H.; Islam, M.S.; Patz, J.A.; Daszak, P.; Luby, S.P. The role of landscape composition and configuration on Pteropus giganteus roosting ecology and nipah virus spillover risk in Bangladesh. Am. J. Trop. Med. Hyg. 2014, 90, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, L.; Chen, N.; Sarkar, S. Vector status of Aedes species determines geographical risk of autochthonous Zika virus establishment. PLoS Negl. Trop. Dis. 2017, 11, e0005487. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S. Origin of the west nile virus responsible for an outbreak of encephalitis in the Northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abudurexiti, A.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.; et al. Taxonomy of the order Bunyavirales: Update 2019. Arch. Virol. 2019, 164, 1949–1965. [Google Scholar] [CrossRef] [Green Version]
- Maes, P.; Adkins, S.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.; Briese, T.; et al. Taxonomy of the order Bunyavirales: Second update 2018. Arch. Virol. 2019, 164, 927–941. [Google Scholar] [CrossRef] [Green Version]
- Laenen, L.; Vergote, V.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Maes, P. Hantaviridae: Current classification and future perspectives. Viruses 2019, 11, 788. [Google Scholar] [CrossRef] [Green Version]
- Arai, S.; Yanagihara, R. Genetic diversity and geographic distribution of bat-borne hantaviruses. In Bats and Viruses: Current Research and Future Trends; Corrales-Aguilar, E., Schwemmle, M., Eds.; Caister Academic Press: Norfolk, UK, 2020; pp. 59–86. [Google Scholar]
- Avšič-Županc, T.; Saksida, A.; Korva, M. Hantavirus infections. Clin. Microbiol. Infect. 2019, 21, e6–e16. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.-W.; Amyx, H.L.; Yanagihara, R.; Gajdusek, D.C.; Goldgaber, D.; Gibbs, C.J. Partial characterization of prospect hill virus isolated from meadow voles in the United States. J. Infect. Dis. 1985, 152, 826–829. [Google Scholar] [CrossRef]
- Duchin, J.S.; Koster, F.T.; Peters, C.; Simpson, G.L.; Tempest, B.; Zaki, S.R.; Ksiazek, T.G.; Rollin, P.E.; Nichol, S.; Umland, E.T.; et al. Hantavirus pulmonary syndrome: A clinical description of 17 patients with a newly recognized disease. N. Engl. J. Med. 1994, 330, 949–955. [Google Scholar] [CrossRef] [PubMed]
- López, N.; Padula, P.; Rossi, C.; Lázaro, M.E.; Franze-Fernández, M.T. Genetic edentification of a new hantavirus causing severe pulmonary syndrome in Argentina. Virology 1996, 220, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.D.; Forshey, B.M.; Vallejo, E.; Agudo, R.; Vargas, J.; Blazes, D.L.; Guevara, C.; Laguna-Torres, V.A.; Halsey, E.S.; Kochel, T.J. Novel Strain of Andes virus associated with fatal human infection, Central Bolivia. Emerg. Infect. Dis. 2012, 18, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Bohlman, M.C.; Morzunov, S.P.; Meissner, J.; Taylor, M.B.; Ishibashi, K.; Rowe, J.; Levis, S.; Enria, D.; Jeor, S.C.S. Analysis of hantavirus genetic diversity in argentina: S segment-derived phylogeny. J. Virol. 2002, 76, 3765–3773. [Google Scholar] [CrossRef] [Green Version]
- Milazzo, M.L.; Cajimat, M.N.; Richter, M.H.; Bradley, R.D.; Fulhorst, C.F. Muleshoe virus and other hantaviruses associated with neotomine or sigmodontine rodents in Texas. Vector-Borne Zoonotic Dis. 2017, 17, 720–729. [Google Scholar] [CrossRef]
- Song, J.-W.; Baek, L.-J.; Gajdusek, D.; Yanagihara, R.; Gavrilovskaya, I.; Luft, B.; Mackow, E.; Hjelle, B. Isolation of pathogenic hantavirus from white-footed mouse (Peromyscus leucopus). Lancet 1994, 344, 1637. [Google Scholar] [CrossRef]
- Forbes, K.M.; Sironen, T.; Plyusnin, A. Hantavirus maintenance and transmission in reservoir host populations. Curr. Opin. Virol. 2018, 28, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Douglass, R.J.; Semmens, W.J.; Mills, J.N.; Zanto, S.N.; Bond, C.W.; Wilson, T.; Van Horn, R. Longitudinal studies of Sin Nombre virus in deer mouse-dominated ecosystems of Montana. Am. J. Trop. Med. Hyg. 2001, 65, 33–41. [Google Scholar] [CrossRef]
- Douglass, R.; Calisher, C.H.; Wagoner, K.D.; Mills, J.N. Sin Nombre virus infection of deer mice in Montana: Characteristics of newly infected mice, incidence, and temporal pattern of infection. J. Wildl. Dis. 2007, 43, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Botten, J.; Mirowsky, K.; Ye, C.; Gottlieb, K.; Saavedra, M.; Ponce, L.; Hjelle, B. Shedding and intracage transmission of sin nombre hantavirus in the deer mouse (Peromyscus maniculatus) model. J. Virol. 2002, 76, 7587–7594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlen, L.; Tödtmann, J.; Specht, S.; Kallies, R.; Papies, J.; Müller, M.A.; Junglen, S.; Drosten, C.; Eckerle, I. Epithelial cell lines of the cotton rat (Sigmodon hispidus) are highly susceptible in vitro models to zoonotic Bunya-, Rhabdo-, and Flaviviruses. Virol. J. 2016, 13, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaheri, A.; Strandin, T.; Hepojoki, J.; Sironen, T.; Henttonen, H.; Mäkelä, S.; Mustonen, J. Uncovering the mysteries of hantavirus infections. Nat. Rev. Genet. 2013, 11, 539–550. [Google Scholar] [CrossRef]
- De Oliveira, R.C.; Guterres, A.; Fernandes, J.; D’Andrea, P.; Bonvicino, C.R.; de Lemos, E.R.S. Hantavirus reservoirs: Current status with an emphasis on data from Brazil. Viruses 2014, 6, 1929–1973. [Google Scholar] [CrossRef] [Green Version]
- Milazzo, M.L.; Duno, G.; Utrera, A.; Richter, M.H.; Duno, F.; de Manzione, N.; Fulhorst, C.F. Natural host relationships of hantaviruses native to Western Venezuela. Vector-Borne Zoonotic Dis. 2010, 10, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Plyusnin, A.; Morzunov, S.P. Virus evolution and genetic diversity of hantaviruses and their rodent hosts. In Hantaviruses; Schmaljohn, C.S., Nichol, S.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 256, pp. 47–75. [Google Scholar]
- Pitts, R.M.; Mauldin, M.R.; Thompson, C.W.; Choate, J.R. Evidence of hantavirus exposure in rodents from North Texas. West. N. Am. Nat. 2013, 73, 386–391. [Google Scholar] [CrossRef]
- Colombo, V.C.; Brignone, J.; Sen, C.; Previtali, M.A.; Martin, M.L.; Levis, S.; Monje, L.D.; Gonzalez-Ittig, R.; Beldomenico, P.M. Orthohantavirus genotype Lechiguanas in Oligoryzomys nigripes (Rodentia: Cricetidae): New evidence of host-switching. Acta Trop. 2019, 191, 133–138. [Google Scholar] [CrossRef]
- Milazzo, M.L.; Cajimat, M.N.; Romo, H.E.; Estrada-Franco, J.G.; Iñiguez-Dávalos, L.I.; Bradley, R.D.; Fulhorst, C.F. Geographic distribution of hantaviruses associated with neotomine and sigmodontine rodents, Mexico. Emerg. Infect. Dis. 2012, 18, 571–576. [Google Scholar] [CrossRef]
- Kuenzi, A.J.; Morrison, M.L.; Madhav, N.K.; Mills, J.N. Brush mouse (Peromyscus boylii) population dynamics and hantavirus infection during a warm, drought period in southern Arizona. J. Wildl. Dis. 2007, 43, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Torrez-Martinez, N.; Irwin, W.; Harrison, F.J.; Davis, R.; Ascher, M.; Jay, M.; Hjelle, B. Isla Vista virus: A genetically novel hantavirus of the California vole Microtus californicus. J. Gen. Virol. 1995, 76, 3195–3199. [Google Scholar] [CrossRef]
- Rosa, E.S.; Mills, J.N.; Padula, P.; ElKhoury, M.R.; Ksiazek, T.G.; Mendes, W.S.; Santos, E.D.; Araújo, G.C.; Martinez, V.P.; Rosa, J.F.; et al. Newly recognized hantaviruses associated with hantavirus pulmonary syndrome in Northern Brazil: Partial genetic characterization of viruses and serologic implication of likely reservoirs. Vector-Borne Zoonotic Dis. 2005, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-K.; Owen, R.D.; Sánchez-Hernández, C.; Romero-Almaraz, M.D.L.; Jonsson, C.B. Genetic characterization and phylogeny of a hantavirus from Western Mexico. Virus Res. 2008, 131, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Ruiz, C.; Díaz, F.J.; Rodas, J.D. Recent evidence of hantavirus circulation in the American tropic. Viruses 2014, 6, 1274–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, J.D.; Utrera, A.; Fulhorst, C.F. The delicate pygmy rice rat (Oligoryzomys delicatus) is the principal host of maporal virus (family Bunyaviridae, genus Hantavirus). Vector-Borne Zoonotic Dis. 2011, 11, 691–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.-P.; Lin, X.-D.; Wang, W.; Tian, J.-H.; Cong, M.-L.; Zhang, H.-L.; Wang, M.-R.; Zhou, R.-H.; Wang, J.-B.; Li, M.-H.; et al. Phylogeny and origins of hantaviruses harbored by bats, insectivores, and rodents. PLoS Pathog. 2013, 9, e1003159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, S.; Bennett, S.N.; Sumibcay, L.; Cook, J.A.; Song, J.-W.; Hope, A.; Parmenter, C.; Nerurkar, V.R.; Yates, T.L.; Yanagihara, R. Short report: Phylogenetically distinct hantaviruses in the masked shrew (Sorex cinereus) and dusky shrew (Sorex monticolus) in the United States. Am. J. Trop. Med. Hyg. 2008, 78, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Vapalahti, O.; Lundkvist, Å.; Fedorov, V.; Conroy, C.J.; Hirvonen, S.; Plyusnina, A.; Nemirov, K.; Fredga, K.; Cook, J.A.; Niemimaa, J.; et al. Isolation and characterization of a hantavirus from lemmus sibiricus: Evidence for host switch during hantavirus evolution. J. Virol. 1999, 73, 5586–5592. [Google Scholar] [CrossRef] [Green Version]
- Albariño, C.G.; Guerrero, L.W.; Chakrabarti, A.K.; Rollin, P.E.; Nichol, S.T. Complete genome sequences of Monongahela hantavirus from Pennsylvania, USA. Microbiol. Resour. Announc. 2018, 7, e00928-18. [Google Scholar] [CrossRef] [Green Version]
- Monroe, M.C.; Morzunov, S.P.; Johnson, A.M.; Bowen, M.D.; Artsob, H.; Yates, T.; Peters, C.; Rollin, P.E.; Ksiazek, T.G.; Nichol, S.T. Genetic diversity and distribution of Peromyscus-borne hantaviruses in North America. Emerg. Infect. Dis. 1999, 5, 75–86. [Google Scholar] [CrossRef]
- Sinclair, J.R.; Bell, M.; Nichol, S.T.; Montgomery, J.M.; Mills, J.N.; Ksiazek, T.G.; McCombs, K.; Hutson, C.L.; Carroll, D.S.; Comer, J.A.; et al. Two cases of hantavirus pulmonary syndrome in Randolph county, West Virginia: A coincidence of time and place? Am. J. Trop. Med. Hyg. 2007, 76, 438–442. [Google Scholar] [CrossRef]
- Rhodes, L., III. Hantavirus pulmonary syndrome associated with Monongahela virus, Pennsylvania. Emerg. Infect. Dis. 2000, 6, 616–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delfraro, A.; Tomé, L.; D’Elía, G.; Clara, M.; Achával, F.; Russi, J.C.; Rodonz, J.R.A. Juquitiba-like hantavirus from 2 honrelated rodent species, Uruguay. Emerg. Infect. Dis. 2008, 14, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Saasa, N.; Sánchez-Hernández, C.; Romero-Almaraz, M.D.L.; Guerrero-Ibarra, E.; Almazán-Catalán, A.; Yoshida, H.; Miyashita, D.; Ishizuka, M.; Sanada, T.; Seto, T.; et al. Ecology of hantaviruses in Mexico: Genetic identification of rodent host species and spillover infection. Virus Res. 2012, 168, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Henderson, W.W.; Monroe, M.C.; Jeor, S.C.S.; Thayer, W.P.; Rowe, J.E.; Peters, C.; Nichol, S.T. Naturally occurring sin Nombre virus genetic reassortants. Virology 1995, 214, 602–610. [Google Scholar] [CrossRef] [Green Version]
- Firth, C.; Tokarz, R.; Simith, D.B.; Nunes, M.R.; Bhat, M.; Rosa, E.S.T.; Medeiros, D.B.A.; Palacios, G.; Vasconcelos, P.F.C.; Lipkin, W.I. Diversity and distribution of hantaviruses in South America. J. Virol. 2012, 86, 13756–13766. [Google Scholar] [CrossRef] [Green Version]
- Mills, J.N.; Childs, J.E. Ecologic studies of rodent reservoirs: Their relevance for human health. Emerg. Infect. Dis. 1998, 4, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Rollin, P.E.; Ksiazek, T.G.; Elliott, L.H.; Ravkov, E.V.; Martin, M.L.; Morzunov, S.; Livingstone, W.; Monroe, M.; Glass, G.; Ruo, S.; et al. Isolation of black creek canal virus, a new hantavirus from Sigmodon hispidus in Florida. J. Med. Virol. 1995, 46, 35–39. [Google Scholar] [CrossRef]
- Ksiazek, T.G.; Martin, M.L.; Groves, M.G.; Nichol, S.T.; Peters, C.J.; Monroe, M.C.; Mills, J.N.; Rollin, P.E.; Johnson, A.M.; Wozniak, A.; et al. Isolation, genetic diversity, and geographic distribution of Bayou virus (Bunyaviridae: Hantavirus). Am. J. Trop. Med. Hyg. 1997, 57, 445–448. [Google Scholar] [CrossRef]
- Holsomback, T.S.; Van Nice, C.J.; Clark, R.N.; McIntyre, N.E.; Abuzeineh, A.A.; Salazar-Bravo, J. Socio-ecology of the marsh rice rat (Oryzomys palustris) and the spatio-temporal distribution of Bayou virus in coastal Texas. Geospat. Health 2013, 7, 289. [Google Scholar] [CrossRef] [Green Version]
- Vincent, M.J.; Quiroz, E.; Gracia, F.; Sanchez, A.J.; Ksiazek, T.G.; Kitsutani, P.T.; Ruedas, L.A.; Tinnin, D.S.; Cáceres, L.; Garcia, A.; et al. Hantavirus pulmonary syndrome in Panama: Identification of novel hantaviruses and their likely reservoirs. Virology 2000, 277, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Raboni, S.M.; Hoffmann, F.G.; Oliveira, R.C.; Teixeira, B.R.; Bonvicino, C.R.; Stella, V.; Carstensen, S.; Bordignon, J.; D’Andrea, P.; De Lemos, E.R.S.; et al. Phylogenetic characterization of hantaviruses from wild rodents and hantavirus pulmonary syndrome cases in the state of Parana (southern Brazil). J. Gen. Virol. 2009, 90, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Matheus, S.; Lavergne, A.; De Thoisy, B.; Dussart, P.; Lacoste, V. Complete genome sequence of a novel hantavirus variant of Rio Mamoré virus, Maripa Virus, from French Guiana. J. Virol. 2012, 86, 5399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Thoisy, B.; Matheus, S.; Guitet, S.; Donato, D.; Clément, L.; Lavergne, A.; Brunaux, O.; Guidez, A.; Catzeflis, F.; Lacoste, V.; et al. Maripa hantavirus in French Guiana: Phylogenetic position and predicted spatial distribution of rodent hosts. Am. J. Trop. Med. Hyg. 2014, 90, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Matheus, S.; Djossou, F.; Moua, D.; Bourbigot, A.M.; Hommel, D.; Lacoste, V.; Dussart, P.; Lavergne, A. Hantavirus pulmonary syndrome, French Guiana. Emerg. Infect. Dis. 2010, 16, 739–741. [Google Scholar] [CrossRef]
- Montoya-Ruiz, C.; Cajimat, M.N.B.; Milazzo, M.L.; Diaz, F.J.; Rodas, J.D.; Valbuena, G.; Fulhorst, C.F. Phylogenetic relationship of Necoclí virus to other South American hantaviruses (Bunyaviridae: Hantavirus). Vector-Borne Zoonotic Dis. 2015, 15, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef]
- Samson, F.; Knopf, F. Prairie conservation in North America. BioScience 1994, 44, 418–421. [Google Scholar] [CrossRef] [Green Version]
- Fuhlendorf, S.D.; Davis, C.A.; Elmore, R.D.; Goodman, L.E.; Hamilton, R.G. Perspectives on grassland conservation efforts: Should we rewild to the past or conserve for the future? Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170438. [Google Scholar] [CrossRef] [Green Version]
- Geluso, K.; Geluso, K.N.; Andersen, B.R. Distribution of the northern pygmy mouse (Baiomys taylori) in southwestern New Mexico, with notes on reproduction. Occas. Pap. Tex. Tech. Univ. Mus. 2017, 349, 12. [Google Scholar]
- Padula, P.; Figueroa, R.; Navarrete, M.; Pizarro, E.; Cadiz, R.; Bellomo, C.; Jofre, C.; Zaror, L.; Rodriguez, E.; Murua, R. Transmission study of Andes hantavirus infection in wild sigmodontine rodents. J. Virol. 2004, 78, 11972–11979. [Google Scholar] [CrossRef] [Green Version]
- Safronetz, D.; Drebot, M.A.; Artsob, H.; Cote, T.; Makowski, K.; Lindsay, R. Sin nombre virus shedding patterns in naturally infected deer mice (Peromyscus maniculatus) in relation to duration of infection. Vector-Borne Zoonotic Dis. 2008, 8, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, L.; Sironen, T.; Tonteri, E.; Bäck, A.T.; Razzauti, M.; Karlsson, M.; Wahlström, M.; Niemimaa, J.; Henttonen, H.; Lundkvist, Å. Life-long shedding of Puumala hantavirus in wild bank voles (Myodes glareolus). J. Gen. Virol. 2015, 96, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Hörnfeldt, B.; Evander, M.; Magnusson, M.; Olsson, G.; Ecke, F. Dynamics and drivers of hantavirus prevalence in rodent populations. Vector-Borne Zoonotic Dis. 2014, 14, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.N.; Ksiazek, T.G.; Peters, C.; Childs, J.E. Long-term studies of hantavirus reservoir populations in the Southwestern United States: A synthesis. Emerg. Infect. Dis. 1999, 5, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Yahnke, C.J.; Meserve, P.L.; Mills, J.N.; Ksiazek, T.G. Patterns of infection with Laguna Negra virus in wild populations of Calomys laucha in the central Paraguayan chaco. Am. J. Trop. Med. Hyg. 2001, 65, 768–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadell, M.V.; Bellomo, C.; Martín, A.S.; Padula, P.; Villafañe, I.G. Hantavirus ecology in rodent populations in three protected areas of Argentina: Hantavirus ecology in protected areas. Trop. Med. Int. Health 2011, 16, 1342–1352. [Google Scholar] [CrossRef]
- Walsh, A.S.; Louis, T.A.; Glass, G.E. Detecting multiple levels of effect during survey sampling using a Bayesian approach: Point prevalence estimates of a hantavirus in hispid cotton rats (Sigmodon hispidus). Ecol. Model. 2007, 205, 29–38. [Google Scholar] [CrossRef]
- Emlen, S.; Oring, L. Ecology, sexual selection, and the evolution of mating systems. Science 1977, 197, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Vestal, B.M.; Sehnell, G.D. Influence of environmental complexity and space on social interactions of mice (Mus musculus and Peromyscus leucopus). J. Comp. Psychol. 1986, 100, 143–154. [Google Scholar] [CrossRef]
- Hasler, J.F.; Banks, E. The behavioral and somatic effects of ovariectomy and replacement therapy in female collared lemmings (Dicrostonyx groenlandicus). Horm. Behav. 1976, 7, 59–74. [Google Scholar] [CrossRef]
- Ruffer, D.G. Sexual behaviour of the northern grasshopper mouse (Onychomys leucogaster). Anim. Behav. 1965, 13, 447–452. [Google Scholar] [CrossRef]
- Johnston, R.E. Chemical communication in rodents: From pheromones to individual recognition. J. Mammal. 2003, 84, 1141–1162. [Google Scholar] [CrossRef] [Green Version]
- Hjelle, B.; Torres-Pérez, F. Hantaviruses in the Americas and their role as emerging pathogens. Viruses 2010, 2, 2559–2586. [Google Scholar] [CrossRef] [Green Version]
- Ostfeld, R.S. Limiting resources and territoriality in microtine rodents. Am. Nat. 1985, 126, 1–15. [Google Scholar] [CrossRef]
- Wolff, J.; Peterson, J. An offspring-defense hypothesis for territoriality in female mammals. Ethol. Ecol. Evol. 1998, 10, 227–239. [Google Scholar] [CrossRef]
- Frank, D.H.; Heske, E.J. Seasonal changes in space use patterns in the southern grasshopper mouse, Onychomys torridus torridus. J. Mammal. 1992, 73, 292–298. [Google Scholar] [CrossRef]
- Priotto, J.; Steinmann, A.; Polop, J. Factors affecting home range size and overlap in Calomys venustus (Muridae: Sigmodontinae) in Argentine agroecosystems. Mamm. Biol. 2002, 67, 97–104. [Google Scholar] [CrossRef]
- Schradin, C.; Schubert, M.; Pillay, N. Winter huddling groups in the striped mouse. Can. J. Zool. 2006, 84, 693–698. [Google Scholar] [CrossRef] [Green Version]
- Madison, D.M.; Fitzgerald, R.W.; McShea, W.J. Dynamics of social nesting in overwintering meadow voles (Microtus pennsylvanicus): Possible consequences for population cycling. Behav. Ecol. Sociobiol. 1984, 15, 9–17. [Google Scholar] [CrossRef]
- Wolff, J.O.; Durr, D.S. Winter nesting behavior of Peromyscus leucopus and Peromyscus maniculatus. J. Mammal. 1986, 67, 409–412. [Google Scholar] [CrossRef]
- Vaughan, T.A. Vertebrates inhabiting pocket gopher burrows in Colorado. J. Mammal. 1961, 42, 171. [Google Scholar] [CrossRef]
- Stapp, P. Rodent communities in active and inactive colonies of black-tailed prairie dogs in shortgrass steppe. J. Mammal. 2007, 88, 241–249. [Google Scholar] [CrossRef]
- Kraft, J.P.; Stapp, P. Movements and burrow use by northern grasshopper mice as a possible mechanism of plague spread in prairie dog colonies. J. Mammal. 2013, 94, 1087–1093. [Google Scholar] [CrossRef] [Green Version]
- Diatta, G.; Duplantier, J.; Granjon, L.; Bâ, K.; Chauvancy, G.; Ndiaye, M.; Trape, J.-F. Borrelia infection in small mammals in West Africa and its relationship with tick occurrence inside burrows. Acta Trop. 2015, 152, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kollath, D.R.; Teixeira, M.M.; Funke, A.; Miller, K.J.; Barker, B.M. Investigating the role of animal burrows on the ecology and distribution of Coccidioides spp. in Arizona soils. Mycopathologia 2019, 1–15. [Google Scholar] [CrossRef]
- Buck, C.L.; Barnes, B. Temperatures of hibernacula and changes in body composition of arctic ground squirrels over winter. J. Mammal. 1999, 80, 1264–1276. [Google Scholar] [CrossRef] [Green Version]
- Šumbera, R.; Chitaukali, W.N.; Elichová, M.; Kubová, J.; Burda, H. Microclimatic stability in burrows of an Afrotropical solitary bathyergid rodent, the silvery mole-rat (Heliophobius argenteocinereus). J. Zool. 2004, 263, 409–416. [Google Scholar] [CrossRef]
- Carver, S.; Mills, J.N.; Parmenter, C.A.; Parmenter, R.; Richardson, K.S.; Harris, R.; Douglass, R.J.; Kuenzi, A.J.; Luis, A.D. Toward a mechanistic understanding of environmentally forced zoonotic disease emergence: Sin Nombre hantavirus. BioScience 2015, 65, 651–666. [Google Scholar] [CrossRef] [Green Version]
- Yates, T.L.; Mills, J.N.; Parmenter, C.A.; Ksiazek, T.G.; Parmenter, R.; Castle, J.R.V.; Calisher, C.H.; Nichol, S.T.; Abbott, K.D.; Young, J.C.; et al. The ecology and evolutionary history of an emergent disease: hantavirus pulmonary syndrome. BioScience 2002, 52, 989–998. [Google Scholar] [CrossRef] [Green Version]
- Mills, J.N.; Schmidt, K.; Ellis, B.A.; Calderon, G.; Enria, D.A.; Ksiazek, T.G. A longitudinal study of hantavirus infection in three sympatric reservoir species in agroecosystems on the Argentine Pampa. Vector-Borne Zoonotic Dis. 2007, 7, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Adler, F.R.; Pearce-Duvet, J.M.C.; Dearing, M.D. How host population dynamics translate into time-lagged prevalence: An investigation of Sin Nombre virus in deer mice. Bull. Math. Biol. 2007, 70, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Adler, F.R.; Clay, C.A.; Lehmer, E.M. The role of heterogeneity in the persistence and prevalence of Sin Nombre virus in deer mice. Am. Nat. 2008, 172, 855–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padula, P.; Edelstein, A.; Miguel, S.; Lopez, N.; Rossi, C.; Rabinovich, R. Hantavirus pulmonary syndrome outbreak in Argentina: Molecular evidence for person-to-person transmission of andes virus. Virology 1998, 241, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toro, J.; Vega, J.D.; Khan, A.S.; Mills, J.N.; Padula, P.; Terry, W.; Yadón, Z.; Valderrama, R.; Ellis, B.A.; Pavletic, C.; et al. An outbreak of hantavirus pulmonary syndrome, Chile, 1997. Emerg. Infect. Dis. 1998, 4, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valdebenito, C.; Calvo, M.; Vial, C.; Mansilla, R.; Marco, C.; Palma, R.E.; Vial, P.A.; Valdivieso, F.; Mertz, G.; Garrido, M.F. Person-to-person household and nosocomial transmission of andes hantavirus, Southern Chile, 2011. Emerg. Infect. Dis. 2014, 20, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Mardones, J.; Villagra, E. Prevalencia de anticuerpos anti-hantavirus en personal de salud en contacto directo con pacientes portadores del síndrome pulmonar por hantavirus: Temuco 1997 a 1999. Rev. Méd. Chile 2000, 128, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, J. Assessment of person-to-person transmission of hantavirus pulmonary syndrome in a Chilean hospital setting. J. Hosp. Infect. 1998, 40, 281–285. [Google Scholar] [CrossRef]
- Bharadwaj, M.; Hjelle, B.; Torrez-Martinez, N.; Botten, J. Rio Mamore virus: Genetic characterization of a newly recognized hantavirus of the pygmy rice rat, Oligoryzomys microtis, from Bolivia. Am. J. Trop. Med. Hyg. 1997, 57, 368–374. [Google Scholar] [CrossRef]
- Armstrong, L.R.; Zaki, S.R.; Goldoft, M.J.; Todd, R.L.; Khan, A.S.; Khabbaz, R.F.; Ksiazek, T.G.; Peters, C.J. Hantavirus pulmonary syndrome associated with entering or cleaning rarely used, rodent-infested structures. J. Infect. Dis. 1995, 172, 1166. [Google Scholar] [CrossRef]
- Munshi-South, J.; Kharchenko, K. Rapid, pervasive genetic differentiation of urban white-footed mouse (Peromyscus leucopus) populations in New York City: Genetics of urban white-footed mice. Mol. Ecol. 2010, 19, 4242–4254. [Google Scholar] [CrossRef]
- Harris, S.E.; Munshi-South, J. Signatures of positive selection and local adaptation to urbanization in white-footed mice (Peromyscus leucopus). Mol. Ecol. 2017, 26, 6336–6350. [Google Scholar] [CrossRef] [PubMed]
- Clément, J.; Maes, P.; Van Ranst, M. Hantaviruses in the old and new world. Perspect. Med. Virol. 2006, 16, 161–177. [Google Scholar]
- Wilson, A.C.; Fenton, B.; Malloch, G.L.; Boag, B.; Hubbard, S.; Begg, G. Urbanisation versus agriculture: A comparison of local genetic diversity and gene flow between wood mouse Apodemus sylvaticus populations in human-modified landscapes. Ecography 2015, 39, 87–97. [Google Scholar] [CrossRef]
- Kerins, J.L.; Koske, S.E.; Kazmierczak, J.; Austin, C.; Gowdy, K.; Dibernardo, A. Outbreak of Seoul virus among rats and rat owners—United States and Canada, 2017. Morb. Mortal. Wkly. Rep. 2018, 67, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Childs, J.E.; Korch, G.W.; Glass, G.E.; Leduc, J.W.; Shah, K.V. Epizootiology of hantavirus infections in Baltimore: Isolation of a virus from Norway rats, and characteristics of infected rat populations. Am. J. Epidemiol. 1987, 126, 55–68. [Google Scholar] [CrossRef]
- Williams, S.H.; Che, X.; Paulick, A.; Guo, C.; Lee, B.; Muller, D.; Uhlemann, A.-C.; Lowy, F.D.; Corrigan, R.M.; Lipkin, W.I. New York City house mice (Mus musculus) as potential reservoirs for pathogenic bacteria and antimicrobial resistance determinants. mBio 2018, 9, e00624-18. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.H.; Che, X.; Garcia, J.A.; Klena, J.D.; Lee, B.; Muller, D.; Ulrich, W.; Corrigan, R.M.; Nichol, S.; Jain, K.; et al. Viral diversity of house mice in New York City. mBio 2018, 9, e01354-17. [Google Scholar] [CrossRef] [Green Version]
- Delfraro, A.; Clara, M.; Tome, L.; Achával, F.; Levis, S.; Calderon, G.; Enria, D.; Lozano, M.; Russi, J.; Arbiza, J. Yellow pygmy rice rat (Oligoryzomys flavescens) and hantavirus pulmonary syndrome in Uruguay. Emerg. Infect. Dis. 2003, 9, 846–852. [Google Scholar] [CrossRef]
- Wilkinson, D.A.; Marshall, J.C.; French, N.P.; Hayman, D.T.S. Habitat fragmentation, biodiversity loss and the risk of novel infectious disease emergence. J. R. Soc. Interface 2018, 15, 20180403. [Google Scholar] [CrossRef] [Green Version]
- Rulli, M.C.; Santini, M.; Hayman, D.T.S.; D’Odorico, P. The nexus between forest fragmentation in Africa and Ebola virus disease outbreaks. Sci. Rep. 2017, 7, 41613. [Google Scholar] [CrossRef] [Green Version]
- Morzunov, S.P.; Feldmann, H.; Spiropoulou, C.F.; Semenova, V.A.; Rollin, P.E.; Ksiazek, T.G.; Peters, C.J.; Nichol, S.T. A newly recognized virus associated with a fatal case of hantavirus pulmonary syndrome in Louisiana. J. Virol. 1995, 69, 1980–1983. [Google Scholar] [CrossRef] [Green Version]
- Ravkov, E.V.; Rollin, P.E.; Ksiazek, T.G.; Peters, C.J.; Nichol, S.T. Genetic and serologic analysis of black creek canal virus and its association with human disease and Sigmodon hispidus infection. Virology 1995, 210, 482–489. [Google Scholar] [CrossRef]
- Palma, R.E.; Polop, J.J.; Owen, R.D.; Mills, J.N. Ecology of rodent-associated hantaviruses in the southern cone of South America: Argentina, Chile, Paraguay, and Uruguay. J. Wildl. Dis. 2012, 48, 267–281. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Bravo, J.; Armién, B.; Suzán, G.; Armien, A.; A Ruedas, L.; Avila, M.; Zaldívar, Y.; Pascale, J.M.; Gracia, F.; Yates, T.L. Serosurvey of wild rodents for hantaviruses in Panama, 2000–2002. J. Wildl. Dis. 2004, 40, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Levis, S.; Morzunov, S.P.; Rowe, J.E.; Enria, D.; Pini, N.; Calderon, G.; Sabattini, M.; Jeor, S.C.S. Genetic diversity and epidemiology of hantaviruses in Argentina. J. Infect. Dis. 1998, 177, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, C.B.; Figueiredo, L.T.M.; Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitsutani, P.T.; Denton, R.W.; Fritz, C.L.; Murray, R.A.; Todd, R.L.; Pape, W.J.; Frampton, J.W.; Young, J.C.; Khan, A.S.; Peters, C.J.; et al. Acute Sin Nombre hantavirus infection without pulmonary syndrome, United States. Emerg. Infect. Dis. 1999, 5, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Bennett, S.N.; Dizney, L.; Sumibcay, L.; Arai, S.; Ruedas, L.A.; Song, J.-W.; Yanagihara, R. Host switch during evolution of a genetically distinct hantavirus in the American shrew mole (Neurotrichus gibbsii). Virology 2009, 388, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.J.; Bennett, S.N.; Hope, A.G.; Cook, J.A.; Yanagihara, R. Shared ancestry between a newfound mole-borne hantavirus and hantaviruses harbored by cricetid rodents. J. Virol. 2011, 85, 7496–7503. [Google Scholar] [CrossRef] [Green Version]
- Arai, S.; Song, J.-W.; Sumibcay, L.; Bennett, S.N.; Nerurkar, V.R.; Parmenter, C.; Cook, J.A.; Yates, T.L.; Yanagihara, R. Hantavirus in Northern short-tailed shrew, United States. Emerg. Infect. Dis. 2007, 13, 1420–1423. [Google Scholar] [CrossRef]


| Virus Species | Virus Strain | Virus Abbreviation | No. GenBank Submissions (Nov 9 2019) | Year Described | Human Disease | Discovery Source |
|---|---|---|---|---|---|---|
| Andes orthohantavirus | Andes virus | ANDV | 285 | 1996 | Yes | HCPS |
| Castelo dos Sonhos virus | CASV | 11 | 1999 | Yes | HCPS | |
| Lechiguanas virus | LECV/LECHV | 26 | 1997 | Yes | HCPS | |
| Oran virus | ORNV | 11 | 1998 | Yes | HCPS | |
| Bayou orthohantavirus | Bayou virus | BAYV | 13 | 1995 | Yes | HCPS |
| Catacamas virus | CATV | 3 | 2006 | No | Rodent | |
| Black Creek Canal orthohantavirus | Black Creek Canal virus | BCCV | 8 | 1995 | Yes | Rodent |
| Caño Delgadito orthohantavirus | Caño Delgadito virus | CADV | 17 | 1997 | No 1 | Rodent |
| Choclo orthohantavirus | Choclo virus | CHOV | 12 | 2000 | Yes | HCPS |
| El Moro Canyon orthohantavirus | Carrizal virus | CARV | 9 | 2012 | No | Rodent |
| El Moro Canyon virus | ELMCV | 35 | 1994 | No 1 | Rodent | |
| Huitzilac virus | HUIV | 4 | 2012 | No | Rodent | |
| Laguna Negra orthohantavirus | Laguna Negra virus | LANV | 35 | 1997 | Yes | HCPS |
| Maripa virus | MARV | 16 | 2012 | Yes | HCPS | |
| Rio Mamoré virus | RIOMV | 15 | 1997 | Yes | Rodent | |
| Maporal orthohantavirus | Maporal virus | MAPV | 10 | 2004 | No | Rodent |
| Montano orthohantavirus | Montano virus | MTNV | 60 | 2012 | No | Rodent |
| Necocli orthohantavirus | Necocli virus | NECV | 10 | 2011 | No | Rodent |
| Prospect Hill orthohantavirus | Prospect Hill virus | PHV | 24 | 1985 | No | Rodent |
| Sin Nombre orthohantavirus | New York virus | NYV | 4 | 1995 2 | Yes | HCPS |
| Sin Nombre virus | SNV | 228 | 1994 | Yes | HCPS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mull, N.; Jackson, R.; Sironen, T.; Forbes, K.M. Ecology of Neglected Rodent-Borne American Orthohantaviruses. Pathogens 2020, 9, 325. https://doi.org/10.3390/pathogens9050325
Mull N, Jackson R, Sironen T, Forbes KM. Ecology of Neglected Rodent-Borne American Orthohantaviruses. Pathogens. 2020; 9(5):325. https://doi.org/10.3390/pathogens9050325
Chicago/Turabian StyleMull, Nathaniel, Reilly Jackson, Tarja Sironen, and Kristian M. Forbes. 2020. "Ecology of Neglected Rodent-Borne American Orthohantaviruses" Pathogens 9, no. 5: 325. https://doi.org/10.3390/pathogens9050325
APA StyleMull, N., Jackson, R., Sironen, T., & Forbes, K. M. (2020). Ecology of Neglected Rodent-Borne American Orthohantaviruses. Pathogens, 9(5), 325. https://doi.org/10.3390/pathogens9050325






