An Investigation of the Effect of Catecholamines and Glucocorticoids on the Growth and Pathogenicity of Campylobacter jejuni
Abstract
:1. Introduction
2. Results
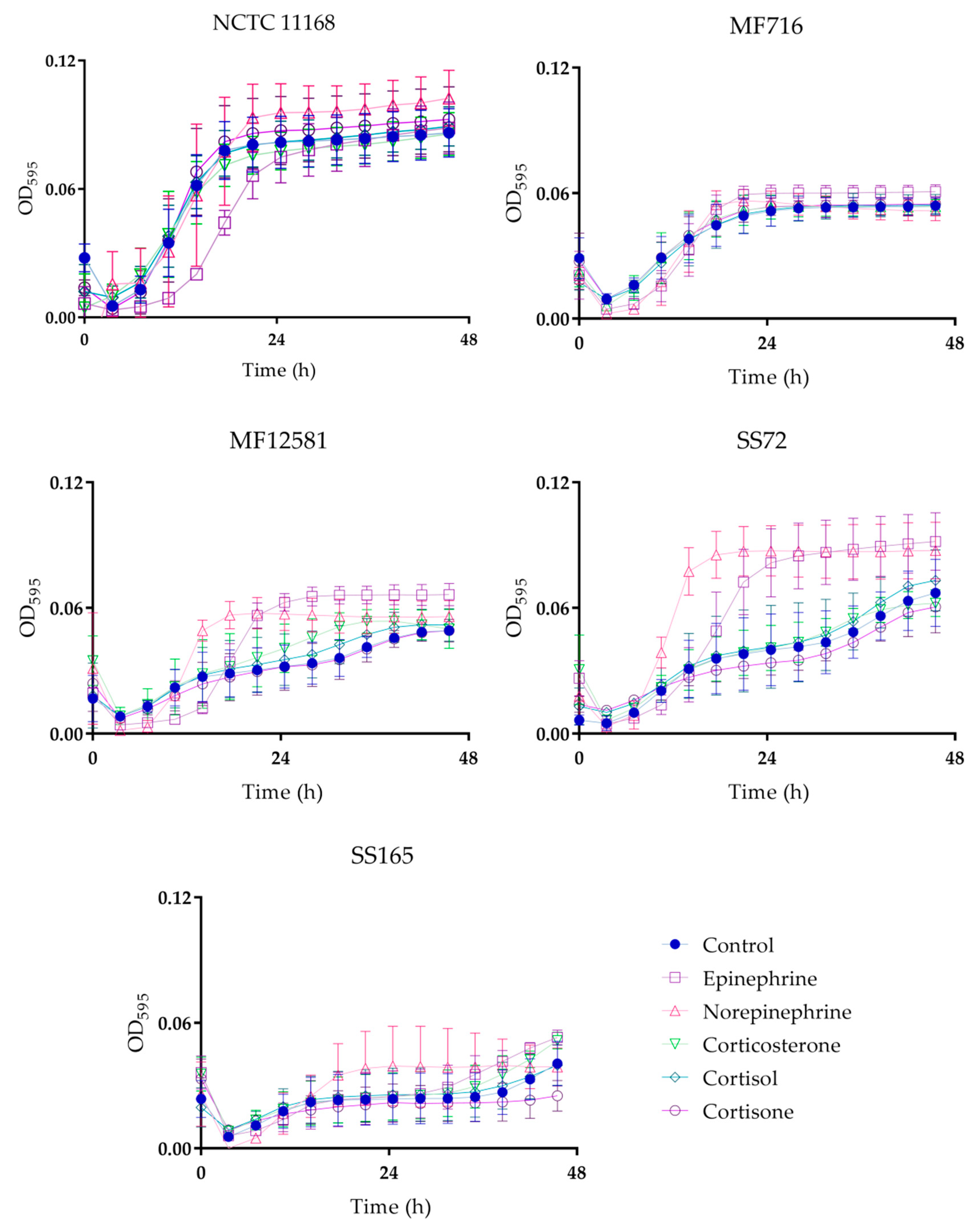
2.1. Bacterial Growth
2.2. Motility
2.3. Adhesion and Invasion of Caco-2 Cells
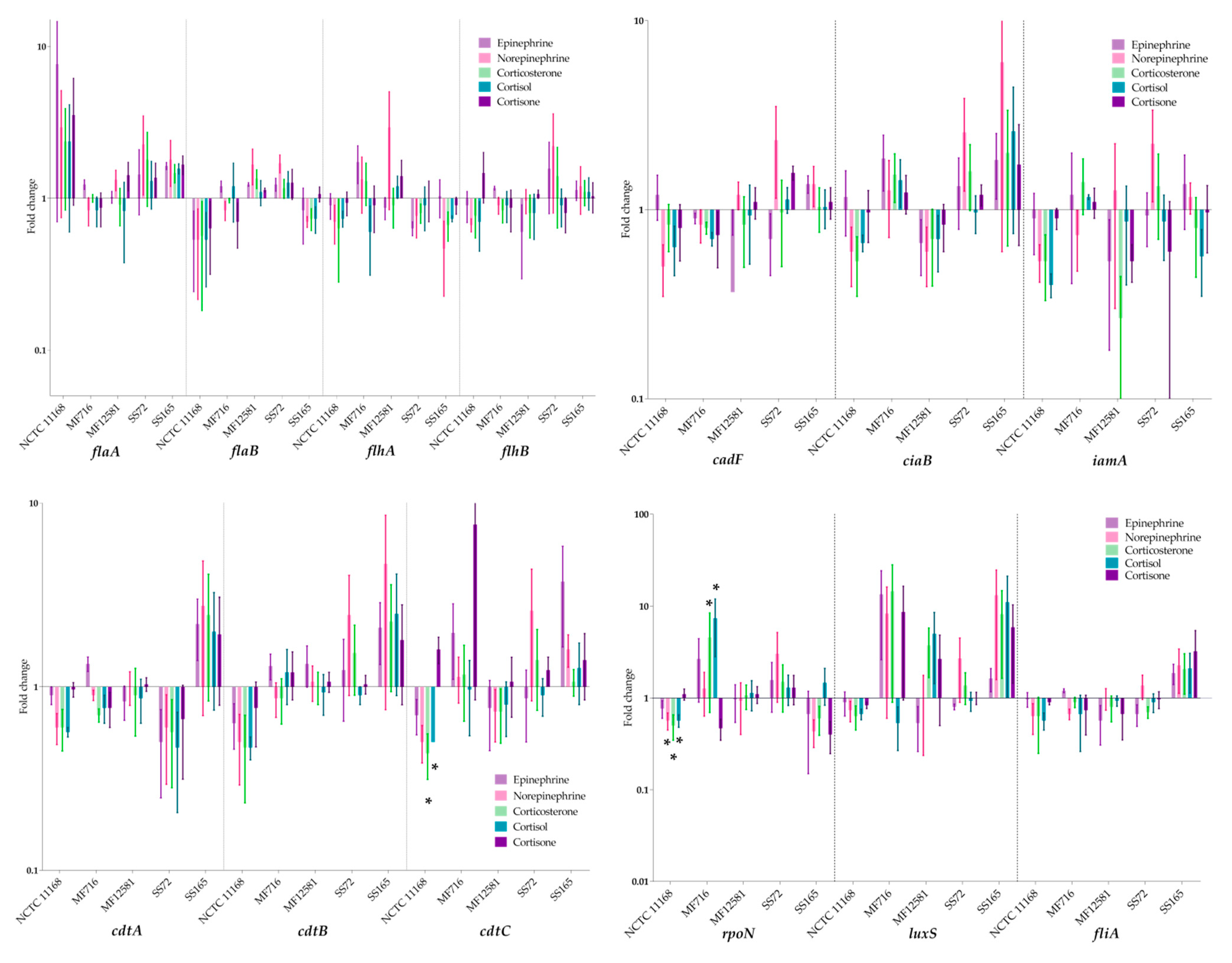
2.4. Expression of Virulence Genes
3. Discussion
4. Materials and Methods
4.1. Bacterial Growth Conditions
4.2. Bacterial Growth Assay
4.3. Motility Assay
4.4. Adhesion and Invasion Assay
4.4.1. Cultivation of Caco-2 Cells
4.4.2. Infection of Caco-2 Cells
4.4.3. Adhesion Assay
4.4.4. Invasion Assay
4.5. Virulence Gene Expression RT-qPCR
4.5.1. Bacterial Growth Conditions
4.5.2. RNA Extraction, Purification and cDNA Synthesis
4.5.3. RT-qPCR
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Campylobacter Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 3 June 2020).
- European Food Safety Authority. Scientific Topic: Campylobacter. Available online: https://www.efsa.europa.eu/en/topics/topic/campylobacter (accessed on 31 March 2020).
- Bolton, D. Campylobacter virulence and survival factors. Food Microbiol. 2015, 48, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony Multiplex PCR Assay for Identification and Differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, T.; O’Brien, S.; Madsen, M. Campylobacters as zoonotic pathogens: A food production perspective. Int. J. Food Microbiol. 2007, 117, 237–257. [Google Scholar] [CrossRef]
- Callicott, K.; Friðriksdóttir, V.; Reiersen, J.; Lowman, R.; Bisaillon, J.-R.; Gunnarsson, E.; Berndtson, E.; Hiett, K.L.; Needleman, D.S.; Stern, N.J.; et al. Lack of Evidence for Vertical Transmission of Campylobacter spp. in Chickens. Appl. Environ. Microbiol. 2006, 72, 5794–5798. [Google Scholar] [CrossRef] [Green Version]
- Cawthraw, S.A.; Wassenaar, T.M.; Ayling, R.; Newell, D.G. Increased colonization potential of Campylobacter jejuni strain 81116 after passage through chickens and its implication on the rate of transmission within flocks. Epidemiol. Infect. 1996, 117, 213–215. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation. Risk assessment of Campylobacter spp. in broiler chickens: Technical Report. Microbiol. Risk Assess. Ser. 2009, 12, 30–37. [Google Scholar]
- Blaser, M.J. Epidemiologic and clinical features of Campylobacter jejuni infections. J. Infect. Dis. 1997, 176, S103–S105. [Google Scholar] [CrossRef] [Green Version]
- Man, S.M. The clinical importance of emerging Campylobacter species. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 669–685. [Google Scholar] [CrossRef]
- Engberg, J. Clinical Aspects of Campylobacter jejuni and Campylobacter coli Infections. In Campylobacter, 3rd ed.; American Society for Microbiology: Washington, DC, USA, 2008; pp. 99–121. [Google Scholar]
- Baqar, S.; Tribble, D.R.; Carmolli, M.; Sadigh, K.; Poly, F.; Porter, C.; Larsson, C.J.; Pierce, K.K.; Guerry, P.; Darsley, M.; et al. Recrudescent Campylobacter jejuni Infection in an Immunocompetent Adult following Experimental Infection with a Well-Characterized Organism. Clin. Vaccine Immunol. 2009, 17, 80–86. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Gharaibeh, R.Z.; Newsome, R.C.; Pope, J.L.; Dougherty, M.W.; Tomkovich, S.; Pons, B.J.; Mirey, G.; Vignard, J.; Hendrixson, D.R.; et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut 2018, 68, 289–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogan, T.A.; O Thomas, A.; Rees, L.E.N.; Taylor, A.H.; A Jepson, M.; Williams, P.H.; Ketley, J.; Humphrey, T.J. Norepinephrine increases the pathogenic potential of Campylobacter jejuni. Gut 2006, 56, 1060–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroori, S.V.; Cogan, T.A.; Humphrey, T. Effect of Noradrenaline on the Virulence Properties of Campylobacter Species. Int. J. Microbiol. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Wu, C.; Guo, F.; Cui, G.; Zeng, X.; Yang, B.; Lin, J. Transcriptomic analysis of Campylobacter jejuni NCTC 11168 in response to epinephrine and norepinephrine. Front. Microbiol. 2015, 6, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freestone, P.P.; Sandrini, S.M.; Haigh, R.D.; Lyte, M. Microbial endocrinology: How stress influences susceptibility to infection. Trends Microbiol. 2008, 16, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Grover, M. Role of gut pathogens in development of irritable bowel syndrome. Indian J. Med. Res. 2014, 139, 11–18. [Google Scholar] [PubMed]
- Villageliũ, D.N.; Lyte, M. Microbial endocrinology: Why the intersection of microbiology and neurobiology matters to poultry health. Poult. Sci. 2017, 96, 2501–2508. [Google Scholar] [CrossRef]
- Lyte, M. The effect of stress on microbial growth. Anim. Heal. Res. Rev. 2014, 15, 172–174. [Google Scholar] [CrossRef]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef] [Green Version]
- Ridlon, J.M.; Ikegawa, S.; Alves, J.; Zhou, B.; Kobayashi, A.; Iida, T.; Mitamura, K.; Tanabe, G.; Serrano, M.; De Guzman, A.; et al. Clostridium scindens: A human gut microbe with a high potential to convert glucocorticoids into androgens. J. Lipid Res. 2013, 54, 2437–2449. [Google Scholar] [CrossRef] [Green Version]
- Elmi, A.; Watson, E.; Sandu, P.; Gundogdu, O.; Mills, D.C.; Inglis, N.F.; Manson, E.; Imrie, L.; Bajaj-Elliott, M.; Wren, B.W.; et al. Campylobacter jejuni Outer Membrane Vesicles Play an Important Role in Bacterial Interactions with Human Intestinal Epithelial Cells. Infect. Immun. 2012, 80, 4089–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louwen, R.; Heikema, A.; Van Belkum, A.; Ott, A.; Gilbert, M.; Ang, W.; Endtz, H.P.; Bergman, M.P.; Nieuwenhuis, E.E. The Sialylated Lipooligosaccharide Outer Core in Campylobacter jejuni Is an Important Determinant for Epithelial Cell Invasion. Infect. Immun. 2008, 76, 4431–4438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassenaar, T.M.; Bleumink-Pluym, N.M.; Newell, D.G.; Nuijten, P.J.; A Van Der Zeijst, B. Differential flagellin expression in a flaA flaB+ mutant of Campylobacter jejuni. Infect. Immun. 1994, 62, 3901–3906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermans, D.; Van Deun, K.; Martel, A.; Van Immerseel, F.; Messens, W.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 2011, 42, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buelow, D.R.; Christensen, J.E.; Neal-McKinney, J.M.; Konkel, M.E. Campylobacter jejuni survival within human epithelial cells is enhanced by the secreted protein CiaI. Mol. Microbiol. 2011, 80, 1296–1312. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.J.; Nolan, C.; Wang, S.P.; Shelton, W.R.; Blaser, M.J. Persistent Campylobacter jejuni Infection in an Immunocompromised Patient. Ann. Intern. Med. 1984, 100, 832–834. [Google Scholar] [CrossRef]
- Freestone, P. Communication between Bacteria and Their Hosts. Scientifica 2013, 2013, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Xu, F.; Lin, J. Molecular, Antigenic, and Functional Characteristics of Ferric Enterobactin Receptor CfrA in Campylobacter jejuni. Infect. Immun. 2009, 77, 5437–5448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sopinka, N.M.; Patterson, L.D.; Redfern, J.C.; Pleizier, N.; Belanger, C.B.; Midwood, J.D.; Crossin, G.T.; Cooke, S.J. Manipulating glucocorticoids in wild animals: Basic and applied perspectives. Conserv. Physiol. 2015, 3. [Google Scholar] [CrossRef]
- McKay, L.I.; Cidlowski, J.A. Physiologic and Pharmacologic Effects of Corticosteroids; BC Decker Inc.: Hamilton, ON, Canada, 2003. [Google Scholar]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akcalı, A.; Huck, O.; Buduneli, N.; Davideau, J.-L.; Köse, T.; Tenenbaum, H. Exposure of Porphyromonas gingivalis to cortisol increases bacterial growth. Arch. Oral Boil. 2014, 59, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Declercq, A.M.; Aerts, J.; Ampe, B.; Haesebrouck, F.; De Saeger, S.; Decostere, A. Cortisol directly impacts Flavobacterium columnare in vitro growth characteristics. Vet. Res. 2016, 47, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duran-Pinedo, A.E.; Solbiati, J.; Frias-Lopez, J. The stress hormone cortisol induces virulence in the oral microbiome. Biofilms Microbiomes. 2018, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.E.; Kontoyiannis, D.P. Invasive aspergillosis in glucocorticoid-treated patients. Med. Mycol. 2009, 47, S271–S281. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Kontoyiannis, D.P. Glucocorticoids and invasive fungal infections. Lancet 2003, 362, 1828–1838. [Google Scholar] [CrossRef]
- Seligmann, E. Virulence Enhancement of Candida albicans by Antibiotics and Cortisone. Exp. Boil. Med. 1953, 83, 778–781. [Google Scholar] [CrossRef]
- Plummer, P.J. LuxS and quorum-sensing in Campylobacter. Front. Microbiol. 2012, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Xu, Z.; Zhou, Y.; Sun, L.; Liu, Z.; Chen, H.; Zhou, R. Global Effects of Catecholamines on Actinobacillus pleuropneumoniae Gene Expression. PLoS ONE 2012, 7, e31121. [Google Scholar] [CrossRef]
- Sperandio, V.; Torres, A.G.; Jarvis, B.; Nataro, J.P.; Kaper, J.B. Bacteria-host communication: The language of hormones. Proc. Natl. Acad. Sci. USA 2003, 100, 8951–8956. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Fall, K.; Fang, F.; Erlendsdóttir, H.; Lu, D.; Mataix-Cols, D.; De La Cruz, L.F.; D’Onofrio, B.M.; Lichtenstein, P.; Gottfreðsson, M.; et al. Stress related disorders and subsequent risk of life threatening infections: Population based sibling controlled cohort study. BMJ 2019, 367, l5784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkholder, K.M.; Thompson, K.L.; Einstein, M.E.; Applegate, T.; Patterson, J.A. Influence of Stressors on Normal Intestinal Microbiota, Intestinal Morphology, and Susceptibility to Salmonella Enteritidis Colonization in Broilers. Poult. Sci. 2008, 87, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Iannetti, L.; Neri, D.; Santarelli, G.A.; Cotturone, G.; Vulpiani, M.P.; Salini, R.; Antoci, S.; Di Serafino, G.; Di Giannatale, E.; Pomilio, F.; et al. Animal welfare and microbiological safety of poultry meat: Impact of different at-farm animal welfare levels on at-slaughterhouse Campylobacter and Salmonella contamination. Food Control. 2020, 109, 106921. [Google Scholar] [CrossRef]
- Whyte, P.; Collins, J.D.; McGill, K.; Monahan, C.; O’Mahony, H. The Effect of Transportation Stress on Excretion Rates of Campylobacters in Market-age Broilers. Poult. Sci. 2001, 80, 817–820. [Google Scholar] [CrossRef]
- Koolman, L.; Whyte, P.; Bolton, D. An investigation of broiler caecalCampylobactercounts at first and second thinning. J. Appl. Microbiol. 2014, 117, 876–881. [Google Scholar] [CrossRef]
- Koolman, L.; Whyte, P.; Burgess, C.; Bolton, D. Virulence gene expression, adhesion and invasion of Campylobacter jejuni exposed to oxidative stress (H2O2). Int. J. Food Microbiol. 2016, 220, 33–38. [Google Scholar] [CrossRef]
- Davis, L.; Young, K.; DiRita, V. Genetic Manipulation ofCampylobacter jejuni. Curr. Protoc. Microbiol. 2008. [Google Scholar] [CrossRef] [Green Version]
- Tellmann, G. The E-Method: A highly accurate technique for gene-expression analysis. Nat. Methods 2006, 3, i–ii. [Google Scholar] [CrossRef]


| Isolate | Source |
|---|---|
| C. jejuni subsp. jejuni NCTC 11168 | Human |
| C. jejuni MF716 | Human |
| C. jejuni MF12581 | Human |
| C. jejuni SS72 | Broiler |
| C. jejuni SS165 | Broiler |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truccollo, B.; Whyte, P.; Bolton, D.J. An Investigation of the Effect of Catecholamines and Glucocorticoids on the Growth and Pathogenicity of Campylobacter jejuni. Pathogens 2020, 9, 555. https://doi.org/10.3390/pathogens9070555
Truccollo B, Whyte P, Bolton DJ. An Investigation of the Effect of Catecholamines and Glucocorticoids on the Growth and Pathogenicity of Campylobacter jejuni. Pathogens. 2020; 9(7):555. https://doi.org/10.3390/pathogens9070555
Chicago/Turabian StyleTruccollo, Brendha, Paul Whyte, and Declan J. Bolton. 2020. "An Investigation of the Effect of Catecholamines and Glucocorticoids on the Growth and Pathogenicity of Campylobacter jejuni" Pathogens 9, no. 7: 555. https://doi.org/10.3390/pathogens9070555




