Current Status and Trends in Prophylaxis and Management of Anthrax Disease
Abstract
1. Introduction
2. Anthrax Pathogenesis
2.1. Virulence Factors
2.2. Immunology
2.3. Clinical Forms of Anthrax Disease
2.3.1. Cutaneous
2.3.2. Gastrointestinal
2.3.3. Inhalational
2.3.4. Intravenous/Injectional Anthrax
3. Summary of Animal Research
4. Current Guidelines for Anthrax Management and Prophylaxis
4.1. World Health Organization
4.2. Centers for Disease Control and Prevention
4.3. United States Department of Defense (DOD)
4.4. United States Department of Agriculture (USDA)
5. Regulatory Aspects of Anthrax Medical Countermeasures (MCMs) Development under the U.S. Food and Drug Administration (FDA)
6. Pre-Exposure Prophylaxis (PrEP)—Vaccination Prior to Anthrax Exposure
6.1. Historical Overview of Anthrax Vaccine Development
6.2. BioThrax Vaccine—The Only FDA-Licensed Anthrax Vaccine in the United States
7. Post-Exposure Prophylaxis (PEP)—Vaccination or Treatment after Exposure but Prior to Onset of Symptoms
7.1. Use of Animal Rule for Approval of Anthrax MCMs
7.2. Antibiotics
7.3. Post-Exposure Vaccination
7.4. Antibody-Based Therapeutics
7.4.1. Anthrasil AIGIV
7.4.2. Raxibacumab
7.4.3. Obiltoxaximab
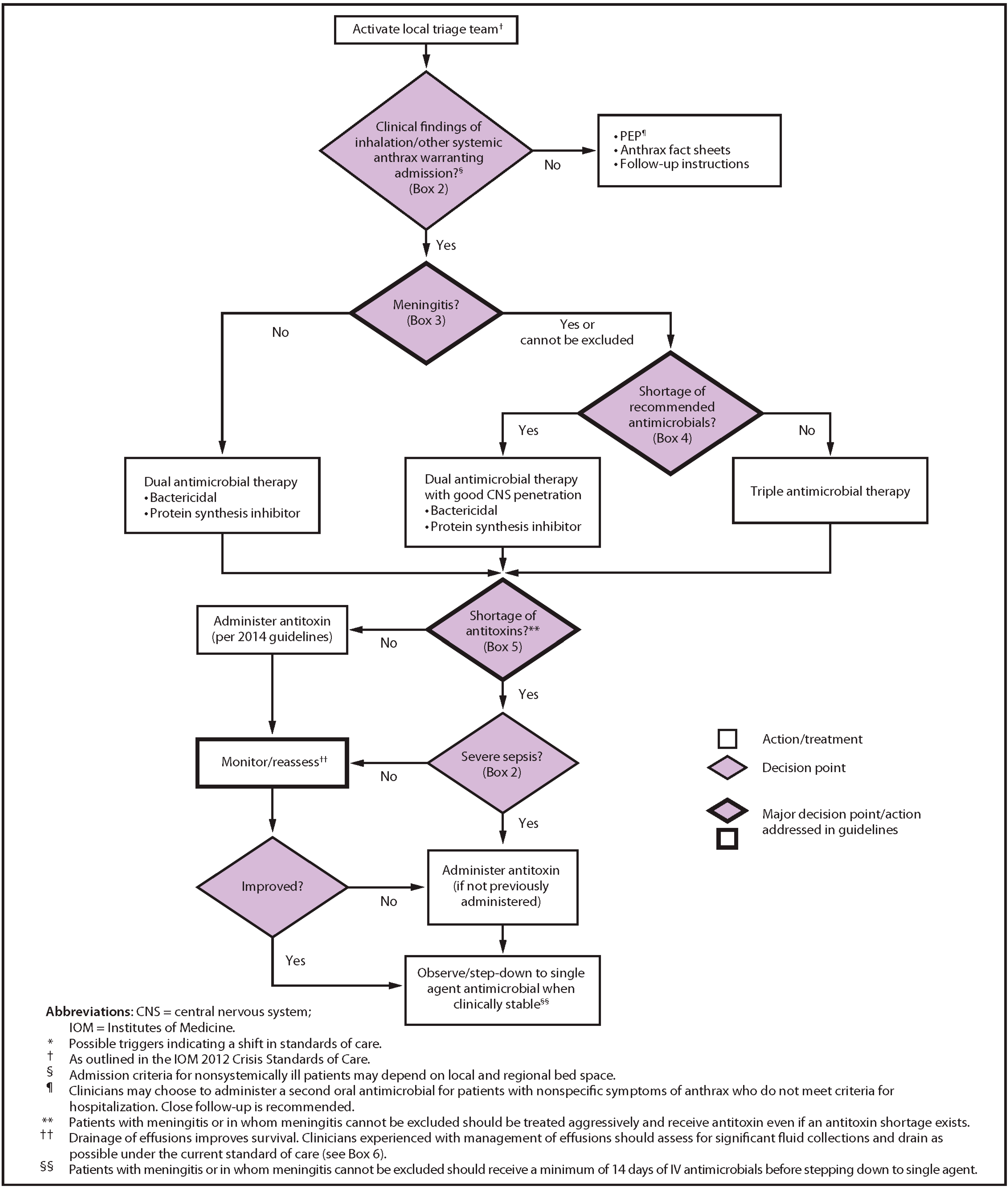
8. Treatment of Symptomatic Disease
8.1. Antibiotics
8.2. Antitoxin Therapy
9. Next-Generation Product Candidates
9.1. AV7909
9.2. Recombinant Protective Antigen-Based Vaccines
9.3. Novel Antibiotics
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sternbach, G. The history of anthrax. J. Emerg. Med. 2003, 24, 463–467. [Google Scholar] [CrossRef]
- Doganay, M. Anthrax. In Infectious Diseases; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1123–1128. [Google Scholar]
- Frischknecht, F. The history of biological warfare: Human experimentation, modern nightmares and lone madmen in the twentieth century. EMBO Rep. 2003, 4, 47–52. [Google Scholar] [CrossRef]
- Moayeri, M.; Leppla, S.H. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Asp. Med. 2009, 30, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, A.M.; Vietri, N.J. Anthrax. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Content Repository Only: London, UK, 2020; pp. 571–576. [Google Scholar]
- WHO. Anthrax. In Humans and Animals; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar] [CrossRef]
- Swick, M.C.; Koehler, T.M.; Driks, A. Surviving Between Hosts: Sporulation and Transmission. Microbiol. Spectr. 2016, 4, 529–566. [Google Scholar] [CrossRef]
- Cote, C.; Welkos, S.L. Anthrax Toxins in Context of Bacillus anthracis Spores and Spore Germination. Toxins 2015, 7, 3167–3178. [Google Scholar] [CrossRef]
- Leppla, S.H.; Robbins, J.B.; Schneerson, R.; Shiloach, J. Development of an improved vaccine for anthrax. J. Clin. Investig. 2002, 110, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Welkos, S. Plasmid-associated virulence factors of non-toxigenic (pX01−) Bacillus anthracis. Microb. Pathog. 1991, 10, 183–198. [Google Scholar] [CrossRef]
- Abrami, L.; Reig, N.; Van Der Goot, G. Anthrax toxin: The long and winding road that leads to the kill. Trends Microbiol. 2005, 13, 72–78. [Google Scholar] [CrossRef]
- Park, J.M.; Greten, F.R.; Li, Z.-W.; Karin, M. Macrophage Apoptosis by Anthrax Lethal Factor Through p38 MAP Kinase Inhibition. Science 2002, 297, 2048–2051. [Google Scholar] [CrossRef]
- Baldari, C.T.; Tonello, F.; Paccani, S.R.; Montecucco, C. Anthrax toxins: A paradigm of bacterial immune suppression. Trends Immunol. 2006, 27, 434–440. [Google Scholar] [CrossRef]
- Agrawal, A.; Lingappa, J.; Leppla, S.H.; Agrawal, S.; Jabbar, A.; Quinn, C.; Pulendran, B. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 2003, 424, 329–334. [Google Scholar] [CrossRef]
- Alileche, A.; Serfass, E.R.; Muehlbauer, S.M.; Porcelli, S.A.; Brojatsch, J. Anthrax Lethal Toxin-Mediated Killing of Human and Murine Dendritic Cells Impairs the Adaptive Immune Response. PLoS Pathog. 2005, 1, 19. [Google Scholar] [CrossRef]
- Paccani, S.R.; Tonello, F.; Ghittoni, R.; Natale, M.; Muraro, L.; D’Elios, M.M.; Tang, W.-J.; Montecucco, C.; Baldari, C.T. Anthrax toxins suppress T lymphocyte activation by disrupting antigen receptor signaling. J. Exp. Med. 2005, 201, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Choo, M.-K.; Sano, Y.; Kim, C.; Yasuda, K.; Li, X.; Lin, X.; Stenzel-Poore, M.; Alexopoulou, L.; Ghosh, S.; Latz, E.; et al. TLR sensing of bacterial spore-associated RNA triggers host immune responses with detrimental effects. J. Exp. Med. 2017, 214, 1297–1311. [Google Scholar] [CrossRef]
- Ingram, R.; Metan, G.; Maillere, B.; Doganay, M.; Ozkul, Y.; Kim, L.U.; Baillie, L.; Dyson, H.; Williamson, E.D.; Chu, K.K.; et al. Natural Exposure to Cutaneous Anthrax Gives Long-Lasting T Cell Immunity Encompassing Infection-Specific Epitopes. J. Immunol. 2010, 184, 3814–3821. [Google Scholar] [CrossRef] [PubMed]
- Inglesby, T.V.; O’Toole, T.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Friedlander, A.M.; Gerberding, J.; Hauer, J.; Hughes, J.; et al. Anthrax as a biological weapon, 2002: Updated recommendations for management. Jama 2002, 287, 2236–2252. [Google Scholar] [CrossRef] [PubMed]
- Zasada, A.A. Injectional anthrax in human: A new face of the old disease. Adv. Clin. Exp. Med. 2018, 27, 553–558. [Google Scholar] [CrossRef]
- Shadomy, S.; Idrissi, A.E.; Raizman, E.; Bruni, M.; Palamara, E.; Pittiglio, C.; Lubroth, J. Anthrax outbreaks: A warning for improved prevention, control and heightened awareness. EMPRES Watch 2016, 37, 1–8. [Google Scholar]
- European Centre for Disease Control and Prevention. Anthrax. Annual Epidemiolocal Report for 2016. 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2016-anthrax.pdf (accessed on 18 October 2019).
- CDC. Anthrax. Available online: https://www.cdc.gov/anthrax/index.html (accessed on 10 October 2019).
- Brachman, P.S. Anthrax. Ann. N. Y. Acad. Sci. 1970, 174, 730. [Google Scholar] [CrossRef]
- Cieslak, T.J.; Eitzen, E.M. Clinical and epidemiologic principles of anthrax. Emerg. Infect. Dis. 1999, 5, 552–555. [Google Scholar] [CrossRef]
- Kuehnert, M.J.; Doyle, T.; Hill, H.A.; Bridges, C.B.; Jernigan, J.A.; Dull, P.M.; Reissman, D.B.; Ashford, D.A.; Jernigan, D.B. Clinical Features that Discriminate Inhalational Anthrax from Other Acute Respiratory Illnesses. Clin. Infect. Dis. 2003, 36, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Shafazand, S.; Doyle, R.; Ruoss, S.; Weinacker, A.; Raffin, T.A. Inhalational anthrax: Epidemiology, diagnosis, and management. Chest 1999, 116, 1369–1376. [Google Scholar] [CrossRef]
- Abramova, F.A.; Grinberg, L.M.; Yampolskaya, O.V.; Walker, D.H. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc. Natl. Acad. Sci. USA 1993, 90, 2291–2294. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, J.A.; Stephens, D.S.; Ashford, D.A.; Omenaca, C.; Topiel, M.S.; Galbraith, M.; Tapper, M.; Fisk, T.L.; Zaki, S.; Popovic, T.; et al. Bioterrorism-related inhalational anthrax: The first 10 cases reported in the United States. Emerg. Infect. Dis. 2001, 7, 933–944. [Google Scholar] [CrossRef]
- National Anthrax Outbreak Control Team. An Outbreak of Anthrax Among Drug Users in Scotland, December 2009 to December 2010. 2011. Available online: https://www.hps.scot.nhs.uk/web-resources-container/an-outbreak-of-anthrax-among-drug-users-in-scotland-december-2009-to-december-2010-a-report-on-behalf-of-the-national-anthrax-outbreak-control-team/ (accessed on 18 October 2019).
- Price, E.P.; Seymour, M.L.; Sarovich, D.S.; Latham, J.; Wolken, S.R.; Mason, J.; Vincent, G.; Drees, K.P.; Beckstrom-Sternberg, S.M.; Phillippy, A.; et al. Molecular Epidemiologic Investigation of an Anthrax Outbreak among Heroin Users, Europe. Emerg. Infect. Dis. 2012, 18, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Doganay, M.; Metan, G.; Alp, E. A review of cutaneous anthrax and its outcome. J. Infect. public Health 2010, 3, 98–105. [Google Scholar] [CrossRef]
- Maddah, G.; Abdollahi, A.; Katebi, M. Gastrointestinal anthrax: Clinical experience in 5 cases. Casp. J. Intern. Med. 2013, 4, 672–676. [Google Scholar]
- Bower, W.A.; Hendricks, K.; Pillai, S.; Guarnizo, J.; Meaney-Delman, D. Clinical Framework and Medical Countermeasure Use During an Anthrax Mass-Casualty Incident. MMWR. Recomm. Rep. 2015, 64, 1–22. [Google Scholar] [CrossRef]
- Hendricks, K.A.; Wright, M.E.; Shadomy, S.V.; Bradley, J.S.; Morrow, M.G.; Pavia, A.T.; Rubinstein, E.; Holty, J.-E.C.; Messonnier, N.E.; Smith, T.L.; et al. Centers for Disease Control and Prevention Expert Panel Meetings on Prevention and Treatment of Anthrax in Adults. Emerg. Infect. Dis. 2014, 20. [Google Scholar] [CrossRef]
- Powell, A.G.; Crozier, J.E.; Hodgson, H.; Galloway, D.J. A case of septicaemic anthrax in an intravenous drug user. BMC Infect. Dis. 2011, 11, 21. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry Animal Models—Essential Elements to Address Efficacy Under the Animal Rule 2015. Available online: https://www.fda.gov/media/88625/download (accessed on 5 May 2020).
- Welkos, S.; Bozue, J.A.; Twenhafel, N.; Cote, C.K. Animal Models for the Pathogenesis, Treatment, and Prevention of Infection by Bacillus anthracis. Microbiol. Spectr. 2015, 3, 269–311. [Google Scholar] [CrossRef] [PubMed]
- Glassman, H.N. Industrially acquired anthrax. Discuss. Bacteriol. Rev. 1966, 30, 657–659. [Google Scholar] [CrossRef]
- Savransky, V.; Sanford, D.C.; Syar, E.; Austin, J.L.; Tordoff, K.P.; Anderson, M.S.; Stark, G.V.; Barnewall, R.E.; Briscoe, C.M.; Lemiale-Biérinx, L.; et al. Pathology and pathophysiology of inhalational anthrax in a guinea pig model. Infect. Immun. 2013, 81, 1152–1163. [Google Scholar] [CrossRef]
- Goossens, P.L. Animal models of human anthrax: The Quest for the Holy Grail. Mol. Asp. Med. 2009, 30, 467–480. [Google Scholar] [CrossRef]
- Chand, H.S.; Drysdale, M.; Lovchik, J.; Koehler, T.M.; Lipscomb, M.F.; Lyons, C.R. Discriminating Virulence Mechanisms among Bacillus anthracis Strains by Using a Murine Subcutaneous Infection Model. Infect. Immun. 2008, 77, 429–435. [Google Scholar] [CrossRef][Green Version]
- Leffel, E.K.; Pitt, L. Anthrax. In Biodefense: Research Methodology and Animal Models; Swearengen, J.R., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 77–95. [Google Scholar]
- Ivins, B. Experimental anthrax vaccines: Efficacy of adjuvants combined with protective antigen against an aerosol Bacillus anthracis spore challenge in guinea pigs. Vaccine 1995, 13, 1779–1784. [Google Scholar] [CrossRef]
- Fellows, P.F.; Linscott, M.K.; Little, S.F.; Gibbs, P.; Ivins, B.E. Anthrax vaccine efficacy in golden Syrian hamsters. Vaccine 2002, 20, 1421–1424. [Google Scholar] [CrossRef]
- Zaucha, G.M.; Pitt, L.M.; Estep, J.; Ivins, B.; Friedlander, A.M. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch. pathol. Lab. Med. 1998, 122, 982–992. [Google Scholar]
- Vasconcelos, D.; Barnewall, R.; Babin, M.; Hunt, R.; Estep, J.; Nielsen, C.; Carnes, R.; Carney, J. Pathology of Inhalation Anthrax in Cynomolgus Monkeys (Macaca fascicularis). Lab. Investig. 2003, 83, 1201–1209. [Google Scholar] [CrossRef]
- Fritz, D.L.; Jaax, N.K.; Lawrence, W.B.; Davis, K.J.; Pitt, M.L.; Ezzell, J.W.; Friedlander, A.M. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab. Investig. 1995, 73, 691–702. [Google Scholar]
- Rossi, C.A.; Ulrich, M.; Norris, S.; Reed, D.S.; Pitt, L.M.; Leffel, E.K. Identification of a Surrogate Marker for Infection in the African Green Monkey Model of Inhalation Anthrax. Infect. Immun. 2008, 76, 5790–5801. [Google Scholar] [CrossRef][Green Version]
- Arora, G.; Misra, R.; Sajid, A. Model Systems for Pulmonary Infectious Diseases: Paradigms of Anthrax. Curr. Top. Med. Chem. 2019, 17, 2077–2099. [Google Scholar] [CrossRef] [PubMed]
- Loving, C.L.; Kennett, M.; Lee, G.M.; Grippe, V.K.; Merkel, T.J. Murine Aerosol Challenge Model of Anthrax . Infect. Immun. 2007, 75, 2689–2698. [Google Scholar]
- Henning, L.N.; Comer, J.E.; Stark, G.V.; Ray, B.D.; Tordoff, K.P.; Knostman, K.A.B.; Meister, G.T. Development of an Inhalational Bacillus anthracis Exposure Therapeutic Model in Cynomolgus Macaques. Clin. Vaccine Immunol. 2012, 19, 1765–1775. [Google Scholar] [CrossRef]
- Comer, J.E.; Ray, B.D.; Henning, L.N.; Stark, G.V.; Barnewall, R.E.; Mott, J.M.; Meister, G.T. Characterization of a Therapeutic Model of Inhalational Anthrax Using an Increase in Body Temperature in New Zealand White Rabbits as a Trigger for Treatment. Clin. Vaccine Immunol. 2012, 19, 1517–1525. [Google Scholar] [CrossRef]
- Yamamoto, B.J.; Shadiack, A.M.; Carpenter, S.; Sanford, D.; Henning, L.N.; Gonzales, N.; O’Connor, E.; Casey, L.S.; Serbina, N.V. Obiltoxaximab Prevents Disseminated Bacillus anthracis Infection and Improves Survival during Pre- and Postexposure Prophylaxis in Animal Models of Inhalational Anthrax. Antimicrob. Agents Chemother. 2016, 60, 5796–5805. [Google Scholar] [CrossRef] [PubMed]
- Henning, L.N.; Carpenter, S.; Stark, G.V.; Serbina, N.V. Development of Protective Immunity in New Zealand White Rabbits Challenged with Bacillus anthracis Spores and Treated with Antibiotics and Obiltoxaximab, a Monoclonal Antibody against Protective Antigen. Antimicrob. Agents Chemother. 2018, 62, e01590-17. [Google Scholar] [CrossRef]
- Rankin, R.; Pontarollo, R.; Ioannou, X.; Krieg, A.M.; Hecker, R.; Babiuk, L.; Hurk, S.V.D.L.-V.D. CpG Motif Identification for Veterinary and Laboratory Species Demonstrates That Sequence Recognition Is Highly Conserved. Antisense Nucleic Acid Drug Dev. 2001, 11, 333–340. [Google Scholar] [CrossRef]
- Darling, R.G.; Catlett, C.L.; Huebner, K.D.; Jarrett, D.G. Threats in bioterrorism I: CDC category A agents. Emerg. Med. Clin. N. Am. 2002, 20, 273–309. [Google Scholar] [CrossRef]
- Bower, W.; Schiffer, J.; Atmar, R.L.; Keitel, W.A.; Friedlander, A.M.; Liu, L.; Yu, Y.; Stephens, D.S.; Quinn, C.P.; Hendricks, K. Use of Anthrax Vaccine in the United States: Recommendations of the Advisory Committee on Immunization Practices, 2019. MMWR Recomm. Rep. 2019, 68, 1–14. [Google Scholar] [CrossRef]
- Department of Defense. Clarifying Guidance for Smallpox and Anthrax Vaccine Immunization Programs 2015. Available online: https://wct.army.mil/documents/policies/Clarifying_Guidance_for_Smallpox_and_Anthrax_Vaccine_Immunization_Programs.pdf (accessed on 22 October 2019).
- Spickler, Anna Rovid. Anthrax. Available online: http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php (accessed on 10 May 2020).
- FDA. Drug and Biologic Animal Rule Approvals. 2019. Available online: https://www.fda.gov/media/107839/download (accessed on 12 February 2020).
- Brady, R.A.; Verma, A.; Meade, B.D.; Burns, D.L. Analysis of Antibody Responses to Protective Antigen-Based Anthrax Vaccines through Use of Competitive Assays. Clin. Vaccine Immunol. 2010, 17, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- FDA. Medical Countermeasures Initiatve (MCMi). Available online: https://www.fda.gov/emergency-preparedness-and-response/counterterrorism-and-emerging-threats/medical-countermeasures-initiative-mcmi (accessed on 24 October 2019).
- Brachman, P.S. Inhalation anthrax. Ann. N. Y. Acad. Sci. 1980, 353, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Sterne, M. Avirulent anthrax vaccine. Onderstepoort J. Vet. Sci. Anim. Ind. 1946, 21, 41–43. [Google Scholar] [PubMed]
- Brachman, P.S.; Gold, H.; Plotkin, S.A.; Fekety, R.; Werrin, M.; Ingraham, N.R. Field Evaluation of Human Anthrax Vaccine. J. Occup. Environ. Med. 1962, 4, 499. [Google Scholar] [CrossRef]
- Brachman, P.S. Bioterrorism: An update with a focus on anthrax. Am. J. Epidemiol. 2002, 155, 981–987. [Google Scholar] [CrossRef]
- Schiffer, J.M.; McNeil, M.M.; Quinn, C. Recent developments in the understanding and use of anthrax vaccine adsorbed: Achieving more with less. Expert Rev. Vaccines 2016, 15, 1151–1162. [Google Scholar] [CrossRef]
- Marano, N.; Plikaytis, B.D.; Martin, S.W.; Rose, C.; Semenova, V.A.; Martin, S.K.; Freeman, A.E.; Li, H.; Mulligan, M.J.; Parker, S.D.; et al. Effects of a Reduced Dose Schedule and Intramuscular Administration of Anthrax Vaccine Adsorbed on Immunogenicity and Safety at 7 Months, A Randomized Trial. Jama 2008, 300, 1532–1543. [Google Scholar] [CrossRef]
- Quinn, C.; Sabourin, C.L.; Niemuth, N.A.; Li, H.; Semenova, V.A.; Rudge, T.L.; Mayfield, H.J.; Schiffer, J.; Mittler, R.S.; Ibegbu, C.C.; et al. A Three-Dose Intramuscular Injection Schedule of Anthrax Vaccine Adsorbed Generates Sustained Humoral and Cellular Immune Responses to Protective Antigen and Provides Long-Term Protection against Inhalation Anthrax in Rhesus Macaques. Clin. Vaccine Immunol. 2012, 19, 1730–1745. [Google Scholar] [CrossRef]
- Wright, J.G.; Plikaytis, B.D.; Rose, C.E.; Parker, S.D.; Babcock, J.; Keitel, W.; El Sahly, H.; Poland, G.A.; Jacobson, R.M.; Keyserling, H.L.; et al. Effect of reduced dose schedules and intramuscular injection of anthrax vaccine adsorbed on immunological response and safety profile: A randomized trial. Vaccine 2014, 32, 1019–1028. [Google Scholar] [CrossRef]
- Beasley, D.W.C.; Brasel, T.L.; Comer, J.E. First vaccine approval under the FDA Animal Rule. npj Vaccines 2016, 1, 16013. [Google Scholar] [CrossRef]
- Longstreth, J.; Skiadopoulos, M.H.; Hopkins, R.J. Licensure strategy for pre- and post-exposure prophylaxis of biothrax vaccine: The first vaccine licensed using the FDA animal rule. Expert Rev. Vaccines 2016, 15, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.L. Licensure of vaccines using the Animal Rule. Curr. Opin. Virol. 2012, 2, 353–356. [Google Scholar] [CrossRef]
- FDA. Pathway to Licensure for Protective Antigen-based Anthrax Vaccines for a Post-exposure Prophylaxis Indication Using the Animal Rule; Briefing Document for the Vaccines and Related Biological Products Advisory Committee Meeting, November 16. 2010. Available online: https://wayback.archive-it.org/7993/20170113080506/http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM232400.pdf (accessed on 10 May 2020).
- Ionin, B.; Hopkins, R.J.; Pleune, B.; Sivko, G.S.; Reid, F.M.; Clement, K.H.; Rudge, T.L.; Stark, G.V.; Innes, A.; Sari, S.; et al. Evaluation of immunogenicity and efficacy of anthrax vaccine adsorbed for postexposure prophylaxis. Clin. Vaccine Immunol. 2013, 20, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.; Little, S.F.; Ivins, B.; Fellows, P.; Barth, J.; Hewetson, J.; Gibbs, P.; Dertzbaugh, M.; Friedlander, A.M. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 2001, 19, 4768–4773. [Google Scholar] [CrossRef]
- Leffel, E.K.; Bourdage, J.S.; Williamson, E.D.; Duchars, M.; Fuerst, T.R.; Fusco, P.C. Recombinant Protective Antigen Anthrax Vaccine Improves Survival when Administered as a Postexposure Prophylaxis Countermeasure with Antibiotic in the New Zealand White Rabbit Model of Inhalation Anthrax. Clin. Vaccine Immunol. 2012, 19, 1158–1164. [Google Scholar] [CrossRef]
- Kao, L.M.; Bush, K.; Barnewall, R.; Estep, J.; Thalacker, F.W.; Olson, P.H.; Drusano, G.L.; Minton, N.; Chien, S.; Hemeryck, A.; et al. Pharmacokinetic Considerations and Efficacy of Levofloxacin in an Inhalational Anthrax (Postexposure) Rhesus Monkey Model. Antimicrob. Agents Chemother. 2006, 50, 3535–3542. [Google Scholar] [CrossRef]
- Vietri, N.J.; Purcell, B.K.; Lawler, J.V.; Leffel, E.K.; Rico, P.; Gamble, C.; Twenhafel, N.A.; Ivins, B.E.; Heine, H.S.; Sheeler, R.; et al. Short-course postexposure antibiotic prophylaxis combined with vaccination protects against experimental inhalational anthrax. Proc. Natl. Acad. Sci. USA 2006, 103, 7813–7816. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Welkos, S.; Pitt, M.L.M.; Ezzell, J.W.; Worsham, P.L.; Rose, K.J.; Ivins, B.; Lowe, J.R.; Howe, G.B.; Mikesell, P.; et al. Postexposure Prophylaxis against Experimental Inhalation Anthrax. J. Infect. Dis. 1993, 167, 1239–1242. [Google Scholar] [CrossRef]
- Kammanadiminti, S.; Patnaikuni, R.K.; Comer, J.; Meister, G.; Sinclair, C.; Kodihalli, S. Combination Therapy with Antibiotics and Anthrax Immune Globulin Intravenous (AIGIV) Is Potentially More Effective than Antibiotics Alone in Rabbit Model of Inhalational Anthrax. PLoS ONE 2014, 9, e106393. [Google Scholar] [CrossRef]
- Mytle, N.; Hopkins, R.J.; Malkevich, N.V.; Basu, S.; Meister, G.T.; Sanford, D.C.; Comer, J.E.; Van Zandt, K.E.; Al-Ibrahim, M.; Kramer, W.G.; et al. Evaluation of intravenous anthrax immune globulin for treatment of inhalation anthrax. Antimicrob. Agents Chemother. 2013, 57, 5684–5692. [Google Scholar] [CrossRef]
- Athamna, A.; Abu-Rashed, N.; Medlej, B.; Bast, D.J.; Rubinstein, E. Selection of Bacillus anthracis isolates resistant to antibiotics. J. Antimicrob. Chemother. 2004, 54, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Pittman, P.; Norris, S.; Barreraoro, J.; Bedwell, D.; Cannon, T.; Mckeejr, K. Patterns of antibody response in humans to the anthrax vaccine adsorbed (AVA) primary (six-dose) series. Vaccine 2006, 24, 3654–3660. [Google Scholar] [CrossRef] [PubMed]
- Kummerfeldt, C.E. Raxibacumab: Potential role in the treatment of inhalational anthrax. Infect. Drug Resist. 2014, 7, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Migone, T.-S.; Subramanian, G.M.; Zhong, J.; Healey, L.M.; Corey, A.; Devalaraja, M.; Lo, L.; Ullrich, S.; Zimmerman, J.; Chen, A.; et al. Raxibacumab for the Treatment of Inhalational Anthrax. N. Engl. J. Med. 2009, 361, 135–144. [Google Scholar] [CrossRef]
- Migone, T.-S.; Bolmer, S.; Zhong, J.; Corey, A.; Vasconcelos, D.; Buccellato, M.; Meister, G. Added Benefit of Raxibacumab to Antibiotic Treatment of Inhalational Anthrax. Antimicrob. Agents Chemother. 2015, 59, 1145–1151. [Google Scholar] [CrossRef]
- Greig, S.L. Obiltoxaximab: First Global Approval. Drugs 2016, 76, 823–830. [Google Scholar] [CrossRef]
- Mohamed, N.; Clagett, M.; Li, J.; Jones, S.; Pincus, S.; D’Alia, G.; Nardone, L.; Babin, M.; Spitalny, G.; Casey, L. A High-Affinity Monoclonal Antibody to Anthrax Protective Antigen Passively Protects Rabbits before and after Aerosolized Bacillus anthracis Spore Challenge. Infect. Immun. 2005, 73, 795–802. [Google Scholar] [CrossRef]
- Biron, B.; Beck, K.; Dyer, D.; Mattix, M.; Twenhafel, N.; Nalca, A. Efficacy of ETI-204 Monoclonal Antibody as an Adjunct Therapy in a New Zealand White Rabbit Partial Survival Model for Inhalational Anthrax. Antimicrob. Agents Chemother. 2015, 59, 2206–2214. [Google Scholar] [CrossRef]
- Lightfoot, N.F.; Scott, R.J.; Turnbull, P.C. Antimicrobial susceptibility of Bacillus anthracis. Salisbury Med. Bull. 1990, 68S, 95–98. [Google Scholar]
- Chen, Y.; Succi, J.; Tenover, F.C.; Koehler, T.M. Beta-lactamase genes of the penicillin-susceptible Bacillus anthracis Sterne strain. J. Bacteriol. 2003, 185, 823–830. [Google Scholar] [CrossRef]
- Huang, E.; Pillai, S.K.; Bower, W.A.; Hendricks, K.A.; Guarnizo, J.T.; Hoyle, J.D.; Gorman, S.E.; Boyer, A.E.; Quinn, C.P.; Meaney-Delman, D. Antitoxin Treatment of Inhalation Anthrax: A Systematic Review. Health Secur. 2015, 13, 365–377. [Google Scholar] [CrossRef]
- Xu, W.; Ohanjanian, L.; Sun, J.; Cui, X.; Suffredini, D.; Li, Y.; Welsh, J.; Eichacker, P.Q. Correction: A systematic review and meta-analysis of preclinical trials testing anti-toxin therapies for B. anthracis infection: A need for more robust study designs and results. PLoS ONE 2017, 12, e0189239. [Google Scholar] [CrossRef]
- Xu, W.; Ohanjandian, L.; Sun, J.; Cui, X.; Suffredini, D.; Li, Y.; Welsh J; Eichacker PQ. A systematic review and meta-analysis of preclinical trials testing anti-toxin therapies for B. anthracis infection: A need for more robust study designs and results. PLoS ONE 2017, 12, e0182879. [Google Scholar] [CrossRef]
- Klinman, D.M.; Xie, H.; Little, S.F.; Currie, D.; Ivins, B.E. CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine 2004, 22, 2881–2886. [Google Scholar] [CrossRef]
- Gu, M.; Hine, P.M.; Jackson, W.J.; Giri, L.; Nabors, G.S. Increased potency of BioThrax® anthrax vaccine with the addition of the C-class CpG oligonucleotide adjuvant CPG. Vaccine 2007, 25, 526–534. [Google Scholar] [CrossRef]
- Savransky, V.; Shearer, J.D.; Gainey, M.R.; Sanford, D.C.; Sivko, G.S.; Stark, G.V.; Li, N.; Ionin, B.; Lacy, M.J.; Skiadopoulos, M.H. Correlation between anthrax lethal toxin neutralizing antibody levels and survival in guinea pigs and nonhuman primates vaccinated with the AV7909 anthrax vaccine candidate. Vaccine 2017, 35, 4952–4959. [Google Scholar] [CrossRef]
- Hopkins, R.J.; Daczkowski, N.F.; Kaptur, P.E.; Muse, D.; Sheldon, E.; LaForce, C.; Sari, S.; Rudge, T.L.; Bernton, E. Randomized, double-blind, placebo-controlled, safety and immunogenicity study of 4 formulations of Anthrax Vaccine Adsorbed plus CPG 7909 (AV7909) in healthy adult volunteers. Vaccine 2013, 31, 3051–3058. [Google Scholar] [CrossRef]
- Hopkins, R.J.; Kalsi, G.; Montalvo-Lugo, V.M.; Sharma, M.; Wu, Y.; Muse, D.D.; Sheldon, E.; Hampel, F.C.; Lemiale, L. Randomized, double-blind, active-controlled study evaluating the safety and immunogenicity of three vaccination schedules and two dose levels of AV7909 vaccine for anthrax post-exposure prophylaxis in healthy adults. Vaccine 2016, 34, 2096–2105. [Google Scholar] [CrossRef]
- Rynkiewicz, D.; Rathkopf, M.; Sim, I.; Waytes, A.T.; Hopkins, R.J.; Giri, L.; DeMuria, D.; Ransom, J.; Quinn, J.; Nabors, G.S.; et al. Marked enhancement of the immune response to BioThrax(R) (Anthrax Vaccine Adsorbed) by the TLR9 agonist CPG 7909 in healthy volunteers. Vaccine 2011, 29, 6313–6320. [Google Scholar] [CrossRef]
- Perry, M.R.; Ionin, B.; Barnewall, R.E.; Vassar, M.L.; Reece, J.J.; Park, S.; Lemiale, L.; Skiadopoulos, M.H.; Shearer, J.D.; Savransky, V. Development of a guinea pig inhalational anthrax model for evaluation of post-exposure prophylaxis efficacy of anthrax vaccines. Vaccine 2020, 38, 2307–2314. [Google Scholar] [CrossRef]
- D’Souza, A.J.M.; Mar, K.D.; Huang, J.; Majumdar, S.; Ford, B.M.; Dyas, B.; Ulrich, R.G.; Sullivan, V.J. Rapid Deamidation of Recombinant Protective Antigen when Adsorbed on Aluminum Hydroxide Gel Correlates with Reduced Potency of Vaccine. J. pharm. Sci. 2013, 102, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Kondakova, O.; Nikitin, N.A.; Evtushenko, E.; Ryabchevskaya, E.; Atabekov, J.; Karpova, O. Vaccines against anthrax based on recombinant protective antigen: Problems and solutions. Expert Rev. Vaccines 2019, 18, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, J.A.; Lin, F.-Y.C.; Chu, C.; Shiloach, J.; Leppla, S.H.; Benavides, G.A.; Karpas, A.; Moayeri, M.; Guo, C.; Robbins, J.B.; et al. Phase 1 Study of a Recombinant Mutant Protective Antigen of Bacillus anthracis. Clin. Vaccine Immunol. 2011, 19, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.K.; Cox, J.; Gillis, A.; VanCott, T.C.; Marovich, M.; Milazzo, M.; Antonille, T.S.; Wieczorek, L.; McKee, K.T.; Metcalfe, K.; et al. Phase I Study of Safety and Immunogenicity of an Escherichia coli-Derived Recombinant Protective Antigen (rPA) Vaccine to Prevent Anthrax in Adults. PLoS ONE 2010, 5, e13849. [Google Scholar] [CrossRef]
- Campbell, J.D.; Clement, K.H.; Wasserman, S.S.; Donegan, S.; Chrisley, L.; Kotloff, K.L. Safety, Reactogenicity, and Immunogenicity of a Recombinant Protective Antigen Anthrax Vaccine Given to Healthy Adults. Hum. Vaccines 2007, 3, 205–211. [Google Scholar] [CrossRef]
- Gorse, G.J.; Keitel, W.; Keyserling, H.; Taylor, D.N.; Lock, M.; Alves, K.; Kenner, J.; Deans, L.; Gurwith, M. Immunogenicity and tolerance of ascending doses of a recombinant protective antigen (rPA102) anthrax vaccine: A randomized, double-blinded, controlled, multicenter trial. Vaccine 2006, 24, 5950–5959. [Google Scholar] [CrossRef]
- Kang, C.K.; Kim, N.-H.; Kim, C.-J.; Rhie, G.-E.; Jo, S.K.; Ahn, M.; Kang, J.; Choe, P.G.; Park, W.B.; Kim, N.-J.; et al. Immunogenicity and safety of a novel recombinant protective antigen anthrax vaccine (GC1109), a randomized, single-blind, placebo controlled phase II clinical study. Vaccine 2019, 37, 3820–3824. [Google Scholar] [CrossRef]
- Bielinska, A.U.; Janczak, K.W.; Landers, J.J.; Makidon, P.; Sower, L.E.; Peterson, J.W.; Baker, J.R. Mucosal Immunization with a Novel Nanoemulsion-Based Recombinant Anthrax Protective Antigen Vaccine Protects against Bacillus anthracis Spore Challenge. Infect. Immun. 2007, 75, 4020–4029. [Google Scholar] [CrossRef]
- Chun, J.-H.; Choi, O.-J.; Cho, M.-H.; Hong, K.-J.; Seong, W.K.; Oh, H.-B.; Rhie, G.-E. Serological Correlate of Protection in Guinea Pigs for a Recombinant Protective Antigen Anthrax Vaccine Produced from Bacillus brevis. Osong Public Health Res. Perspect. 2012, 3, 170–176. [Google Scholar] [CrossRef][Green Version]
- Little, S.F.; Ivins, B.; Webster, W.; Norris, S.; Andrews, G. Effect of aluminum hydroxide adjuvant and formaldehyde in the formulation of rPA anthrax vaccine. Vaccine 2007, 25, 2771–2777. [Google Scholar] [CrossRef]
- Oscherwitz, J.; Yu, F.; Cease, K.B. A synthetic peptide vaccine directed against the 2ss2-2ss3 loop of domain 2 of protective antigen protects rabbits from inhalation anthrax. J. Immunol. 2010, 185, 3661–3668. [Google Scholar] [CrossRef] [PubMed]
- Peachman, K.K.; Li, Q.; Matyas, G.R.; Shivachandra, S.B.; Lovchik, J.; Lyons, R.C.; Alving, C.R.; Rao, V.B.; Rao, M. Anthrax Vaccine Antigen-Adjuvant Formulations Completely Protect New Zealand White Rabbits against Challenge with Bacillus anthracis Ames Strain Spores. Clin. Vaccine Immunol. 2011, 19, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Rhie, G.-E.; Park, Y.-M.; Han, J.-S.; Yu, J.-Y.; Seong, W.-K.; Oh, H.-B. Efficacy of non-toxic deletion mutants of protective antigen from Bacillus anthracis. FEMS Immunol. Med. Microbiol. 2005, 45, 341–347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ribot, W.J.; Powell, B.; Ivins, B.; Little, S.; Johnson, W.; Hoover, T.; Norris, S.; Adamovicz, J.; Friedlander, A.; Andrews, G. Comparative vaccine efficacy of different isoforms of recombinant protective antigen against Bacillus anthracis spore challenge in rabbits. Vaccine 2006, 24, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.; Hodgson, I.; Walker, N.J.; Topping, A.W.; Duchars, M.G.; Mott, J.M.; Estep, J.; LeButt, C.; Flick-Smith, H.C.; Jones, H.E.; et al. Immunogenicity of Recombinant Protective Antigen and Efficacy against Aerosol Challenge with Anthrax. Infect. Immun. 2005, 73, 5978–5987. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.K.; Ahn, B.-E.; Choi, E.H.; Kang, J.E.; An, H.; Oh, M.-D.; Rhie, G.-E. Evaluation of the protective efficacy of recombinant protective antigen vaccine (GC1109)-immunized human sera using passive immunization in a mouse model. Vaccine 2020, 38, 1586–1588. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Mahalingam, M.; Zhu, J.; Moayeri, M.; Kirtley, M.L.; Fitts, E.C.; Andersson, J.A.; Lawrence, W.S.; Leppla, S.H.; Chopra, A.K.; et al. A Bivalent Anthrax–Plague Vaccine That Can Protect against Two Tier-1 Bioterror Pathogens, Bacillus anthracis and Yersinia pestis. Front. Immunol. 2017, 8, 687. [Google Scholar] [CrossRef]
- Kachura, M.; Hickle, C.; Kell, S.A.; Sathe, A.; Calacsan, C.; Kiwan, R.; Hall, B.; Milley, R.; Ott, G.; Coffman, R.L.; et al. A CpG-Ficoll Nanoparticle Adjuvant for Anthrax Protective Antigen Enhances Immunogenicity and Provides Single-Immunization Protection against Inhaled Anthrax in Monkeys. J. Immunol. 2015, 196, 284–297. [Google Scholar] [CrossRef]
- Reed, M.D.; Wilder, J.A.; Mega, W.M.; Hutt, J.A.; Kuehl, P.J.; Valderas, M.W.; Chew, L.L.; Liang, B.C.; Squires, C.H. Immunization with a Recombinant, Pseudomonas fluorescens-Expressed, Mutant Form of Bacillus anthracis-Derived Protective Antigen Protects Rabbits from Anthrax Infection. PLoS ONE 2015, 10, e0130952. [Google Scholar] [CrossRef]
- Oscherwitz, J.; Yu, F.; Jacobs, J.L.; Cease, K.B. Recombinant Vaccine Displaying the Loop-Neutralizing Determinant from Protective Antigen Completely Protects Rabbits from Experimental Inhalation Anthrax. Clin. Vaccine Immunol. 2013, 20, 341–349. [Google Scholar] [CrossRef][Green Version]
- Fay, M.P.; Follmann, D.A.; Lynn, F.; Schiffer, J.M.; Stark, G.V.; Kohberger, R.; Quinn, C.P.; Nuzum, E.O.; Stark, G.V.; Stark, G.; et al. Anthrax Vaccine-Induced Antibodies Provide Cross-Species Prediction of Survival to Aerosol Challenge. Sci. Transl. Med. 2012, 4, 151ra126. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.M.; Macdonald, L.D.; Rajagopalan, R.; Sivko, G.S.; Valderas, M.W.; Rayner, J.; Berger, B.J.; Sammatur, L.; Stanford, M.M. Single dose of DPX-rPA, an enhanced-delivery anthrax vaccine formulation, protects against a lethal Bacillus anthracis spore inhalation challenge. npj Vaccines 2019, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.-H.; Wang, W.; Dai, S.-Q.; Liu, T.-Y.; Tan, J.-J.; Qu, G.-L.; Li, Y.-X.; Ling, Y.; Liu, G.; Fu, X.-Q.; et al. Daptomycin exerts rapid bactericidal activity against Bacillus anthracis without disrupting membrane integrity. Acta pharmacol. Sin. 2013, 35, 211–218. [Google Scholar] [CrossRef]
- Steenbergen, J.; Tanaka, S.K.; Miller, L.L.; Halasohoris, S.A.; Hershfield, J.R. In Vitro and In Vivo Activity of Omadacycline against Two Biothreat Pathogens, Bacillus anthracis and Yersinia pestis. Antimicrob. Agents Chemother. 2017, 61, e02434-16. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.D.; Palmer, M. The action mechanism of daptomycin. Bioorg. Med. Chem. 2016, 24, 6253–6268. [Google Scholar] [CrossRef]
- Honeyman, L.; Ismail, M.; Nelson, M.L.; Bhatia, B.; Bowser, T.E.; Chen, J.; Mechiche, R.; Ohemeng, K.; Verma, A.K.; Cannon, E.P.; et al. Structure-activity relationship of the aminomethylcyclines and the discovery of omadacycline. Antimicrob. Agents Chemother. 2015, 59, 7044–7053. [Google Scholar] [CrossRef]
- Żakowska, D.; Bartoszcze, M.; Niemcewicz, M.; Bielawska-Drózd, A.; Knap, J.; Cieślik, P.; Chomiczewski, K.; Kocik, J. Bacillus anthracis infections—New possibilities of treatment. Ann. Agric. Environ. Med. 2015, 22, 202–207. [Google Scholar] [CrossRef]
- Narayanan, N.; Lacy, C.R.; Cruz, J.; Nahass, M.; Karp, J.; Barone, J.A.; Hermes-DeSantis, E. Disaster Preparedness: Biological Threats and Treatment Options. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 217–234. [Google Scholar] [CrossRef]
- Shepard, C.W.; Soriano-Gabarró, M.; Zell, E.R.; Hayslett, J.; Lukacs, S.; Goldstein, S.; Factor, S.; Jones, J.; Ridzon, R.; Williams, I.; et al. Antimicrobial Postexposure Prophylaxis for Anthrax: Adverse Events and Adherence. Emerg. Infect. Dis. 2002, 8, 1124–1132. [Google Scholar] [CrossRef]
- Artenstein, A.W.; Opal, S.M. Novel Approaches to the Treatment of Systemic Anthrax. Clin. Infect. Dis. 2012, 54, 1148–1161. [Google Scholar] [CrossRef]
| Cutaneous Anthrax | Gastrointestinal Anthrax | Inhalational Anthrax | Intravenous/Injectional Anthrax * | |
|---|---|---|---|---|
| References | [32] | [6,33] | [27,34,35] | [30,36] |
| Occurrence | Endemic areas and middle-income countries | Consumption of undercooked meat in endemic areas | Bioterrorism, bioweapon, sporadic cases in wool handlers, drummers, drum-makers and persons exposed to infected animals | Drug users, industrial countries |
| Incubation period | 1–17 days | 2–5 days | 1–6 days; periods up to 43 days have been observed | 1–10 days |
| Lesion site | Exposure site, mostly superficial | Abdominal pain, vomiting (including hematemesis), hematochezia, and occasional watery diarrhea | Hemorrhagic lymphadenitis, widened mediastinum, meningeal edema and hemorrhage, pleural effusions, pulmonary edema, and hemorrhagic meningitis | Injection site, soft tissue infection with necrosis |
| Severity of infection | Mild to severe, rarely complicated, up to 20% die if not treated | Early diagnosis is difficult, resulting in high mortality | Severe | Severe |
| Diagnosis | Patient history, patient examination, laboratory results | Patient history, patient examination, laboratory results | Early diagnosis is difficult. Patient history, examination, microhemagglutination test specific for PA, X-ray, microbiology (blood, sputum) | Patient history, patient examination, laboratory results |
| Treatment | Antibiotics, supportive care in severe cases | Antibiotics, supportive care | Antibiotics and supportive care, immunoglobulins | Supportive care, of antibiotic combination treatment |
| Duration of antibiotic treatment | 3–5 days | 3–5 days | ≥2–3 weeks, then 60 days from onset of illness | 10–14 days, with up to 60 days for intranasal drug users |
| Surgical intervention | Rarely | Often due to ascites and/or peritonitis | Rarely | Debridement, reconstructive surgery may be required |
| Mortality | <1% | 4–50% | 85–90%. With aggressive treatment, mortality can be reduced to 45% | >30% |
| Species | LD50 Parenteral Route | LD50 Inhalational Route | Primary Pathophysiological Factor(s) | References |
|---|---|---|---|---|
| Mouse | < 10–151 | 14,500 | Bacteremia | [42,43] |
| Guinea Pig | < 10–50 | 16,650–40,000 | Bacteremia, Toxemia | [40,44] |
| Rat | 106 | - | Toxemia (resistant to infection) | [43] |
| Hamster | 10 | - | Bacteremia | [45] |
| Rabbit | - | 105,000 | Toxemia | [46] |
| Cynomolgus Macaque | - | 34,000–110,000 | Toxemia | [47] |
| Rhesus Macaque | - | 30,000–172,000 | Toxemia | [48] |
| African Green Monkey | - | 10,000 | Toxemia | [49] |
| Model | Objective | Role in Establishing TNA Threshold of Protection | Species | References |
|---|---|---|---|---|
| Pre-exposure Prophylaxis (PrEP) | Demonstrate correlation between pre-challenge TNA levels and probability of survival | Appropriate: • Immune response induced solely by vaccination • Protection conferred by both circulating TNA and immune memory • Animals challenged at the time point that approximates when residual spores may germinate | NZW Rabbit | [76,77] |
| Rhesus and Cynomolgus Macaque | [70,76] | |||
| Post-exposure Prophylaxis (PEP) | Demonstrate added benefit of vaccine compared to antibiotic treatment alone, in a post-exposure setting | Limited: • Dynamic model of rapidly progressing disease • Immune response results from both vaccination and infection | NZW Rabbit | [76,78] |
| Rhesus Macaque | [79,80,81] | |||
| Passive Transfer | Demonstrate that neutralizing antibody alone is capable of protection | Limited: • Protection conferred by circulating antibody only • Demonstrates that antibody can protect, but overestimates protective levels of antibody compared with active vaccination | NZW Rabbit AIGIV administered at onset of clinical signs of disease | [82,83] |
| NZW Rabbit AIGIV administered as timed post-exposure prophylaxis | [82,83] | |||
| Cynomolgus Macaque AIGIV administered at onset of clinical signs of disease | [83] |
| Category | Antibiotic | Duration |
|---|---|---|
| Naturally occurring anthrax | First choice *: • Procaine penicillin G, 0.6–1.2 M units IM q 12–24 h Penicillin G, sodium or potassium 4 M units IV q 4–6 h • Amoxicillin 500 mg PO q 6–8 h Alternative *: • Doxycycline 100 mg IV/PO q 12 h Ciprofloxacin 200–400 mg IV q12 h, followed by 500–750 mg PO q12 h | 3–5 days (up to 3–7 days) for cutaneous anthrax without complications; 10–14 days for systemic anthrax † |
| Intravenous/injectional anthrax | Combination of antibiotics, plus surgical debridement, followed by reconstructive surgery if required | 10–14 days, with up to 60 days for intranasal drug users |
| Biological weapon or bio-terrorism-related anthrax | • Ciprofloxacin 200–400 mg IV q 12 h, followed by 500–750 mg PO q12 h • Doxycycline 100 mg IV/PO q12 h | 42–60 days |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savransky, V.; Ionin, B.; Reece, J. Current Status and Trends in Prophylaxis and Management of Anthrax Disease. Pathogens 2020, 9, 370. https://doi.org/10.3390/pathogens9050370
Savransky V, Ionin B, Reece J. Current Status and Trends in Prophylaxis and Management of Anthrax Disease. Pathogens. 2020; 9(5):370. https://doi.org/10.3390/pathogens9050370
Chicago/Turabian StyleSavransky, Vladimir, Boris Ionin, and Joshua Reece. 2020. "Current Status and Trends in Prophylaxis and Management of Anthrax Disease" Pathogens 9, no. 5: 370. https://doi.org/10.3390/pathogens9050370
APA StyleSavransky, V., Ionin, B., & Reece, J. (2020). Current Status and Trends in Prophylaxis and Management of Anthrax Disease. Pathogens, 9(5), 370. https://doi.org/10.3390/pathogens9050370




