Rotavirus A in Brazil: Molecular Epidemiology and Surveillance during 2018–2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Stool Collection and Ethics Statements
2.2. Viral RNA Extraction
2.3. RVA Detection and Quantification
2.4. Genotyping and Sequencing
2.5. Phylogenetic Analysis
2.6. Statistical Analysis
3. Results
3.1. Rotavirus A Epidemiology
3.2. RVA Genotyping
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO | Levels and Trends in Child Mortality Report 2019. Available online: https://www.who.int/maternal_child_adolescent/documents/levels_trends_child_mortality_2019/en/ (accessed on 2 May 2020).
- Liu, L.; Qian, Y.; Zhang, Y.; Zhao, L.; Jia, L.; Dong, H. Epidemiological aspects of rotavirus and adenovirus in hospitalized children with diarrhea: A 5-year survey in Beijing. BMC Infect. Dis. 2016, 16, 508. [Google Scholar] [CrossRef] [PubMed]
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Parashar, U.D.; World Health Organization–Coordinated Global Rotavirus Surveillance Network. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin. Infect. Dis 2016, 62, S96–S105. [Google Scholar] [CrossRef]
- Burnett, E.; Parashar, U.D.; Tate, J.E. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2006–2019. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Aliabadi, N.; Antoni, S.; Mwenda, J.M.; Weldegebriel, G.; Biey, J.N.M.; Cheikh, D.; Fahmy, K.; Teleb, N.; Ashmony, H.A.; Ahmed, H.; et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–2016: Findings from the Global Rotavirus Surveillance Network. Lancet Glob. Health 2019, 7, e893–e903. [Google Scholar] [CrossRef]
- Burke, R.M.; Tate, J.E.; Kirkwood, C.D.; Steele, A.D.; Parashar, U.D. Current and new rotavirus vaccines. Curr. Opin. Infect. Dis. 2019, 32, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Van Ranst, M. Genotype constellation and evolution of group A rotaviruses infecting humans. Curr. Opin. Virol. 2012, 2, 426–433. [Google Scholar] [CrossRef]
- Dóró, R.; Farkas, S.L.; Martella, V.; Bányai, K. Zoonotic transmission of rotavirus: Surveillance and control. Expert Rev. Anti. Infect. Ther. 2015, 13, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Ciarlet, M.; Heiman, E.; Arijs, I.; Delbeke, T.; McDonald, S.M.; Palombo, E.A.; Iturriza-Gómara, M.; Maes, P.; Patton, J.T.; et al. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008, 82, 3204–3219. [Google Scholar] [CrossRef]
- Rotavirus Classification Working Group: RCWG. Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg (accessed on 10 May 2020).
- Dóró, R.; László, B.; Martella, V.; Leshem, E.; Gentsch, J.; Parashar, U.; Bányai, K. Review of global rotavirus strain prevalence data from six years post vaccine licensure surveillance: Is there evidence of strain selection from vaccine pressure? Infect. Genet. Evol. 2014, 28, 446–461. [Google Scholar] [CrossRef]
- Bányai, K.; László, B.; Duque, J.; Steele, A.D.; Nelson, E.A.S.; Gentsch, J.R.; Parashar, U.D. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: Insights for understanding the impact of rotavirus vaccination programs. Vaccine 2012, 30, A122–A130. [Google Scholar] [CrossRef] [PubMed]
- Iturriza-Gómara, M.; Dallman, T.; Bányai, K.; Böttiger, B.; Buesa, J.; Diedrich, S.; Fiore, L.; Johansen, K.; Koopmans, M.; Korsun, N.; et al. Rotavirus genotypes co-circulating in Europe between 2006 and 2009 as determined by EuroRotaNet, a pan-European collaborative strain surveillance network. Epidemiol. Infect. 2011, 139, 895–909. [Google Scholar] [CrossRef] [PubMed]
- de A. Mendes, P.S.; da C. Ribeiro, H.; Mendes, C.M.C. Temporal trends of overall mortality and hospital morbidity due to diarrheal disease in Brazilian children younger than 5 years from 2000 to 2010. J. Pediatr. (Rio J.) 2013, 89, 315–325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gurgel, R.Q.; Alvarez, A.D.J.; Rodrigues, A.; Ribeiro, R.R.; Dolabella, S.S.; Da Mota, N.L.; Santos, V.S.; Iturriza-Gomara, M.; Cunliffe, N.A.; Cuevas, L.E. Incidence of Rotavirus and Circulating Genotypes in Northeast Brazil during 7 Years of National Rotavirus Vaccination. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Gurgel, R.G.; Bohland, A.K.; Vieira, S.C.F.; Oliveira, D.M.P.; Fontes, P.B.; Barros, V.F.; Ramos, M.F.; Dove, W.; Nakagomi, T.; Nakagomi, O.; et al. Incidence of rotavirus and all-cause diarrhea in northeast Brazil following the introduction of a national vaccination program. Gastroenterology 2009, 137, 1970–1975. [Google Scholar] [CrossRef] [PubMed]
- Linhares, A.C.; Velázquez, F.R.; Pérez-Schael, I.; Sáez-Llorens, X.; Abate, H.; Espinoza, F.; López, P.; Macías-Parra, M.; Ortega-Barría, E.; Rivera-Medina, D.M.; et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: A randomised, double-blind, placebo-controlled phase III study. Lancet 2008, 371, 1181–1189. [Google Scholar] [CrossRef]
- Carvalho-Costa, F.A.; de Assis, R.M.S.; Fialho, A.M.; Araújo, I.T.; Silva, M.F.; Gómez, M.M.; Andrade, J.S.; Rose, T.L.; Fumian, T.M.; Volotão, E.M.; et al. The evolving epidemiology of rotavirus A infection in Brazil a decade after the introduction of universal vaccination with Rotarix®. BMC Pediatr. 2019, 19, 42. [Google Scholar] [CrossRef]
- Carvalho-Costa, F.A.; de M. Volotão, E.; de Assis, R.M.S.; Fialho, A.M.; da S.R. de Andrade, J.; Rocha, L.N.; Tort, L.F.L.; da Silva, M.F.M.; Gómez, M.M.; de Souza, P.M.; et al. Laboratory-based rotavirus surveillance during the introduction of a vaccination program, Brazil, 2005–2009. Pediatr. Infect. Dis. J. 2011, 30, S35–S41. [Google Scholar] [CrossRef]
- Luchs, A.; do C.S.T. Timenetsky, M. Group A rotavirus gastroenteritis: Post-vaccine era, genotypes and zoonotic transmission. Einstein (Sao Paulo) 2016, 14, 278–287. [Google Scholar] [CrossRef] [PubMed]
- da Silva Soares, L.; de Fátima Dos Santos Guerra, S.; do Socorro Lima de Oliveira, A.; da Silva Dos Santos, F.; de Fátima Costa de Menezes, E.M.; Mascarenhas, J.; d’Arc, P.; Linhares, A.C. Diversity of rotavirus strains circulating in Northern Brazil after introduction of a rotavirus vaccine: High prevalence of G3P[6] genotype. J. Med. Virol. 2014, 86, 1065–1072. [Google Scholar] [CrossRef]
- Luchs, A.; da Costa, A.C.; Cilli, A.; Komninakis, S.C.V.; de C.C. Carmona, R.; Boen, L.; Morillo, S.G.; Sabino, E.C.; do C.S.T. Timenetsky, M. Spread of the emerging equine-like G3P[8] DS-1-like genetic backbone rotavirus strain in Brazil and identification of potential genetic variants. J. Gen. Virol. 2019, 100, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Zhang, X.; Shi, H.; Chen, J.; Shi, D.; Dong, H.; Feng, L. A G3P[13] porcine group A rotavirus emerging in China is a reassortant and a natural recombinant in the VP4 gene. Transbound. Emerg. Dis. 2018, 65, e317–e328. [Google Scholar] [CrossRef] [PubMed]
- Komoto, S.; Tacharoenmuang, R.; Guntapong, R.; Ide, T.; Sinchai, P.; Upachai, S.; Fukuda, S.; Yoshikawa, T.; Tharmaphornpilas, P.; Sangkitporn, S.; et al. Identification and characterization of a human G9P[23] rotavirus strain from a child with diarrhoea in Thailand: Evidence for porcine-to-human interspecies transmission. J. Gen. Virol. 2017, 98, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Quaye, O.; Roy, S.; Rungsrisuriyachai, K.; Esona, M.D.; Xu, Z.; Tam, K.I.; Banegas, D.J.C.; Rey-Benito, G.; Bowen, M.D. Characterisation of a rare, reassortant human G10P[14] rotavirus strain detected in Honduras. Mem. Inst. Oswaldo Cruz 2018, 113, 9–16. [Google Scholar] [CrossRef]
- Tacharoenmuang, R.; Komoto, S.; Guntapong, R.; Ide, T.; Singchai, P.; Upachai, S.; Fukuda, S.; Yoshida, Y.; Murata, T.; Yoshikawa, T.; et al. Characterization of a G10P[14] rotavirus strain from a diarrheic child in Thailand: Evidence for bovine-to-human zoonotic transmission. Infect. Genet. Evol. 2018, 63, 43–57. [Google Scholar] [CrossRef]
- Gentsch, J.R.; Parashar, U.D.; Glass, R.I. Impact of rotavirus vaccination: The importance of monitoring strains. Future Microbiol. 2009, 4, 1231–1234. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Bilcke, J.; Ciarlet, M.; Martella, V.; Bányai, K.; Rahman, M.; Zeller, M.; Beutels, P.; Van Damme, P.; Van Ranst, M. Rotavirus disease and vaccination: Impact on genotype diversity. Future Microbiol. 2009, 4, 1303–1316. [Google Scholar] [CrossRef]
- Roczo-Farkas, S.; Kirkwood, C.D.; Cowley, D.; Barnes, G.L.; Bishop, R.F.; Bogdanovic-Sakran, N.; Boniface, K.; Donato, C.M.; Bines, J.E. The Impact of Rotavirus Vaccines on Genotype Diversity: A Comprehensive Analysis of 2 Decades of Australian Surveillance Data. J. Infect. Dis. 2018, 218, 546–554. [Google Scholar] [CrossRef]
- Esona, M.D.; Gautam, R.; Tam, K.I.; Williams, A.; Mijatovic-Rustempasic, S.; Bowen, M.D. Multiplexed one-step RT-PCR VP7 and VP4 genotyping assays for rotaviruses using updated primers. J. Virol. Methods 2015, 223, 96–104. [Google Scholar] [CrossRef]
- Gómara, M.I.; Cubitt, D.; Desselberger, U.; Gray, J. Amino acid substitution within the VP7 protein of G2 rotavirus strains associated with failure to serotype. J. Clin. Microbiol. 2001, 39, 3796–3798. [Google Scholar] [CrossRef] [PubMed]
- Gentsch, J.R.; Glass, R.I.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.K.; Bhan, M.K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Justino, M.C.A.; Campos, E.A.; Mascarenhas, J.D.P.; Soares, L.S.; de F.S. Guerra, S.; Furlaneto, I.P.; Pavão, M.J.C.; Maciel, T.S.; Farias, F.P.; Bezerra, O.M.; et al. Rotavirus antigenemia as a common event among children hospitalised for severe, acute gastroenteritis in Belém, northern Brazil. BMC Pediatr. 2019, 19, 193. [Google Scholar] [CrossRef] [PubMed]
- Degiuseppe, J.I.; Stupka, J.A. First assessment of all-cause acute diarrhoea and rotavirus-confirmed cases following massive vaccination in Argentina. Epidemiol. Infect. 2018, 146, 1948–1954. [Google Scholar] [CrossRef]
- Junaid, S.A.; Umeh, C.; Olabode, A.O.; Banda, J.M. Incidence of rotavirus infection in children with gastroenteritis attending Jos university teaching hospital, Nigeria. Virol. J. 2011, 8, 233. [Google Scholar] [CrossRef]
- Nordgren, J.; Bonkoungou, I.J.O.; Nitiema, L.W.; Sharma, S.; Ouermi, D.; Simpore, J.; Barro, N.; Svensson, L. Rotavirus in diarrheal children in rural Burkina Faso: High prevalence of genotype G6P[6]. Infect. Genet. Evol. 2012, 12, 1892–1898. [Google Scholar] [CrossRef]
- Umair, M.; Abbasi, B.H.; Sharif, S.; Alam, M.M.; Rana, M.S.; Mujtaba, G.; Arshad, Y.; Fatmi, M.Q.; Zaidi, S.Z. High prevalence of G3 rotavirus in hospitalized children in Rawalpindi, Pakistan during 2014. PLoS ONE 2018, 13, e0195947. [Google Scholar] [CrossRef]
- Halasa, N.; Piya, B.; Stewart, L.S.; Rahman, H.; Payne, D.C.; Woron, A.; Thomas, L.; Constantine-Renna, L.; Garman, K.; McHenry, R.; et al. The Changing Landscape of Pediatric Viral Enteropathogens in the Post-Rotavirus Vaccine Era. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Rovida, F.; Nepita, E.V.; Giardina, F.; Piralla, A.; Campanini, G.; Baldanti, F. Rotavirus molecular epidemiology in hospitalized patients, Northern Italy, 2015–2018. New Microbiol. 2020, 43, 1–5. [Google Scholar]
- Levy, K.; Hubbard, A.E.; Eisenberg, J.N.S. Seasonality of rotavirus disease in the tropics: A systematic review and meta-analysis. Int. J. Epidemiol. 2009, 38, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Samdan, A.; Ganbold, S.; Guntev, O.; Orosoo, S.; Javzandorj, N.; Gongor, A.; Enkhtuvshin, A.; Demberelsuren, S.; Abdul, W.; Jee, Y.; et al. Hospital-based surveillance for rotavirus diarrhea in Ulaanbaatar, Mongolia, April 2009 through March 2016. Vaccine 2018, 36, 7883–7887. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.; Bostan, N.; Bokhari, H.; Matthijnssens, J.; Yinda, K.C.; Raza, S.; Nawaz, T. Molecular characterization of human group A rotavirus genotypes circulating in Rawalpindi, Islamabad, Pakistan during 2015-2016. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Luchs, A.; Cilli, A.; Morillo, S.G.; de C.C. Carmona, R.; do C.S.T. Timenetsky, M. ROTAVIRUS GENOTYPES CIRCULATING IN BRAZIL, 2007-2012: IMPLICATIONS FOR THE VACCINE PROGRAM. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 305–313. [Google Scholar] [CrossRef]
- Huyen, D.T.T.; Hong, D.T.; Trung, N.T.; Hoa, T.T.N.; Oanh, N.K.; Thang, H.V.; Thao, N.T.T.; Hung, D.M.; Iijima, M.; Fox, K.; et al. Epidemiology of acute diarrhea caused by rotavirus in sentinel surveillance sites of Vietnam, 2012–2015. Vaccine 2018, 36, 7894–7900. [Google Scholar] [CrossRef]
- Hull, J.J.; Teel, E.N.; Kerin, T.K.; Freeman, M.M.; Esona, M.D.; Gentsch, J.R.; Cortese, M.M.; Parashar, U.D.; Glass, R.I.; Bowen, M.D.; et al. United States rotavirus strain surveillance from 2005 to 2008: Genotype prevalence before and after vaccine introduction. Pediatr. Infect. Dis. J. 2011, 30, S42–S47. [Google Scholar] [CrossRef]
- Desai, R.; Parashar, U.D.; Lopman, B.; de Oliveira, L.H.; Clark, A.D.; Sanderson, C.F.B.; Tate, J.E.; Matus, C.R.; Andrus, J.K.; Patel, M.M. Potential intussusception risk versus health benefits from rotavirus vaccination in Latin America. Clin. Infect. Dis. 2012, 54, 1397–1405. [Google Scholar] [CrossRef]
- Clarke, E.; Desselberger, U. Correlates of protection against human rotavirus disease and the factors influencing protection in low-income settings. Mucosal. Immunol. 2015, 8, 1–17. [Google Scholar] [CrossRef]
- Kang, G.; Iturriza-Gomara, M.; Wheeler, J.G.; Crystal, P.; Monica, B.; Ramani, S.; Primrose, B.; Moses, P.D.; Gallimore, C.I.; Brown, D.W.; et al. Quantitation of Group A Rotavirus by Real-Time Reverse-Transcription-Polymerase Chain Reaction. J. Med. Virol. 2004, 73, 118–122. [Google Scholar] [CrossRef]
- da Silva, M.F.M.; Fumian, T.M.; de Assis, R.M.S.; Fialho, A.M.; Carvalho-Costa, F.A.; da Silva Ribeiro de Andrade, J.; Leite, J.P.G. VP7 and VP8* genetic characterization of group A rotavirus genotype G12P[8]: Emergence and spreading in the Eastern Brazilian coast in 2014. J. Med. Virol. 2017, 89, 64–70. [Google Scholar] [CrossRef]
- Kirkwood, C.D.; Roczo-Farkas, S.; Australian Rotavirus Surveillance Group. Australian Rotavirus Surveillance Program annual report, 2013. Commun. Dis. Intell. Q Rep. 2014, 38, E334–E342. [Google Scholar]
- Arana, A.; Montes, M.; Jere, K.C.; Alkorta, M.; Iturriza-Gómara, M.; Cilla, G. Emergence and spread of G3P[8] rotaviruses possessing an equine-like VP7 and a DS-1-like genetic backbone in the Basque Country (North of Spain), 2015. Infect. Genet. Evol. 2016, 44, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Dóró, R.; Marton, S.; Bartókné, A.H.; Lengyel, G.; Agócs, Z.; Jakab, F.; Bányai, K. Equine-like G3 rotavirus in Hungary, 2015–Is it a novel intergenogroup reassortant pandemic strain? Acta. Microbiol. Immunol. Hung. 2016, 63, 243–255. [Google Scholar] [CrossRef]
- Perkins, C.; Mijatovic-Rustempasic, S.; Ward, M.L.; Cortese, M.M.; Bowen, M.D. Genomic Characterization of the First Equine-Like G3P[8] Rotavirus Strain Detected in the United States. Genome. Announc. 2017, 5. [Google Scholar] [CrossRef]
- Kikuchi, W.; Nakagomi, T.; Gauchan, P.; Agbemabiese, C.A.; Noguchi, A.; Nakagomi, O.; Takahashi, T. Detection in Japan of an equine-like G3P[8] reassortant rotavirus A strain that is highly homologous to European strains across all genome segments. Arch. Virol. 2018, 163, 791–794. [Google Scholar] [CrossRef]
- Komoto, S.; Ide, T.; Negoro, M.; Tanaka, T.; Asada, K.; Umemoto, M.; Kuroki, H.; Ito, H.; Tanaka, S.; Ito, M.; et al. Characterization of unusual DS-1-like G3P[8] rotavirus strains in children with diarrhea in Japan. J. Med. Virol. 2018, 90, 890–898. [Google Scholar] [CrossRef]
- Pietsch, C.; Liebert, U.G. Molecular characterization of different equine-like G3 rotavirus strains from Germany. Infect. Genet. Evol. 2018, 57, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.F.S.; Soares, L.S.; Lobo, P.S.; Penha Júnior, E.T.; Sousa Júnior, E.C.; Bezerra, D.A.M.; Vaz, L.R.; Linhares, A.C.; Mascarenhas, J.D.P. Detection of a novel equine-like G3 rotavirus associated with acute gastroenteritis in Brazil. J. Gen. Virol. 2016, 97, 3131–3138. [Google Scholar] [CrossRef]
- Maguire, J.E.; Glasgow, K.; Glass, K.; Roczo-Farkas, S.; Bines, J.E.; Sheppeard, V.; Macartney, K.; Quinn, H.E. Rotavirus Epidemiology and Monovalent Rotavirus Vaccine Effectiveness in Australia: 2010–2017. Pediatrics 2019, 144. [Google Scholar] [CrossRef]
- Ianiro, G.; Delogu, R.; Camilloni, B.; Lorini, C.; Ruggeri, F.M.; Fiore, L. Detection of unusual G6 rotavirus strains in Italian children with diarrhoea during the 2011 surveillance season. J. Med. Virol. 2013, 85, 1860–1869. [Google Scholar] [CrossRef]
- Mladenova, Z.; Nawaz, S.; Ganesh, B.; Iturriza-Gomara, M. Increased detection of G3P[9] and G6P[9] rotavirus strains in hospitalized children with acute diarrhea in Bulgaria. Infect. Genet. Evol. 2015, 29, 118–126. [Google Scholar] [CrossRef]
- Ansari, S.; Sherchand, J.B.; Rijal, B.P.; Parajuli, K.; Mishra, S.K.; Dahal, R.K.; Shrestha, S.; Tandukar, S.; Chaudhary, R.; Kattel, H.P.; et al. Characterization of rotavirus causing acute diarrhoea in children in Kathmandu, Nepal, showing the dominance of serotype G12. J. Med. Microbiol. 2013, 62, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Sherchand, J.B.; Pokhrel, B.M.; Parajuli, K.; Shah, N.; Mishra, S.K.; Sharma, S.; Kattel, H.P.; Khadka, S.; Khatiwada, S.; et al. Molecular epidemiology of Rotavirus causing diarrhea among children less than five years of age visiting national level children hospitals, Nepal. BMC Pediatr. 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.S.; Gurgel, R.Q.; Cavalcante, S.M.M.; Kirby, A.; Café, L.P.; Souto, M.J.; Dolabella, S.S.; de Assis, M.R.; Fumian, T.M.; Miagostovich, M.P.; et al. Acute norovirus gastroenteritis in children in a highly rotavirus-vaccinated population in Northeast Brazil. J. Clin. Virol. 2017, 88, 33–38. [Google Scholar] [CrossRef]




| Region/State | No. of Fecal Samples: Positive/Tested (%) | p-Value (Chi-Square Test) | ||
|---|---|---|---|---|
| Total | 2018 | 2019 | ||
| Southeast | 14/381 (3.7) | 2/168 (1.2) | 12/213 (5.6) | 0.022 |
| Espírito Santo | 1/56 | 2/101 | ||
| Minas Gerais | 1/75 | - | ||
| Rio de Janeiro | - | 10/79 | ||
| Northeast | 81/434 (18.7) | 44/252 (17.5) | 37/182 (20.3) | 0.452 |
| Bahia | 1/98 | 2/95 | ||
| Maranhão | 1/8 | 1/1 | ||
| Paraíba | 20/37 | - | ||
| Pernambuco | 19/68 | 30/61 | ||
| Rio Grande do Norte | - | 1/5 | ||
| Sergipe | 3/41 | 3/20 | ||
| South | 90/720 (12.5) | 39/381 (10.2) | 51/340 (15) | 0.053 |
| Rio Grande do Sul | 16/168 | 38/181 | ||
| Santa Catarina | 23/213 | 13/159 | ||
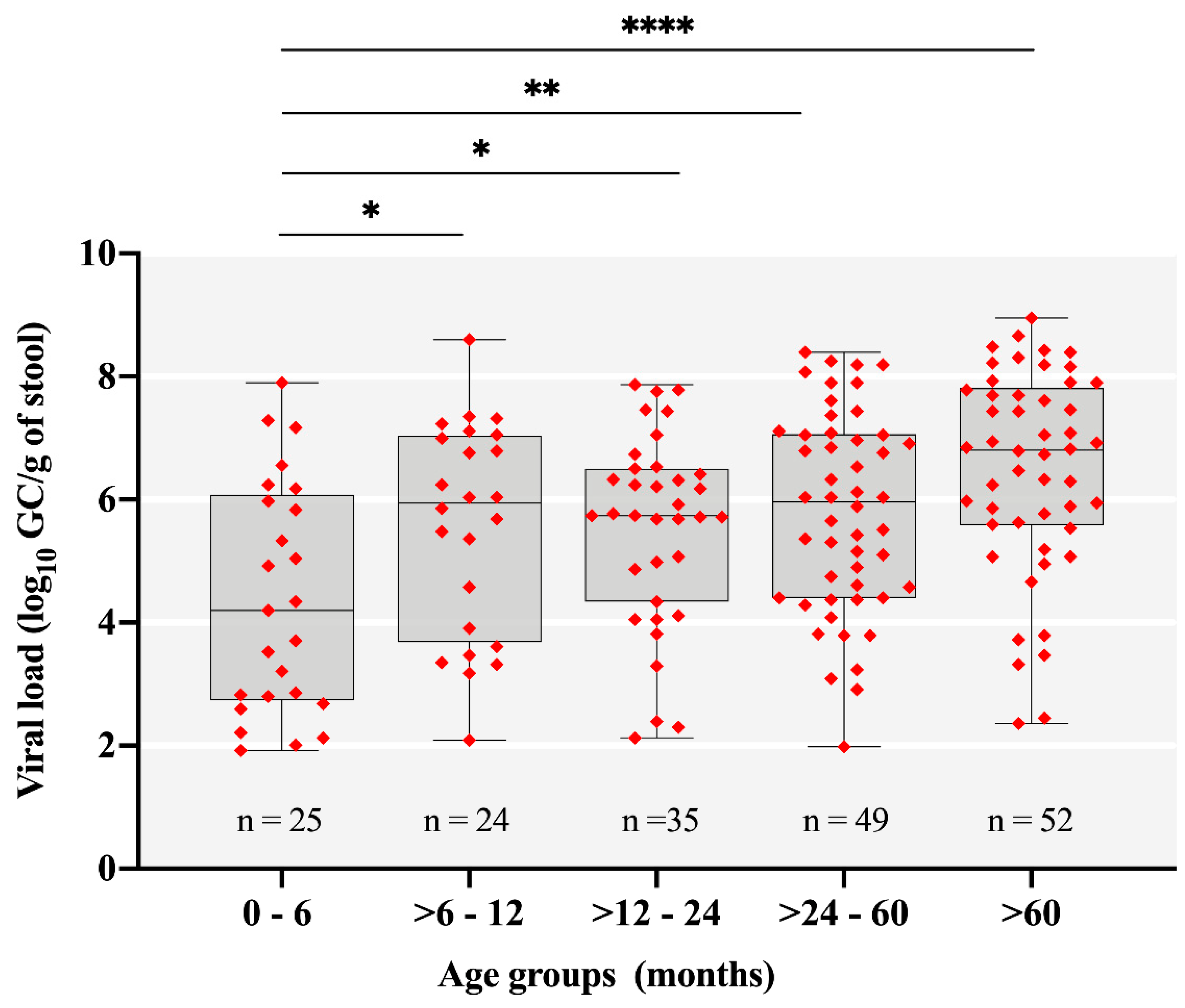
| Age Group (Months) | No. of Fecal Samples: Positive/Tested (%) | p-Value * (Chi-Square Test) | ||
|---|---|---|---|---|
| 2018 | 2019 | Total | ||
| 0–6 | 16/122 (13.1) | 9/101 (8.9) | 25/223 (11.2) | 0.0153 |
| >6–12 | 10/133 (7.5) | 14/116 (12) | 24/249 (9.6) | 0.0021 |
| >12–24 | 17/203 (8.3) | 18/173 (10.4) | 35/376 (9.3) | 0.0003 |
| >24–60 | 26/141 (18.4) | 23/119 (19.3) | 49/260 (18.8) | - |
| >60 | 16/202 (7.9) | 36/227 (15.8) | 52/428 (12.1) | 0.0109 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez, M.B.; Fialho, A.M.; Maranhão, A.G.; Malta, F.C.; Andrade, J.d.S.R.d.; Assis, R.M.S.d.; Mouta, S.d.S.e.; Miagostovich, M.P.; Leite, J.P.G.; Machado Fumian, T. Rotavirus A in Brazil: Molecular Epidemiology and Surveillance during 2018–2019. Pathogens 2020, 9, 515. https://doi.org/10.3390/pathogens9070515
Gutierrez MB, Fialho AM, Maranhão AG, Malta FC, Andrade JdSRd, Assis RMSd, Mouta SdSe, Miagostovich MP, Leite JPG, Machado Fumian T. Rotavirus A in Brazil: Molecular Epidemiology and Surveillance during 2018–2019. Pathogens. 2020; 9(7):515. https://doi.org/10.3390/pathogens9070515
Chicago/Turabian StyleGutierrez, Meylin Bautista, Alexandre Madi Fialho, Adriana Gonçalves Maranhão, Fábio Correia Malta, Juliana da Silva Ribeiro de Andrade, Rosane Maria Santos de Assis, Sérgio da Silva e Mouta, Marize Pereira Miagostovich, José Paulo Gagliardi Leite, and Tulio Machado Fumian. 2020. "Rotavirus A in Brazil: Molecular Epidemiology and Surveillance during 2018–2019" Pathogens 9, no. 7: 515. https://doi.org/10.3390/pathogens9070515
APA StyleGutierrez, M. B., Fialho, A. M., Maranhão, A. G., Malta, F. C., Andrade, J. d. S. R. d., Assis, R. M. S. d., Mouta, S. d. S. e., Miagostovich, M. P., Leite, J. P. G., & Machado Fumian, T. (2020). Rotavirus A in Brazil: Molecular Epidemiology and Surveillance during 2018–2019. Pathogens, 9(7), 515. https://doi.org/10.3390/pathogens9070515





