Abstract
Non-human primates (NHPs) have been shown to be infected by parasites of the genus Plasmodium, the etiological agent of malaria in humans, creating potential risks of zoonotic transmission. Plasmodium brasilianum, a parasite species similar to P. malariae of humans, have been described in NHPs from Central and South America, including Brazil. The merozoite surface protein 1 (MSP1), besides being a malaria vaccine candidate, is highly immunogenic. Due to such properties, we tested this protein for the diagnosis of parasite infection. We used recombinant proteins of P. malariae MSP1, as well as of P. falciparum and P. vivax, for the detection of antibodies anti-MSP1 of these parasite species, in the sera of NHPs collected in different regions of Brazil. About 40% of the NHP sera were confirmed as reactive to the proteins of one or more parasite species. A relatively higher number of reactive sera was found in animals from the Atlantic Forest than those from the Amazon region, possibly reflecting the former more intense parasite circulation among NHPs due to their proximity to humans at a higher populational density. The presence of Plasmodium positive NHPs in the surveyed areas, being therefore potential parasite reservoirs, needs to be considered in any malaria surveillance program.
1. Introduction
The merozoite surface protein 1 (MSP1) is the most abundant protein on the malaria parasite cell surface. It is involved in the erythrocyte invasion process, being one of the most studied targets for a malaria vaccine [1]. MSP1 is synthesized from the onset of schizogony [2] and transported to the surface of the parasite cell, together with the proteins MSP6 and MSP7 as a complex, where it is retained through its glycosyl phosphatidylinositol (GPI) anchor. The egress of merozoites from erythrocytes is accompanied by a primary proteolytic processing of MSP1 that results in a protein complex on the surface of the merozoite cells (reviewed in [1]).
The gene of Plasmodium malariae MSP1 (pmmsp1) encodes a protein of 1751 amino acids, including a 19 amino acid signal peptide [3]. Recently, we analyzed a portion of this gene in P. malariae, and in its non-human primate (NHP) equivalent, P. brasilianum, isolated from different geographic regions of Brazil [4]. Analyses of the pmmsp1 gene was performed through the amplification and sequencing of five fragments, F1 to F5 (F5 also named PmMSP119), equivalent to blocks 3 to 17 of the PfMSP1 (P. falciparum MSP1) and covering 60% of the gene [4]. Except for the fragment F4, all others showed differences to a previously published sequence [4], the PmMSP1 allele MM1A of a parasite isolated from a patient in Cameroon [3]. The most polymorphic region was in the sequence of fragment F2, previously described to include imperfect repeats [3].
The availability of recombinant proteins from P. falciparum and P. vivax has allowed for numerous studies revealing whether individuals have been naturally exposed to malaria parasites, by detecting antibodies to the protein in their sera (reviewed in [5]). For the purposes of studying such exposure in NHPs, we produced recombinant glutathione S-transferase (GST)-fusion proteins out of the characterized P. malariae msp1 gene fragments, named PmMSP1F1, PmMSP1F2, PmMSP1F3, PmMSP1F4 and PmMSP119. The immunization of BALB/c mice with these recombinant proteins elicited a significant humoral immune response, making them potential component candidates for a vaccine against P. malariae. These recombinant proteins were also shown to be very useful as diagnostic markers in epidemiological studies and for the differential diagnosis of P. malariae infection [6].
In this work, we evaluated the diagnostic capability of the P. malariae MSP1 recombinant proteins, together with the MSP119 of P. falciparum (PfMSP119) and of P. vivax (PvMSP119), for the detection of anti-MSP1 in the sera of NHPs from malaria endemic regions of Brazil, the Amazon and Atlantic forests, and from a non-endemic region, the Cerrado, in Central Brazil. Using these recombinant proteins, we also aimed to find the prevalence of antibodies against these parasite species in the sera of NHPs and, thus, the potentiality of these animals as malaria reservoirs.
2. Materials and Methods
2.1. Sera Sampling from Non-Human Primates
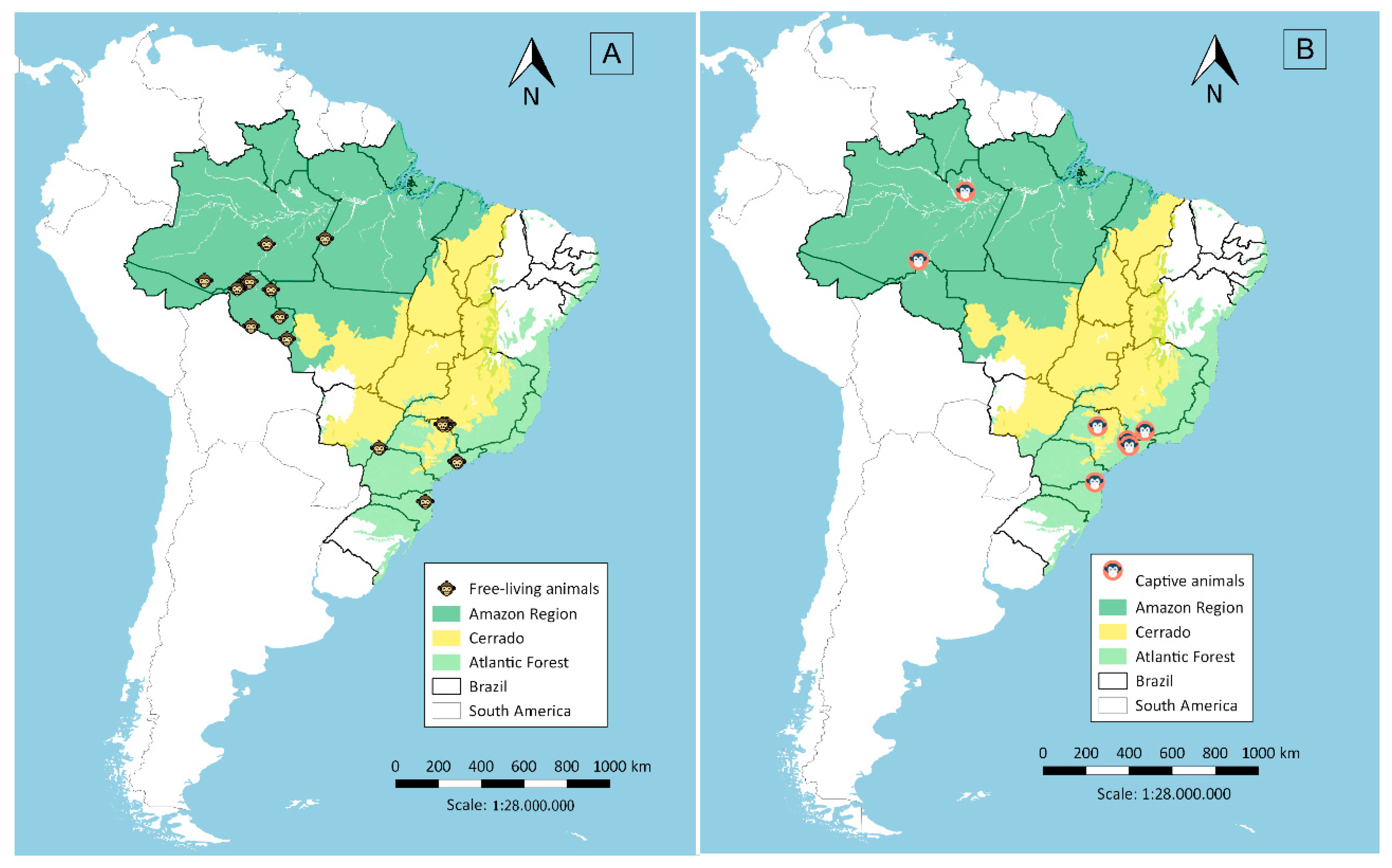
Serum samples were collected in two malaria endemic regions in Brazil, the Amazon and Atlantic Forest regions, and in a non-endemic region, the Cerrado region in Central Brazil. A total of 373 samples were collected from free-living animals and 122 from captive animals. Sera from free-living animals (Figure 1A) were obtained in different localities: (i) in the Amazon Region (n = 155), collected close to Porto Velho city in Rondônia state, from March 2009 to November 2012, during projects related to environmental management for the construction of hydroelectric power plants [7,8]; (ii) in the Atlantic Forest (n = 111), from October 1997 to July 2005, in forest fragments around the municipality of São Paulo [9] and the municipality of Indaial in Santa Catarina state, from June 2001 to February 2015; and (iii) in the Cerrado region (n = 107), from April 2000 to March 2001 and January to December 2009, in the canopy of woods in flooded areas of the lake at Porto Primavera dam during wildlife rescue operations [10]. Sera from captive animals (Figure 1B) were from the Atlantic Forest area (n = 103), 60 being from the São Paulo city Zoo, 31 from the Tietê Ecological Park and 12 from CETAS (Centro de Triagem de Animais Silvestres; Wildlife Rescue and Rehabilitation Center) in Lorena city (n = 08) and Unimonte in São Vicente city (n = 1), as well as from Bauru city Zoo (n = 1) and the Centre for Biological Research in Indaial city (n = 2). From the Amazon Region (n = 19), samples were collected from animals kept close to areas of human habitation, such as the Ecological Park of Porto Velho city, and animals rescued by IBAMA (Brazilian Institute of Environment and Renewable Natural Resources) which had been kept illegally as pets in rural or suburban areas.
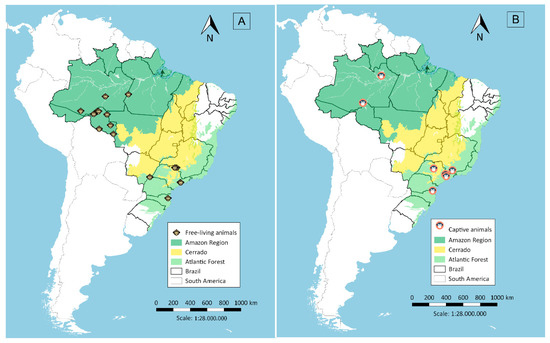
Figure 1.
Sera collection sites in the Amazon Region, Cerrado and the Atlantic Forest, Brazil, from free-living (A) and captive animals (B).
All procedures were approved by the Ethical Committee in Animal Research (CEUA) of the Institute of Tropical Medicine of São Paulo, University of Sao Paulo (number 2014/281A) and were in full compliance with federal permits issued by the Brazilian Ministry of the Environment (SISBIO numbers 14081, 17302, 18861, 24319, 44751, 47812, 50076).
2.2. Recombinant Antigens
GST and GST-recombinant fusion proteins of PmMSP1 (PmMSP1F1, PmMSP1F2, PmMSP1F3, PmMSP1F4, PmMSP119), P. vivax (PvMSP119) and P. falciparum (PfMSP119), representing the polymorphic N-terminal (MSP1F1), the central (MSP1F2, MSP1F3, MSP1F4) and the conserved C-terminal (MSP119) regions [6], were used for detecting anti-parasite antibodies in the sera samples. The GST and GST-fusion proteins were purified on glutathione-Sepharose 4B (GE Healthcare, MilliporeSigma, St. Louis, MO, USA) and the protein concentration was determined with the Bradford protein Assay (Bio-Rad, Hercules, CA, USA).
2.3. Multiplexed Serological Assay
Recombinant proteins were covalently bound to Bio-Plex Pro Magnetic COOH Beads using the BioPlex Amine Coupling Kit (BioPlex Amine Coupling Kit, Bio-Rad, Hercules, CA, USA), following the manufacturer’s instructions. Coupled beads were then used for the analyses of the NHP sera as described [11], with modifications. Briefly, 50 μL of bead suspension, corresponding to 2000 coated beads, was used with each serum sample. Serum samples were diluted 1:50 in assay buffer (PBS 1×, BSA 1%, Tween 20 0.02%) and 50 μL aliquots added to 50 μL of protein coated magnetic beads (final dilution 1:100). Aliquots of 50 μL of biotinylated monkey IgG antibody (MilliporeSigma, St. Louis, MO, USA) (diluted 1:2000) and of phycoerythrin conjugated streptavidin (2 μg/mL) (MilliporeSigma, St. Louis, MO, USA) were used in subsequent incubations. Beads were re-suspended in 125 μL of assay buffer (PBS 1×, BSA 1%, Tween 20 0.02%) and fluorescence measured with the BioPlex200 system (Bio-Rad, Hercules, CA, USA). Results were expressed as median fluorescence intensity (MFI).
Sera were tested in two replicates and positivity evaluated by the measured MFI values of antibodies binding to the recombinant proteins, minus the MFI value of the same serum to non-fusion GST. The cut-off values are presented as the geometric mean of values obtained with a panel of eight negative control sera plus 3× standard deviations. Average cut-off values are shown in Table 1.

Table 1.
Average cut-off values obtained in the multiplex bead assay of Plasmodium recombinant proteins and non-human primate sera.
2.4. Assessment of Coupling Efficiency
To determine the overall efficiency of the Plasmodium spp. GST-MSP1 fusion proteins coupling to the BioPlex carboxylated beads, multiplex assays were performed using a rabbit anti-GST polyclonal IgG antibody (Biotin) (Abcam, Cambridge, MA, USA) to detect the fusion protein on the bead. The dilution of the anti-GST antibody was 1:1000 in assay buffer (PBS 1×, BSA 1%, Tween 20 0.02%) (50 μL/well). The bound anti-GST antibody was detected with R-phycoerythrin-labelled streptavidin and fluorescence was measured on the BioPlex 200 instrument (Bio-Rad, Hercules, CA, USA), as described above.
3. Results
3.1. Testing of Coupling Efficiency of GST-Fusion Proteins to BioPlex Carboxylated Magnetic Beads
Quality control of the coupling was placed on each plate, where the beads that were covalently coated with the different recombinant proteins were tested in the same plate, with biotinylated anti-GST, to evaluate the efficiency of the coupling and verify the reading behavior of each protein. In Figure S1 (see Supplementary information), it was observed that the reading remained stable and very similar for all the proteins with minimal variation between plates, except for fragment 4 (PmMSP1F4), which was unstable and lower in all readings. PvMSP119 presented a similar fluorescence curve but slightly lower, except for Plate 1.
3.2. Antibodies Against MSP1 in the NHP Sera
A total of 495 NHP serum samples were analyzed, belonging to the two groups: (i) free living animals (n = 373); (ii) captive animals (n = 122). Of all samples analyzed, 199 (40.2%) presented IgG antibodies against at least one of the malaria recombinant proteins. Sera that were reactive to the P. malariae PmMSP1F1 were most frequent, with a total of 109 (22%), followed by those reactive to P. vivax MSP119 (19.8%). The least reactive sera were to P. falciparum PfMSP119 (1.6%) (Table 2, Tables S1 and S2).

Table 2.
Percentage of reactive sera (free-living and captive animals, n = 495) to the MSP1 recombinant proteins.
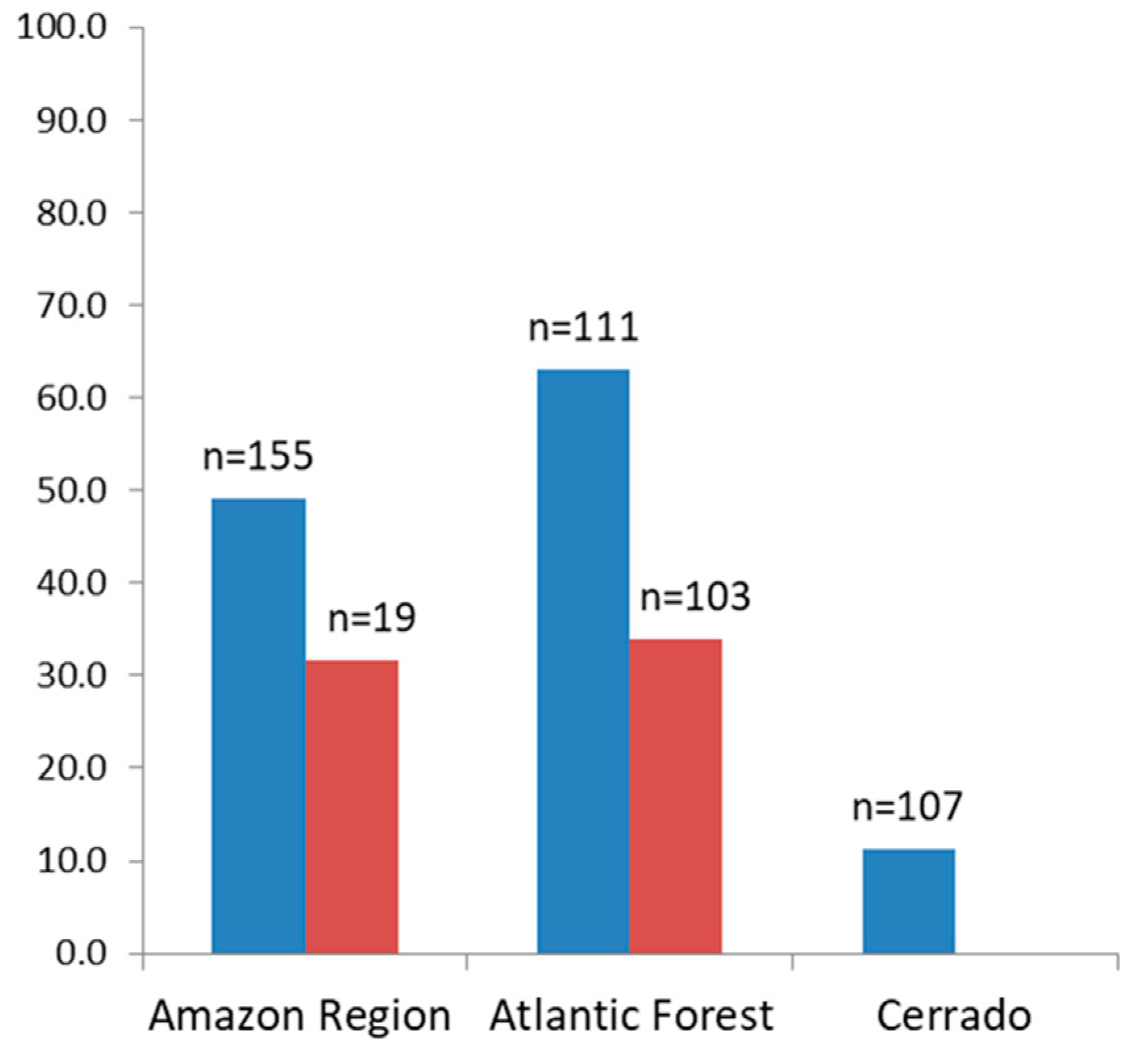
The percentage of sera of free-living animals reacting to the recombinant proteins was higher in animals from the Atlantic Forest (63.1%), compared to those from the Amazon and Cerrado regions, which were 49% and 11.2% respectively (Figure 2).
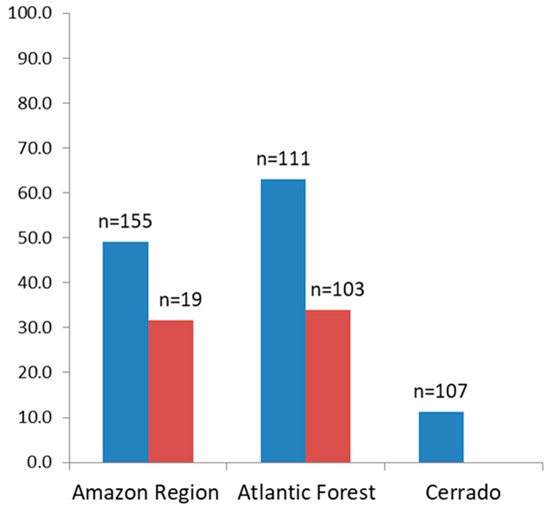
Figure 2.
Percentage of positive sera from free-living (blue) and from captive (red) non-human primates to one or more of the MSP1 recombinant proteins.
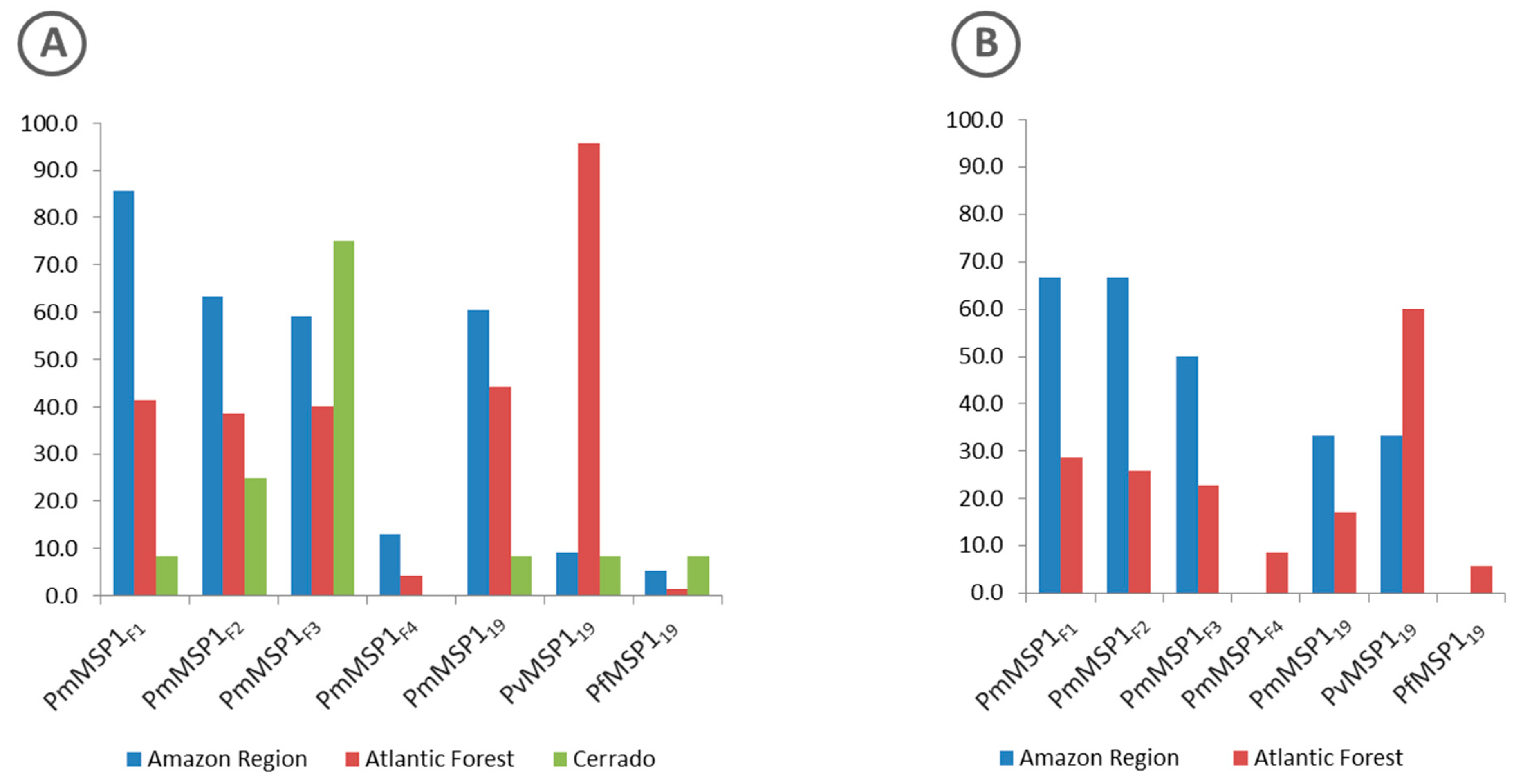
Proteins with the highest rates of reactivity to the sera of free-living animals from all regions (Amazon, Atlantic Forest and Cerrado) were PmMSP1F1, PvMSP119 and PmMSP1F3, respectively (Figure 3A).
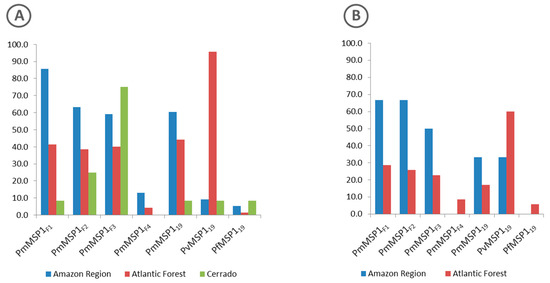
Figure 3.
Percentage of positive sera from free-living (A) and captive (B) non-human primates of the Amazon Region (blue), Atlantic Forest (red) and Cerrado (green) regions to each of the MSP1 recombinant proteins.
As for the sera of animals in captivity, the percentage of sera samples that were reactive to any of the recombinant antigens that were tested was similar for those from the Atlantic Forest (35%) and from the Amazon region (31.6%) (Figure 2). The highest rates of reactive sera from such animals from the Amazon region were to proteins PmMSP1F1 and PmMSP1F2, while animals from the Atlantic Forest were most reactive to PvMSP119 (Figure 3). Two sera of captive animals classified here as Atlantic Forest were positive to PfMSP119 (Figure 3). However, these sera were from animals brought from the Amazon region and kept at the São Paulo Zoo.
Of the 164 positive samples for any of the P. malariae recombinant proteins, 34 (20%) reacted concomitantly to four proteins (PmMSP1F1, PmMSP1F2, PmMSP1F3 and PmMSP119), while 61 (37%) sera reacted to only one of the proteins, with the lowest positivity to PmMSP1F4 (Supplementary information, Figure S2).
3.3. Malaria Exposure History
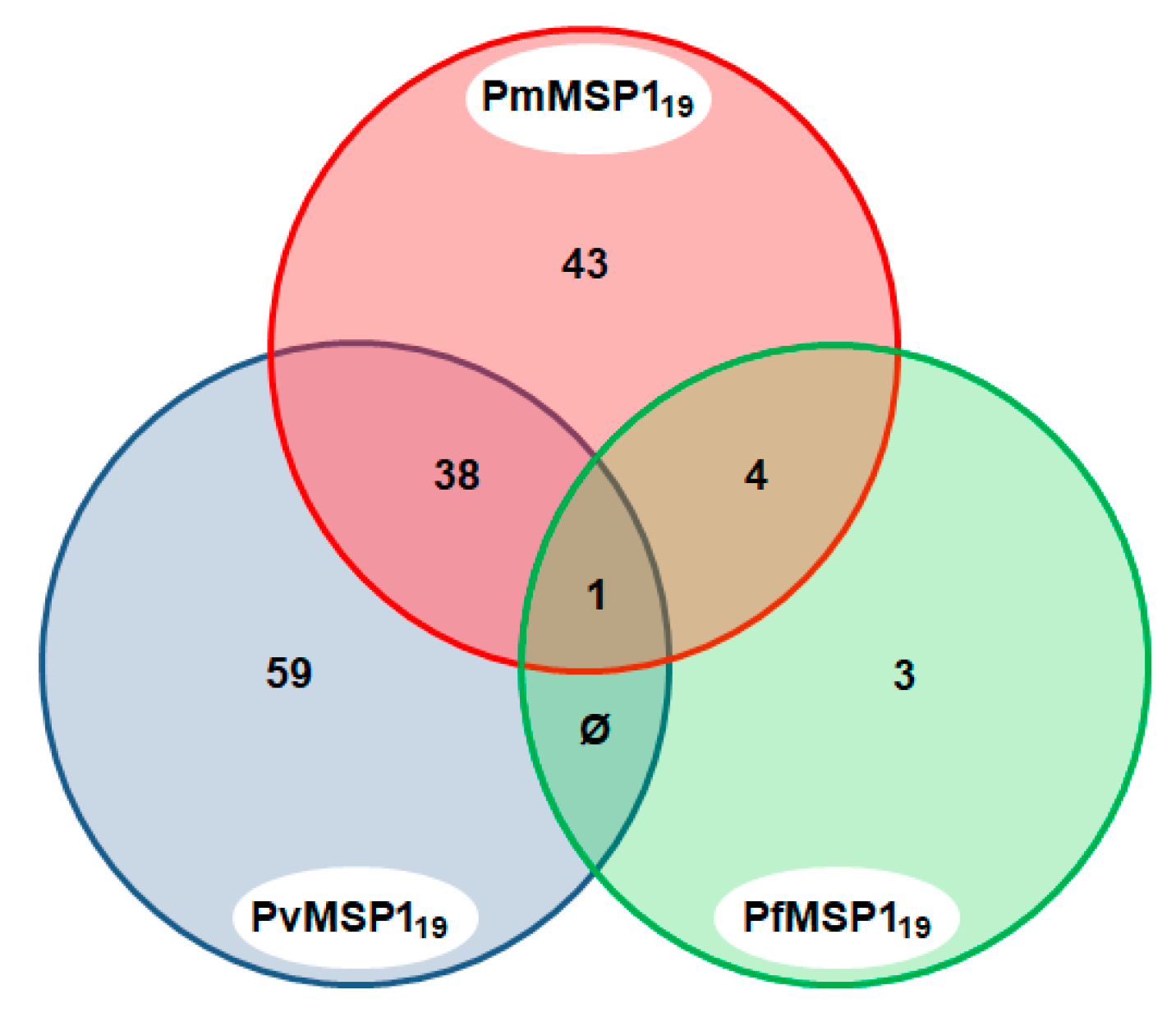
A total of 148 sera (29.9%) were found to be positive to the MSP1 C-terminal region of the different malaria parasites (PmMSP119, PvMSP119 and PfMSP119). Of these, 43 (29.1%) were positive to more than one Plasmodium species: P. falciparum and P. malariae (2.7%); P. vivax and P. malariae (25.7%); and P. falciparum, P. malariae and P. vivax (0.7%), yielding evidence of the lifetime exposure of the animals to these Plasmodium species (Figure 4).
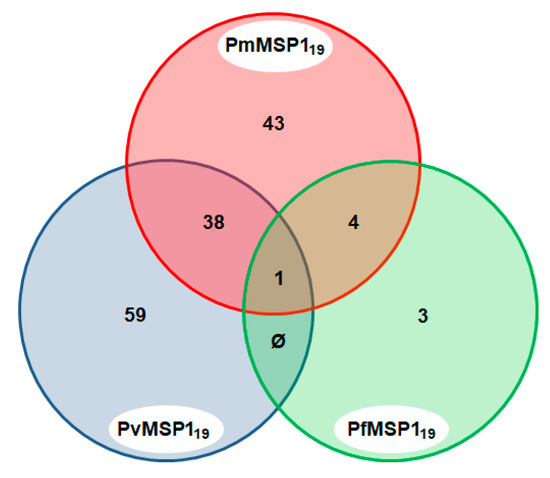
Figure 4.
Venn diagram of sera that were positive to multiple Plasmodium species MSP1. Only sera that were reactive to the MSP1 C-terminal region of the three Plasmodium species, PmMSP119, PvMSP119 and PfMSP119, are shown.
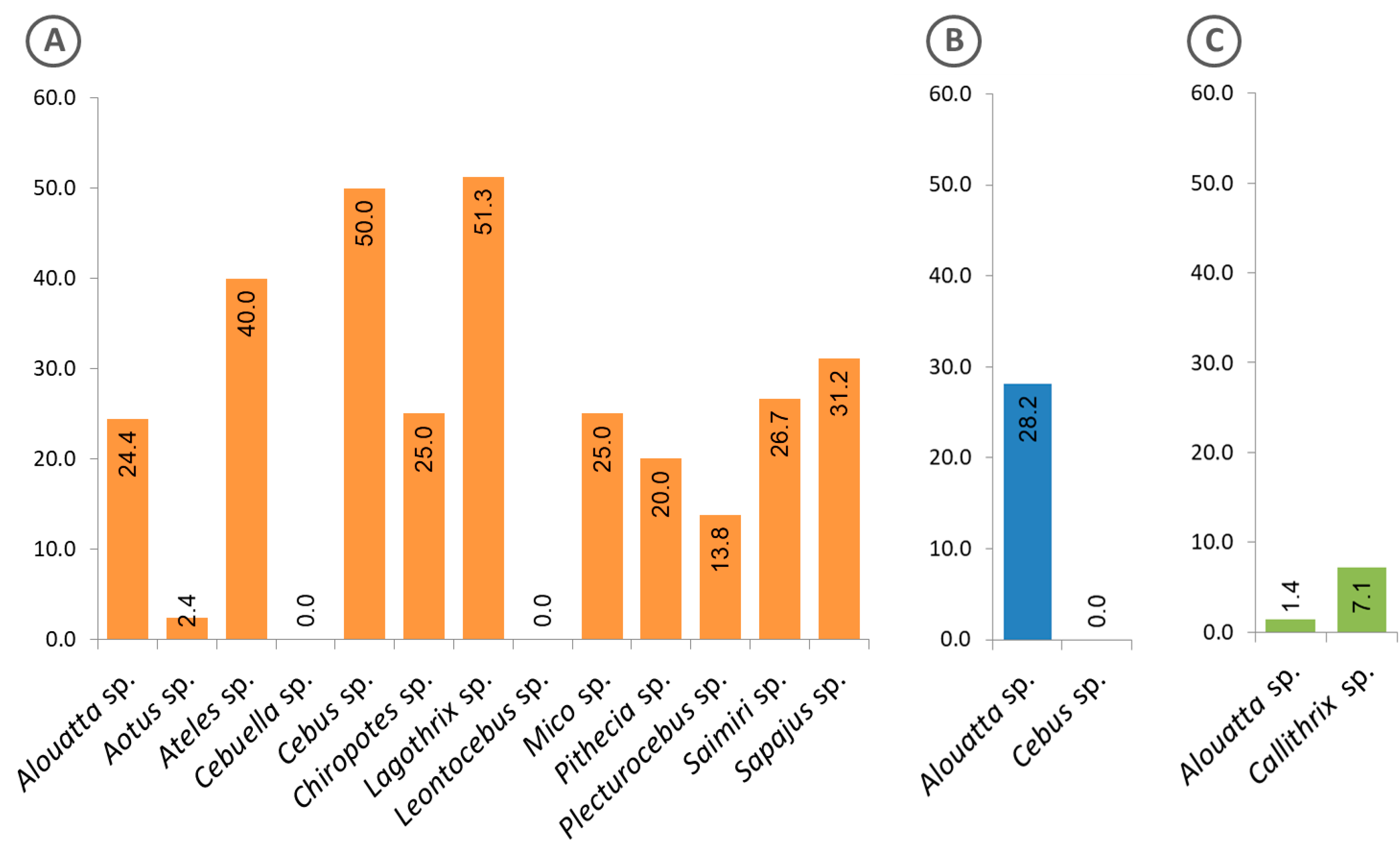
Figure 5 shows the frequency of free-living animals with positive sera, classified by genus and region. Of the 13 genera of animals from the Amazon region, 11 presented positive sera (Figure 5A), while in the Atlantic Forest, only specimens of the genus Alouatta showed such positivity (Figure 5B). Interestingly, in the Cerrado region, sera from animals of another genus (Callithrix) were found positive, in addition to those of the genus Alouatta (Figure 5C).
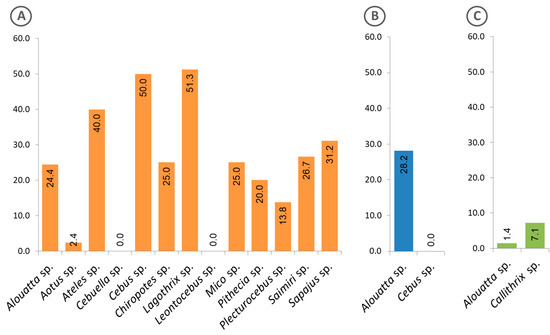
Figure 5.
Frequency of free-living animals classified at genus level, with sera that were reactive to the Plasmodium recombinant MSP1. (A) Animals from the Amazon region, (B) Atlantic Forest and (C) Cerrado. The calculated percentages of positive sera are shown inside the bars.
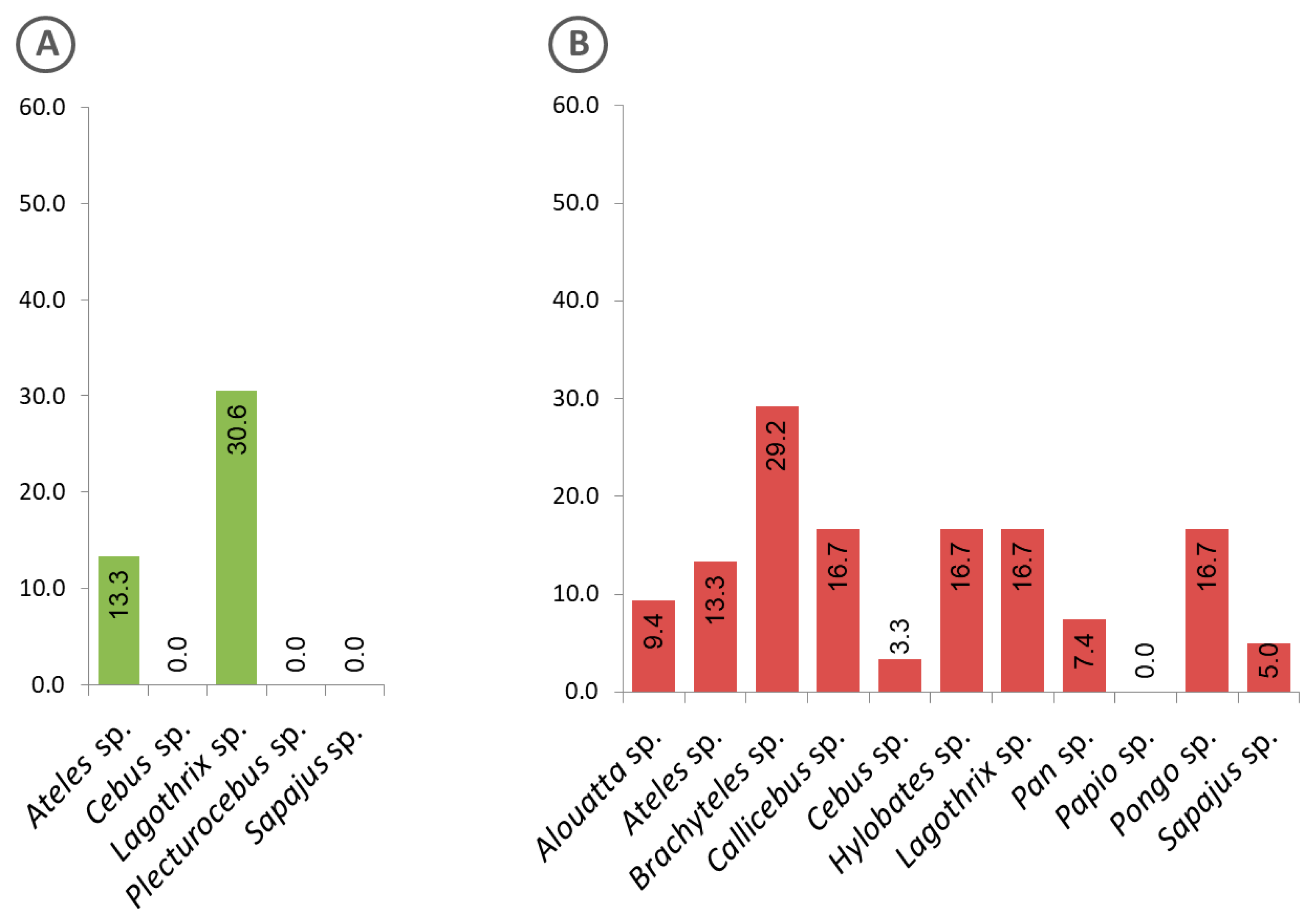
Evidence of exposure to malaria parasites by animals kept in captivity at genus level is shown in Figure 6. While animals of the genus Lagothrix from the Amazon region showed the highest positivity rate (Figure 6A), those in the Atlantic Forest were of the genus Brachyteles (Figure 6B).
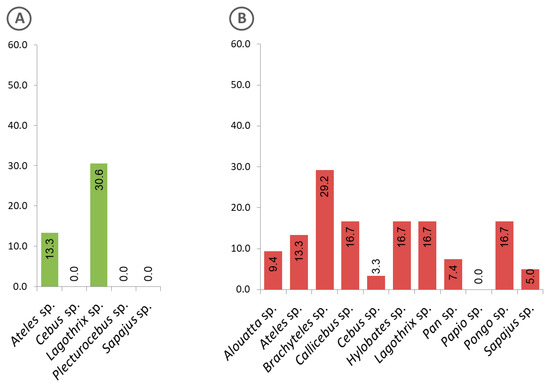
Figure 6.
Frequency of captive animals with sera that were positive to the Plasmodium recombinant MSP1, classified at genus level. (A) Animals from the Amazon region, (B) Atlantic Forest.
4. Discussion
Brazil is home to far more non-human primates than any other country; its 110 species account for about 27%, or one in every four, NHPs in the world. The Amazon region and the Atlantic Forest each house 20% of these taxa, including some endangered species [12,13].
NHPs have been shown to be infected by parasites from the genus Plasmodium, the etiological agent of malaria in humans, creating potential risks of zoonotic transmission and consequent public health concerns. Two species of simian malaria have been described in Brazil: P. brasilianum, a quartan malaria parasite genetically and morphologically similar to P. malariae, first described in bald uakari (Cacajao calvus) in the upper Amazon (Northern Brazil) [14] and P. simium, a tertian malaria parasite genetically and morphologically similar to P. vivax, described in a howler monkey (Alouatta fusca) in the state of São Paulo (southern Brazil) [15].
Recently, many studies have been performed to evaluate the presence of malaria parasites in NHPs, both in the Atlantic Forest and Amazon regions, aiming to understand their roles as reservoirs of malaria [4,7,8,16,17,18,19,20,21,22,23,24]. Some serological studies have also been carried out with the same aim but without using any P. malariae erythrocytic stage antigens [9,10,25]. In the present study, we used five recombinant antigens derived from the MSP1 protein of this parasite species. The recombinant proteins were designed as GST-fusion proteins and successfully used with human sera in a previous epidemiological study [6]. With these five recombinant proteins of P. malariae MSP1 (PmMSP1F1, PmMSP1F2, PmMSP1F3, PmMSP1F4, PmMSP119), as well as with those of P. falciparum (PfMSP119) and P. vivax (PvMSP119), we found that about 40% of the NHP sera, from different areas in Brazil, were reactive to the recombinant antigens, with PmMSP1F1 being the most frequently detected. This might reflect the potentially high immunogenicity of this region of P. malariae MSP1, like those of P. vivax and P. falciparum MSP1, as described in humans [26,27]. Reactivity to the C-terminal portion of PmMSP1 (PmMSP119) was also high, as expected from studies on the identical regions of P. vivax and P. falciparum MSP1 in humans [28]. Sera with antibodies to other regions of P. malariae MSP1 were also found, with the lowest frequency to PmMSP1F4, which might be due to its low binding capacity to the beads, as observed in the coupling efficiency assay.
Serological estimates correlate well with parasitological and entomological measurements in assessing transmission intensity and spatial and demographic risks to malaria [29,30]. The number of positive sera of animals from the Atlantic Forest was higher than that in the Amazon region, showing a likely higher Plasmodium circulation among NHPs from this area. This might also reflect the proximity of NHPs to humans at a higher populational density in the Atlantic Forest. The number of positive NHP sera from Cerrado was low, as expected, due to the low level of malaria incidence in Central Brazil. Another expected finding was the higher prevalence of anti- P. vivax and P. malariae antibodies in the sera of NHPs from the Atlantic Forest, as it has been demonstrated that P. simium, the equivalent of P. vivax, and P. brasilianum, the equivalent of P. malariae, are prevalent in this area, with the former being the most prevalent [18,19,31].
It is important to note here the higher diversity of NHPs from the Amazon region that was found to carry the anti-MSP1 antibodies, as compared to the diversity of NHPs from the Atlantic Forest, where only animals of the genus Alouatta have been found with such antibodies. This singularity has been observed since the description of the first Plasmodium infected animal [9,15,18,32,33]. These results might reflect the different species of mosquito vector present in the animals’ environments in each region, which in turn might reflect the different ecological niches of each component of the malaria system, the humans, the NHPs, the mosquito vectors and the parasites. More research is necessary for assessing all these relationships.
The seropositive NHPs indicate previous infections as well as the potential presence of infected animals in the surveyed areas, which can play a role in the maintenance of human malarias, making it more difficult to eliminate or even control the disease. Moreover, the illegal wildlife trade, the allocation of species for captivity or the effort to conserve them (translocation or reintroduction), promotes the movement of animals and may favor the transmission of disease [34,35]. Thus, it is necessary to carry out a rapid, inexpensive and effective test for Plasmodium diagnosis in NHPs in order to contribute to One Health surveillance. We have demonstrated that the MSP1 recombinant proteins used in this study are useful and important candidates to be included in diagnostic tools for the surveillance, and, ultimately, for the control or elimination, of malaria.
5. Conclusions
This study validates the use of recombinant proteins in multiplex immunoassays to detect IgG antibodies against the MSP1 protein of malaria parasites and demonstrates that this technique is an important tool for making serum-epidemiological surveys of malaria. Lastly, the high prevalence of NHPs with antibodies against P. vivax and P. malariae (as well as P. falciparum in the Amazon region) supports the hypothesis that these animals are potential reservoirs of malaria parasites and that NHPs must be considered in any measure for the control or elimination of the disease.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/7/525/s1, Figure S1. Coupling efficiency of recombinant proteins to magnetic beads. Coupling efficiency of recombinant proteins to magnetic beads was assessed by using biotinylated rabbit anti-GST polyclonal IgG antibody (Abcam, Cambridge, MA, USA), followed by incubation with R-phycoerythrin-labelled streptavidin and fluorescence measurement with the BioPlex 200 instrument (Bio-Rad, Hercules, CA, USA), as described in Materials and Methods. Figure S2. Intersection chart of positive samples against Plasmodium malariae antigens. Table S1. Absolute and relative frequencies of positive free-living non-human primates by Bioplex assay per MSP1 protein. Table S2. Absolute and relative frequencies of positive captive non-human primates by Bioplex assay per MSP1 protein.
Author Contributions
Conceptualization, E.F.M., C.F.-B. and K.K.; Data curation, E.F.M.; Formal analysis, E.F.M. and M.M.H.; Funding acquisition, K.K.; Investigation, E.F.M. and B.d.S.M.; Methodology, E.F.M and C.F.-B.; Project administration, K.K.; Resources, C.F.-B., M.d.S.A., M.S.M., L.S.O., A.M.R.d.C.D., M.G.B., J.L.C.-D., C.R.F.C., M.G.d.S., S.V.S., M.M.H., J.C.d.S.Jr. and K.K.; Supervision, K.K.; Validation, E.F.M. and C.F.-B.; Visualization, E.F.M. and K.K.; Writing—original draft, E.F.M., L.S.O. and K.K.; Writing—review & editing, E.F.M., C.F.-B., M.d.S.A., M.R.M., L.S.O., A.M.R.d.C.D., M.G.B., J.L.C.-D., C.R.F.C., M.G.d.S., S.V.S., M.M.H., J.C.d.S.Jr. and K.K. All authors have read and agree to the published version of the manuscript.
Funding
K.Kirchgatter and J.L.Catão-Dias are recipients of fellowships by the National Research Council (Conselho Nacional de Desenvolvimento Científico e Tecnológico) (CNPq; grants 308678/2018-4 and 304999/2018, respectively). S.V.Santos was supported by a postdoctoral fellowship (PNPD scholarship) from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors would also like to thank São Paulo Research Foundation (FAPESP) for financial support (Grants 2014/10919-4 and 2016/04559-0).
Acknowledgments
The authors would like to thank Meire Ioshie Hiyane and Niels Olsen Saraiva Camara from Department of Immunology, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil, and Departamento de Parques e Áreas Verdes (DEPAVE), Prefeitura Municipal de São Paulo, São Paulo, Brazil. The authors would also like to thank São Paulo Research Foundation (FAPESP) for financial support (Grants 2014/10919-4 and 2016/04559-0).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Holder, A.A. The carboxy-terminus of merozoite surface protein 1: Structure, specific antibodies and immunity to malaria. Parasitology 2009, 136, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Holder, A.A.; Freeman, R.R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J. Exp. Med. 1982, 156, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Birkenmeyer, L.; Muerhoff, A.S.; Dawson, G.J.; Desai, S.M. Isolation and characterization of the MSP1 genes from Plasmodium malariae and Plasmodium ovale. Am. J. Trop. Med. Hyg. 2010, 82, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.O.; Wunderlich, G.; Alves, J.M.; Bueno, M.G.; Röhe, F.; Catão-Dias, J.L.; Neves, A.; Malafronte, R.S.; Curado, I.; Domingues, W.; et al. Merozoite surface protein-1 genetic diversity in Plasmodium malariae and Plasmodium brasilianum from Brazil. BMC Infect. Dis. 2015, 15, 529. [Google Scholar] [CrossRef] [PubMed]
- Healer, J.; Chiu, C.Y.; Hansen, D.S. Mechanisms of naturally acquired immunity to P. falciparum and approaches to identify merozoite antigen targets. Parasitology 2018, 145, 839–847. [Google Scholar] [CrossRef]
- Elizardez, Y.B.; Fotoran, W.L.; Junior, A.J.G.; Curado, I.; Junior, N.K.; Monteiro, E.F.; Romero Neto, I.; Wunderlich, G.; Kirchgatter, K. Recombinant proteins of Plasmodium malariae merozoite surface protein 1 (PmMSP1): Testing immunogenicity in the BALB/c model and potential use as diagnostic tool. PLoS ONE 2019, 14, e0219629. [Google Scholar] [CrossRef]
- Araújo, M.S.; Messias, M.R.; Figueiró, M.R.; Gil, L.H.; Probst, C.M.; Vidal, N.M.; Katsuragawa, T.H.; Krieger, M.A.; Silva, L.H.; Ozaki, L.S. Natural Plasmodium infection in monkeys in the state of Rondônia (Brazilian Western Amazon). Malar. J. 2013, 12, 180. [Google Scholar] [CrossRef]
- Bueno, M.G.; Rohe, F.; Kirchgatter, K.; Di Santi, S.M.; Guimarães, L.O.; Witte, C.L.; Costa-Nascimento, M.J.; Toniolo, C.R.; Catão-Dias, J.L. Survey of Plasmodium spp. in free-ranging neotropical primates from the Brazilian Amazon region impacted by anthropogenic actions. Ecohealth 2013, 10, 48–53. [Google Scholar] [CrossRef]
- Duarte, A.M.; Malafronte, R.; Cerutti, C., Jr.; Curado, I.; de Paiva, B.R.; Maeda, A.Y.; Yamasaki, T.; Summa, M.E.; Neves, D.; de Oliveira, S.G.; et al. Natural Plasmodium infections in Brazilian wild monkeys: Reservoirs for human infections? Acta Trop. 2008, 107, 179–185. [Google Scholar] [CrossRef]
- Duarte, A.M.; Porto, M.A.; Curado, I.; Malafronte, R.S.; Hoffmann, E.H.; de Oliveira, S.G.; da Silva, A.M.; Kloetzel, J.K.; Gomes, A. Widespread occurrence of antibodies against circumsporozoite protein and against blood forms of Plasmodium vivax, P. falciparum and P. malariae in Brazilian wild monkeys. J. Med. Primatol. 2006, 35, 87–96. [Google Scholar] [CrossRef]
- Fernandez-Becerra, C.; Sanz, S.; Brucet, M.; Stanisic, D.I.; Alves, F.P.; Camargo, E.P.; Alonso, P.L.; Mueller, I.; del Portillo, H.A. Naturally-acquired humoral immune responses against the N- and C-termini of the Plasmodium vivax MSP1 protein in endemic regions of Brazil and Papua New Guinea using a multiplex assay. Malar. J. 2010, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, R.A. Primate diversity and the tropical forest: Case studies from Brazil and Madagascar and the importance of the megadiversity countries. In Biodiversity; Wilson, E.O., Peter, F.M., Eds.; National Academy Press: Washington, DC, USA, 1988; pp. 145–154. [Google Scholar]
- Mittermeier, R.A.; Coimbra-Filho, A.F.; Kierulff, M.C.M.; Rylands, A.B.; Mendes, S.L.; Pissinatti, A.; Almeida, L.M. Monkeys of the Atlantic Forest of Eastern Brazil: Pocket Identification Guide; Conservation International Tropical Pocket Guide Series #3; Conservation International: Arlington, VA, USA, 2007. [Google Scholar]
- Gonder, R.; Von Berenberg-Gossler, H. Untersuchungen über malaria-plasmodien der affen. Malaria Intern. Archiv. (Leipzig). 1908, 1, 47–50. [Google Scholar]
- Fonseca, F. Plasmódio de primatas do Brasil. Mem. Inst. Oswaldo Cruz. 1951, 49, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.O.; Bajay, M.M.; Wunderlich, G.; Bueno, M.G.; Röhe, F.; Catão-Dias, J.L.; Neves, A.; Malafronte, R.S.; Curado, I.; Kirchgatter, K. The genetic diversity of Plasmodium malariae and Plasmodium brasilianum from human, simian and mosquito hosts in Brazil. Acta Trop. 2012, 124, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.G.; Catão-dias, J.L.; Laroque, P.O.; Vasconcellos, S.A.; Ferreira Neto, J.S.; Gennari, S.M.; Ferreira, F.; Laurenti, M.D.; Umezawa, E.S.; Kesper, N.; et al. Infectious Diseases In Free-ranging Blonde Capuchins, Sapajus Flavius, In Brazil. Int. J. Primatol. 2017, 38, 1017–1031. [Google Scholar] [CrossRef]
- Costa, D.C.; da Cunha, V.P.; de Assis, G.M.; de Souza Junior, J.C.; Hirano, Z.M.; de Arruda, M.E.; Kano, F.S.; Carvalho, L.H.; de Brito, C.F. Plasmodium simium/Plasmodium vivax infections in southern brown howler monkeys from the Atlantic Forest. Mem. Inst. Oswaldo Cruz 2014, 109, 641–653. [Google Scholar] [CrossRef]
- Alvarenga, D.A.; de Pina-Costa, A.; de Sousa, T.N.; Pissinatti, A.; Zalis, M.G.; Suaréz-Mutis, M.C.; Lourenço-de-Oliveira, R.; Brasil, P.; Daniel-Ribeiro, C.T.; de Brito, C.F. Simian malaria in the Brazilian Atlantic forest: First description of natural infection of capuchin monkeys (Cebinae subfamily) by Plasmodium simium. Malar. J. 2015, 14, 81. [Google Scholar] [CrossRef]
- Alvarenga, D.A.; Pina-Costa, A.; Bianco, C., Jr.; Moreira, S.B.; Brasil, P.; Pissinatti, A.; Daniel-Ribeiro, C.T.; Brito, C.F. New potential Plasmodium brasilianum hosts: Tamarin and marmoset monkeys (family Callitrichidae). Malar. J. 2017, 16, 71. [Google Scholar] [CrossRef]
- Figueiredo, M.A.; Di Santi, S.M.; Figueiredo, T.A.; Machado, R.Z. Natural Plasmodium infection in neotropical primates in the island of São Luís, state of Maranhão, Brazil. Rev. Bras. Parasitol. Vet. 2015, 24, 122–128. [Google Scholar] [CrossRef]
- Assis, G.M.; Alvarenga, D.A.; Costa, D.C.; Souza, J.C., Jr.; Hirano, Z.M.; Kano, F.S.; Sousa, T.N.; Brito, C.F. Detection of Plasmodium in faeces of the New World primate Alouatta clamitans. Mem. Inst. Oswaldo Cruz. 2016, 111, 570–576. [Google Scholar] [CrossRef]
- Brasil, P.; Zalis, M.G.; de Pina-Costa, A.; Siqueira, A.M.; Júnior, C.B.; Silva, S.; Areas, A.L.L.; Pelajo-Machado, M.; de Alvarenga, D.A.M.; da Silva Santelli, A.C.F.; et al. Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: A molecular epidemiological investigation. Lancet Glob. Health 2017, 5, E1038–E1046. [Google Scholar] [CrossRef]
- Silva, T.; Barros, F.; Bahia, M.; Sampaio Junior, F.D.; Santos, S.; Inoue, L.S.; Gonçalves, T.S.; Chiesorin Neto, L.; Faria, D.; Tochetto, C.; et al. Plasmodium vivax and Plasmodium falciparum infection in Neotropical primates in the western Amazon, Brazil. Zoonoses Public. Health 2019, 66, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Duarte, A.M.; Curado, I.; Summa, M.E.; Neves, D.V.; Wunderlich, G.; Malafronte, R.S. Detection of etiological agents of malaria in howler monkeys from Atlantic Forests, rescued in regions of São Paulo city, Brazil. J. Med. Primatol. 2011, 40, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Soares, I.S.; Levitus, G.; Souza, J.M.; Del Portillo, H.A.; Rodrigues, M.M. Acquired immune responses to the N- and C-terminal regions of Plasmodium vivax merozoite surface protein 1 in individuals exposed to malaria. Infect. Immun. 1997, 65, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Polley, S.D.; Tetteh, K.K.; Cavanagh, D.R.; Pearce, R.J.; Lloyd, J.M.; Bojang, K.A.; Okenu, D.M.; Greenwood, B.M.; McBride, J.S.; Conway, D.J. Repeat sequences in block 2 of Plasmodium falciparum merozoite surface protein 1 are targets of antibodies associated with protection from malaria. Infect. Immun. 2003, 71, 1833–1842. [Google Scholar] [CrossRef]
- Priest, J.W.; Plucinski, M.M.; Huber, C.S.; Rogier, E.; Mao, B.; Gregory, C.J.; Candrinho, B.; Colborn, J.; Barnwell, J.W. Specificity of the IgG antibody response to Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale MSP1(19) subunit proteins in multiplexed serologic assays. Malar. J. 2018, 17, 417. [Google Scholar] [CrossRef]
- Drakeley, C.J.; Corran, P.H.; Coleman, P.G.; Tongren, J.E.; McDonald, S.L.; Carneiro, I.; Malima, R.; Lusingu, J.; Manjurano, A.; Nkya, W.M.M.; et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc. Natl. Acad. Sci. USA 2005, 102, 5108–5113. [Google Scholar] [CrossRef]
- Stewart, L.; Gosling, R.; Griffin, J.; Gesase, S.; Campo, J.; Hashim, R.; Masika, P.; Mosha, J.; Bousema, T.; Shekalaghe, S.; et al. Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS ONE 2009, 4, e6083. [Google Scholar] [CrossRef]
- Buery, J.C.; Rodrigues, P.T.; Natal, L.; Salla, L.C.; Loss, A.C.; Vicente, C.R.; Rezende, H.R.; Duarte, A.M.R.C.; Fux, B.; Malafronte, R.D.S.; et al. Mitochondrial genome of Plasmodium vivax/simium detected in an endemic region for malaria in the Atlantic Forest of Espírito Santo state, Brazil: Do mosquitoes, simians and humans harbour the same parasite? Malar. J. 2017, 16, 437. [Google Scholar] [CrossRef]
- Deane, L.M. Simian malaria in Brazil. Mem. Inst. Oswaldo Cruz 1992, 87, 1–20. [Google Scholar] [CrossRef]
- Abreu, F.V.S.; Santos, E.D.; Mello, A.R.L.; Gomes, L.R.; Alvarenga, D.A.M.; Gomes, M.Q.; Vargas, W.P.; Bianco-Júnior, C.; Pina-Costa, A.; Teixeira, D.S.; et al. Howler monkeys are the reservoir of malarial parasites causing zoonotic infections in the Atlantic forest of Rio de Janeiro. PLoS Negl. Trop. Dis. 2019, 13, e0007906. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.; Aguirre, A.A. Infectious diseases and the illegal wildlife trade. Ann. N. Y. Acad. Sci. 2008, 1149, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Kock, R.A.; Woodford, M.H.; Rossiter, P.B. Disease risk associated with the translocation of wildlife. Revue Scientifique et Technique 2010, 29, 329–350. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).