Investigation of Streptomyces scabies Causing Potato Scab by Various Detection Techniques, Its Pathogenicity and Determination of Host-Disease Resistance in Potato Germplasm
Abstract
:1. Introduction
2. Results
2.1. Diversity and Isolation Frequency of Streptomyces Species from Different Regions
2.2. Phenotypic Characterization
2.3. Pathogenicity Tests/Virulence Confirmation Assays
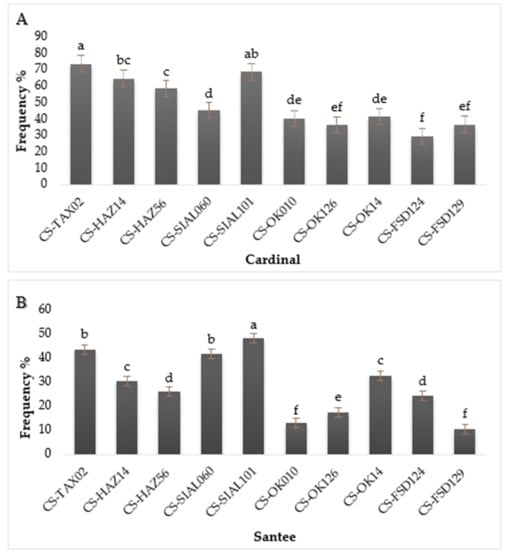
2.4. Screening of Potato Germplasm against S. scabies
2.5. Measurement of the Percentage of Potato Scab Incidence
2.6. Measurement of Potato Scab Index and Percent Disease Severity
2.7. Effect of Streptomyces scabies on Growth Parameters of Potato Cultivars
2.8. Molecular Detection of Streptomyces scabies (Gel Electrophoresis and PCR Amplification)
2.9. Sequence Analysis
3. Discussion
4. Materials and Methods
4.1. Diversity and Isolation of Streptomyces Species from Different Regions
4.2. Phenotypic Characterization
4.3. Pathogenicity Tests/Virulence Confirmation Assays
4.4. Screening of Potato Germplasm against Streptomyces scabies
4.5. Percentage of Disease Incidence
4.6. Percentage of Potato Scab Index and Disease Severity
- 0 = No symptom,
- 1 = Very small lesions,
- 2 = Small superficial lesions,
- 3 = Periderm broken,
- 4 = Light pitted
- 5 = Deep pitted
4.7. Effect of Streptomyces scabies on Growth Parameters of Potato Cultivars
4.8. Molecular Detection of S. scabies (Gel Electrophoresis and PCR Amplification)
4.9. Sequence Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spooner, D.M.; Mclean, K.; Ramsay, G.; Waugh, R.; Bryan, G.J. A single domestication for potato based on multilocus amplified fragment length polymorphism genotyping. Proc. Natl. Acad. Sci. USA 2005, 102, 14694–14699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeed, A.; Muhammad, Z. Potato production in Pakistan: Challenges and prospective management strategies–A review. Pak. J. Bot. 2018, 50, 2077–2084. [Google Scholar]
- Anwar, D.; Shabbir, D.; Shahid, M.H.; Samreen, W. Determinants of Potato Prices and Its Forecasting: A Case Study of Punjab, Pakistan. 16 September 2015. Available online: https://mpra.ub.uni-muenchen.de/66678/ (accessed on 20 August 2020).
- Enciso-Rodriguez, F.; Douches, D.; Lopez-Cruz, M.; Coombs, J.; de Los Campos, G. Genomic selection for late blight and common scab resistance in tetraploid potato (Solanum tuberosum). G3 Gene Genomes Genet. 2018, 8, 2471–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAOSTAT. FAOSTAT Database Collections; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Gondal, A.S.; Javed, N.; Khan, S.A.; Hyder, S. Genotypic diversity of potato germplasm against root knot nematode (Meloidogyne incognita) infection in Pakistan. Int. J Phytopathol. 2012, 1, 27–38. [Google Scholar] [CrossRef]
- Ashraf, A.; Rauf, A.; Abbas, M.F.; Rehman, R. Isolation and identification of Verticillium dahliae causing wilt on potato in Pakistan. Pak. J. Phytopathol. 2012, 24, 112–116. [Google Scholar]
- Mehboob, S.; Khan, M.; Rehman, A.; Idrees, M. Role of epidemiological and biochemical factors against early blight of potato. Int. J. Phytopathol. 2013, 2, 8–13. [Google Scholar] [CrossRef]
- Al-Mughrabi, K.I.; Vikram, A.; Poirier, R.; Jayasuriya, K.; Moreau, G. Management of common scab of potato in the field using biopesticides, fungicides, soil additives, or soil fumigants. Biocontrol Sci. Technol. 2016, 26, 125–135. [Google Scholar] [CrossRef]
- Bencheikh, M.; Setti, B. Characterization of Streptomyces scabies isolated from common scab lesions on potato tubers by morphological, biochemical and pathogenicity tests in Chlef region in western Algeria. Sci. Technol. C Biotechnol. 2007, 26, 61–67. [Google Scholar]
- Ventura, M.; Canchaya, C.; Tauch, A.; Chandra, G.; Fitzgerald, G.F.; Chater, K.F.; van Sinderen, D. Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 2007, 71, 495–548. [Google Scholar]
- Flärdh, K.; Buttner, M.J. Streptomyces morphogenetics: Dissecting differentiation in a filamentous bacterium. Nat. Rev. Microbiol. 2009, 7, 36. [Google Scholar]
- Braun, S.; Gevens, A.; Charkowski, A.; Allen, C.; Jansky, S. Potato common scab: A review of the causal pathogens, management practices, varietal resistance screening methods, and host resistance. Am. J. Potato Res. 2017, 94, 283–296. [Google Scholar] [CrossRef]
- Dees, M.; Sletten, A.; Hermansen, A. Isolation and characterization of Streptomyces species from potato common scab lesions in Norway. Plant Pathol. 2013, 62, 217–225. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Y.; Yan, R.; Ha, S.; Lin, T.; Cheng, Y.; Hu, B. Occurrence and control approach of potato common scab (caused by Streptomyces scabiei) in Nei Menggu. Chin. Potato J. 2013, 27, 56–59. [Google Scholar]
- Slabbert, R.; Klerk, A.D.; Pretorius, E. Isolation of the phytotoxin thaxtomin A associated with Streptomyces scabies (common scab) in potatoes. J. S. Afr. Soc. Hortic. Sci. 1994, 4, 33–34. [Google Scholar]
- Ahmad, I.; Soomro, M.; Khalid, S.; Iftikhar, S.; Munir, A.; Burney, K. Recent distributional trends of potato diseases in Pakistan. In Research and Development of Potato Production in Pakistan. Proceedings of the National Seminar Held at NARC, Islamabad, Pakistan, 23–25 April 1995; Pakistan Agricultural Research Council: Islamabad, Pakistan, 1995; pp. 117–125. [Google Scholar]
- Kalantar Zadeh, M.; Shahidi Bonjar, G.; Rashid Farrokhi, P.; Ghasemi, A.; Aghighi, S.; Mahdavi, M. Antagonistic potential of two native Streptomyces strains in biocontrol of the major causals of common scab of potato in Iran. Asian J. Plant Sci. 2006, 5, 5–8. [Google Scholar]
- Lysenko, Y.N.; Pluzhnikova, I. A system of potato protection from diseases with the use of biological control agents. Kartof. Ovoshchi 2005, 3, 28–29. [Google Scholar]
- Pasiecznik, N.; Smith, I.; Watson, G.; Brunt, A.; Ritchie, B.; Charles, L. CABI/EPPO distribution maps of plant pests and plant diseases and their important role in plant quarantine. Eppo Bull. 2005, 35, 1–7. [Google Scholar] [CrossRef]
- Kers, J.A.; Cameron, K.D.; Joshi, M.V.; Bukhalid, R.A.; Morello, J.E.; Wach, M.J.; Gibson, D.M.; Loria, R. A large, mobile pathogenicity island confers plant pathogenicity on Streptomyces species. Mol. Microbiol. 2005, 55, 1025–1033. [Google Scholar] [CrossRef]
- Ahmad, M.S.; El-Gendy, A.O.; Ahmed, R.R.; Hassan, H.M.; El-Kabbany, H.M.; Merdash, A.G. Exploring the antimicrobial and antitumor potentials of Streptomyces sp. AGM12-1 isolated from Egyptian soil. Front. Microbiol. 2017, 8, 438. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wang, C.; Gui, P.; Liu, H.; Khalaf, S.M.; Elsayed, E.A.; Wadaan, M.A.; Hozzein, W.N.; Zhu, W. Identification, bioactivity, and productivity of actinomycins from the marine-derived Streptomyces heliomycini. Front. Microbiol. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Challis, G.L.; Hopwood, D.A. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc. Natl. Acad. Sci. USA 2003, 100, 14555–14561. [Google Scholar] [CrossRef] [Green Version]
- Dees, M.W.; Wanner, L.A. In search of better management of potato common scab. Potato Res. 2012, 55, 249–268. [Google Scholar] [CrossRef]
- Francis, I.; Holsters, M.; Vereecke, D. The Gram-positive side of plant–microbe interactions. Environ. Microbiol. 2010, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kinkel, L.L.; Schlatter, D.C.; Bakker, M.G.; Arenz, B.E. Streptomyces competition and co-evolution in relation to plant disease suppression. Res. Microbiol. 2012, 163, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Seipke, R.F.; Kaltenpoth, M.; Hutchings, M.I. Streptomyces as symbionts: An emerging and widespread theme? Fems Microbiol. Rev. 2012, 36, 862–876. [Google Scholar]
- Loria, R.; Kers, J.; Joshi, M. Evolution of plant pathogenicity in Streptomyces. Annu. Rev. Phytopathol. 2006, 44, 469–487. [Google Scholar] [CrossRef]
- Hiltunen, L.H.; Alanen, M.; Laakso, I.; Kangas, A.; Virtanen, E.; Valkonen, J. Elimination of common scab sensitive progeny from a potato breeding population using thaxtomin A as a selective agent. Plant Pathol. 2011, 60, 426–435. [Google Scholar]
- Jansky, S.; Douches, D.; Haynes, K. Germplasm release: Three tetraploid potato clones with resistance to common scab. Am. J. Potato Res. 2018, 95, 178–182. [Google Scholar]
- Kobayashi, Y.O.; Kobayashi, A.; Maeda, M.; Takenaka, S. Isolation of antagonistic Streptomyces sp. against a potato scab pathogen from a field cultivated with wild oat. J. Gen. Plant Pathol. 2012, 78, 62–72. [Google Scholar]
- Loria, R.; Coombs, J.; Yoshida, M.; Kers, J.; Bukhalid, R. A paucity of bacterial root diseases: Streptomyces succeeds where others fail. Physiol. Mol. Plant Pathol. 2003, 62, 65–72. [Google Scholar]
- Diallo, S.; Crépin, A.; Barbey, C.; Orange, N.; Burini, J.-F.; Latour, X. Mechanisms and recent advances in biological control mediated through the potato rhizosphere. Fems Microbiol. Ecol. 2011, 75, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, L.J.; Morris, W.L.; Hedley, P.E.; Shepherd, T.; Davies, H.V.; Millam, S.; Taylor, M.A. Metabolic engineering of high carotenoid potato tubers containing enhanced levels of β-carotene and lutein. J. Exp. Bot. 2005, 56, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Q.; Yin, J.; Rosenzweig, N.; Douches, D.; Hao, J.J. Culture-based assessment of microbial communities in soil suppressive to potato common scab. Plant Dis. 2012, 96, 712–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatri, B.B.; Tegg, R.; Brown, P.H.; Wilson, C. Temporal association of potato tuber development with susceptibility to common scab and Streptomyces scabiei-induced responses in the potato periderm. Plant Pathol. 2011, 60, 776–786. [Google Scholar] [CrossRef]
- Hill, J.; Lazarovits, G. A mail survey of growers to estimate potato common scab prevalence and economic loss in Canada. Can. J. Plant Pathol. 2005, 27, 46–52. [Google Scholar] [CrossRef]
- Hossain, S.; Rasul, G.; Mian, M.K.; Haque, M.M.; Akanda, A.M. Yield Potential of Twelve Potato (Solanum tuberosum) Varieties Grown from Different Generations of Seed. Agriculturists 2015, 13, 120–132. [Google Scholar] [CrossRef]
- Mukhtar, H.; Ijaz, S.; Ikram-ul-Haq. Production of antitumor antibiotic by Streptomyces capoamus. Pak. J. Bot. 2012, 44, 445–452. [Google Scholar]
- Bais, Y.; Nimbekar, T.; Wanjari, B.; Timande, S. Isolation of antibacterial compound from marine soil Actinomycetes. Int. J. Biomed. Adv. Res. 2012, 3, 193–196. [Google Scholar]
- Park, Y.; Kim, S.; Cho, J. Conductive Environment and Ecology of Common Scab (Streptomyces scabies) of Potato. J. Korean Soc. Hortic. Sci. 2002, 43, 607–612. [Google Scholar]
- Lin, C.; Tsai, C.-H.; Chen, P.-Y.; Wu, C.-Y.; Chang, Y.-L.; Yang, Y.-L.; Chen, Y.-L. Biological control of potato common scab by Bacillus amyloliquefaciens Ba01. PLoS ONE 2018, 13, e0196520. [Google Scholar] [CrossRef] [Green Version]
- Pedley, K.F.; Martin, G.B. Molecular basis of Pto-mediated resistance to bacterial speck disease in tomato. Annu. Rev. Phytopathol. 2003, 41, 215–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, K.G.; Wanner, L.A.; Thill, C.A.; Bradeen, J.M.; Miller, J.; Novy, R.G.; Whitworth, J.L.; Corsini, D.L.; Vinyard, B.T. Common scab trials of potato varieties and advanced selections at three US locations. Am. J. Potato Res. 2010, 87, 261–276. [Google Scholar] [CrossRef]
- Lerat, S.; SIMAO-BEAUNOIR, A.M.; Beaulieu, C. Genetic and physiological determinants of Streptomyces scabies pathogenicity. Mol. Plant Pathol. 2009, 10, 579–585. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [Green Version]
- Lapaz, M.; Huguet-Tapia, J.; Siri, M.; Verdier, E.; Loria, R.; Pianzzola, M. Genotypic and phenotypic characterization of Streptomyces species causing potato common scab in Uruguay. Plant Dis. 2017, 101, 1362–1372. [Google Scholar] [CrossRef] [Green Version]
- Hamedo, H.A.; Makhlouf, A.H. Identification and characterization of actinomycetes for biological control of bacterial scab of Streptomyces scabies isolated from potato. J. Biol. Agric. Healthcare 2013, 3, 142–153. [Google Scholar]
- Barakate, M.; Ouhdouch, Y.; Oufdou, K.; Beaulieu, C. Characterization of rhizospheric soil streptomycetes from Moroccan habitats and their antimicrobial activities. World J. Microbiol. Biotechnol. 2002, 18, 49–54. [Google Scholar] [CrossRef]
- Han, J.; Cheng, J.; Yoon, T.; Song, J.; Rajkarnikar, A.; Kim, W.G.; Yoo, I.D.; Yang, Y.; Suh, J. Biological control agent of common scab disease by antagonistic strain Bacillus sp. sunhua. J. Appl. Microbiol. 2005, 99, 213–221. [Google Scholar] [CrossRef]
- Jonit, N.; Low, Y.; Tan, G. Xanthomonas oryzae pv. oryzae, biochemical tests, rice (Oryza sativa), Bacterial Leaf Blight (BLB) disease, Sekinchan. J. Appl. Environ. Microb. 2016, 4, 63–69. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Atiq, M.; Khalid, A.R.; Hussian, W.; Nawaz, A.; Asad, S.; Ahmad, T.M. Genetic potential of potato germplasm against common scab disease caused by Streptomyces scabies. Pak. J. Phytopathol. 2013, 25, 27–30. [Google Scholar]
- St-Onge, R.; Goyer, C.; Coffin, R.; Filion, M. Genetic diversity of Streptomyces spp. causing common scab of potato in eastern Canada. Syst. Appl. Microbiol. 2008, 31, 474–484. [Google Scholar] [PubMed]
- Vismara, P.; Capezio, S.; Bedogni, C. Common scab performance of tetraploid potato genotypes from Argentina. Adv. Plants Agric. Res. 2016, 5, 544–549. [Google Scholar]
- Sarwar, A.; Latif, Z.; Zhang, S.; Zhu, J.; Zechel, D.L.; Bechthold, A. Biological control of potato common scab with rare isatropolone C compound produced by plant growth promoting Streptomyces A1RT. Front. Microbiol. 2018, 9, 1126. [Google Scholar]
- Takeuchi, T.; Sawada, H.; Tanaka, F.; Matsuda, I. Phylogenetic analysis of Streptomyces spp. causing potato scab based on 16S rRNA sequences. Int. J. Syst. Evol. Microbiol. 1996, 46, 476–479. [Google Scholar]
- Wanner, L.A. A survey of genetic variation in Streptomyces isolates causing potato common scab in the United States. Phytopathology 2006, 96, 1363–1371. [Google Scholar] [PubMed] [Green Version]
- Zhang, K.; Yuan-Ying, S.; Cai, L. An optimized protocol of single spore isolation for fungi. Cryptogam. Mycol. 2013, 34, 349–356. [Google Scholar]
- Chaudhry, Z.; Rashid, H. Isolation and characterization of Ralstonia solanacearum from infected tomato plants of soan skesar valley of Punjab. Pak. J. Bot. 2011, 43, 2979–2985. [Google Scholar]
- Naher, N.; Hossain, M.; Bashar, M. Survey on the incidence and severity of common scab of potato in Bangladesh. J. Asiat. Soc. Bangladesh Sci. 2013, 39, 35–41. [Google Scholar]
- Bukhalid, R.; Takeuchi, T.; Labeda, D.; Loria, R. Horizontal transfer of the plant virulence gene, nec1, and flanking sequences among genetically distinct Streptomyces strains in the Diastatochromogenes cluster. Appl. Environ. Microbiol. 2002, 68, 738–744. [Google Scholar]
















| Area | Location | Disease Incidence (%) | Percent Scab Index |
|---|---|---|---|
| Taxila (Rawalpindi) | Thatha | 2% | 3.1% |
| Usman Khattir | 3% | 3.2% | |
| Ghari sikandar | 16% | 6.7% | |
| Khurum Paracha | 12% | 5.5% | |
| Labthatoo | 5% | 3.6% | |
| Average | 8% | 4.42% | |
| Hazro (Attock) | Waisa | 13% | 9.6% |
| Mallah | 3% | 2.6% | |
| Shamsabad | 11% | 8.2% | |
| Sirka | 2% | 1.9% | |
| Shadi khan | 9% | 4.6% | |
| Kalu kalan | 5% | 2.5% | |
| Average | 7% | 4.90% | |
| Sialkot | Kishan garh | 22% | 13.7% |
| Hussain pur | 23% | 13.9% | |
| Randhir | 34% | 18.7% | |
| Habibpur | 41% | 21.2% | |
| Chicharwali | 21% | 13.2% | |
| Average | 28% | 16.14% | |
| Kasur | Qutba | 40% | 22.6% |
| Faqeerwala | 38% | 21.8% | |
| Dolaywala | 42% | 23.1% | |
| Average | 40% | 22.5% | |
| Okara | Moza Ameer | 36% | 23.8% |
| Salwal | 45% | 17.5% | |
| Qadirabad | 52% | 25.2% | |
| Average | 44.33% | 22.16% | |
| Faisalabad | Jaranwala | 32% | 15.1% |
| Ram Diwali | 26% | 14.9% | |
| Karamsar | 24% | 18.7% | |
| Average | 27.33% | 16.23% | |
| Isolates | Potato Cultivars | Location of Sampling | Suggested Identification |
|---|---|---|---|
| CS-TAX01 | Cardinal | Taxila (Rawalpindi) | Streptomyces scabies |
| CS-TAX02 | Cardinal | Taxila (Rawalpindi) | Streptomyces acidiscabies |
| CS-TAX002 | Asterix | Taxila (Rawalpindi) | Streptomyces scabies |
| CS-TAX004 | Lady Rosetta | Taxila (Rawalpindi) | Streptomyces scabies |
| CS-TAX06 | Lady Rosetta | Taxila (Rawalpindi) | Streptomyces griseoflavus |
| CS-TAX08 | Faisalabad red | Taxila (Rawalpindi) | Streptomyces scabies |
| CS-TAX010 | Faisalabad red | Taxila (Rawalpindi) | Streptomyces griseoflavus |
| CS-TAX12 | Faisalabad red | Taxila (Rawalpindi) | Streptomyces griseoflavus |
| CS-TAX13 | Faisalabad white | Taxila (Rawalpindi) | Streptomyces scabies |
| CS-HAZ14 | Faisalabad white | Hazro (Attock) | Streptomyces scabies |
| CS-HAZ15 | Sadaf | Hazro (Attock) | Streptomyces acidiscabies |
| CS-HAZ18 | Sadaf | Hazro (Attock) | Streptomyces acidiscabies |
| CS-HAZ20 | Sadaf | Hazro (Attock) | Streptomyces acidiscabies |
| CS-HAZ24 | Sadaf | Hazro (Attock) | Streptomyces acidiscabies |
| CS-HAZ40 | Sadaf | Hazro (Attock) | Streptomyces acidiscabies |
| CS-HAZ56 | Sadaf | Hazro (Attock) | Streptomyces acidiscabies |
| CS-HAZ58 | Tourag | Hazro (Attock) | Streptomyces acidiscabies |
| CS-HAZ59 | Tourag | Hazro (Attock) | Streptomyces acidiscabies |
| CS-HAZ62 | Tourag | Hazro (Attock) | Streptomyces scabies |
| CS-SIAL060 | Tourag | Sialkot | Streptomyces scabies |
| CS-SIAL70 | Diamant | Sialkot | Streptomyces scabies |
| CS-SIAL72 | Diamant | Sialkot | Streptomyces scabies |
| CS-SIAL74 | Diamant | Sialkot | Streptomyces scabies |
| CS-SIAL76 | Diamant | Sialkot | Streptomyces scabies |
| CS-SIAL78 | Diamant | Sialkot | Streptomyces griseoflavus |
| CS-SIAL80 | Diamant | Sialkot | Streptomyces griseoflavus |
| CS-SIAL90 | Diamant | Sialkot | Streptomyces griseoflavus |
| CS-SIAL101 | Kuroda | Sialkot | Streptomyces griseoflavus |
| CS-SIAL110 | Kuroda | Sialkot | Streptomyces griseoflavus |
| CS-OK108 | Kuroda | Okara | Streptomyces griseoflavus |
| CS-OK0101 | Kuroda | Okara | Streptomyces scabies |
| CS-OK120 | Kuroda | Okara | Streptomyces scabies |
| CS-OK124 | Asterix | Okara | Streptomyces scabies |
| CS-OK126 | Asterix | Okara | Streptomyces scabies |
| CS-OK128 | Asterix | Okara | Streptomyces scabies |
| CS-OK130 | Faisalabad red | Okara | Streptomyces scabies |
| CS-OK143 | Faisalabad white | Okara | Streptomyces griseoflavus |
| CS-OK131 | Faisalabad white | Okara | Streptomyces griseoflavus |
| CS-OK121 | Diamant | Okara | Streptomyces griseoflavus |
| CS-FSD124 | Sadaf | Faisalabad | Streptomyces griseoflavus |
| CS-FSD126 | Sadaf | Faisalabad | Streptomyces acidiscabies |
| CS-FSD123 | Diamant | Faisalabad | Streptomyces acidiscabies |
| CS-FSD128 | Kuroda | Faisalabad | Streptomyces acidiscabies |
| CS-FSD129 | Lady Rosetta | Faisalabad | Streptomyces acidiscabies |
| CS-FSD130 | Lady Rosetta | Faisalabad | Streptomyces acidiscabies |
| CS-FSD139 | Sante | Faisalabad | Streptomyces scabies |
| CS-FSD149 | Sante | Faisalabad | Streptomyces scabies |
| CS-FSD0149 | Sante | Faisalabad | Streptomyces scabies |
| CS-FSD143 | Diamant | Faisalabad | Streptomyces scabies |
| CS-FSD146 | Diamant | Faisalabad | Streptomyces scabies |
| Culture Media | Growth | Colony Color | Aerial Mycelium |
|---|---|---|---|
| nutrient agar | Moderate | Creamy White | Abundant |
| Potato dextrose agar | Moderate | Creamy White | Abundant |
| Yeast malt agar | Well Abundant | Creamy White | Well Abundant |
| King’s B media | Moderate | White to Creamy | Poor |
| Oat meal agar | Moderate | White | Abundant |
| Potato yeast extract | Moderate | Brown | Abundant |
| Czapek media | Moderate | White | Abundant |
| Isolates | Spore Shape | Spore Color | Septations | Spore Size (μm) | |
|---|---|---|---|---|---|
| Length (Min–Max) Mean | Width(Min–Max) Mean | ||||
| CS-TAX01 | Pointed from one end and blunted from another end | Hyaline | 2 celled actinomycetes conidia | (9–18) 13.5 | (5–6) 5.5 |
| CS-TAX002 | Pointed from one end and blunted from other end | Hyaline | 2 celled actinomycetes conidia | (9–15) 12 | (5–6) 5.5 |
| CS-TAX004 | Pointed from one end | Hyaline | 2 celled actinomycetes conidia | (9–20) 14.5 | (6–7) 6.5 |
| CS-TAX08 | Pointed from one end | Hyaline | 2 celled actinomycetes conidia | (9–17) 13 | (5–7) 6 |
| CS-TAX13 | Pointed from one end and blunted from other end | Hyaline | 2 celled actinomycetes conidia | (9–20) 14.5 | (6–7) 6.5 |
| CS-HAZ14 | Pointed from one end | Hyaline | 2 celled actinomycetes conidia | (10–20) 15 | (6–7) 6.5 |
| CS-HAZ62 | Pointed from one end | Hyaline | 2 celled actinomycetes conidia | (10–21) 15.5 | (5–7) 6 |
| CS-SIAL060 | Pointed from one end | Hyaline | Single-cell actinomycetes conidia | (8–22) 15 | (3–7) 5 |
| CS-SIAL70 | Pointed from one end and blunted from other end | Falcate | Single-cell actinomycetes conidia | (13–25) 14 | (4–7) 5.5 |
| CS-SIAL72 | Pointed from one end and blunted from other end | Fusiform | Single-cell actinomycetes conidia | (12–22) 17 | (6–8) 7 |
| CS-SIAL74 | Pointed from two ends | Falcate | 2 celled actinomycetes conidia | (15–21) 18 | (2–9) 5.5 |
| CS-SIAL76 | Pointed from two ends | Hyaline | 2 celled actinomycetes conidia | (13–17) 15 | (5–8) 6.5 |
| CS-OK0101 | Pointed from two ends | Hyaline | Single-cell actinomycetes conidia | (13–21) 17 | (5–10) 7.5 |
| CS-OK120 | Pointed from one end and blunted from other end | Hyaline | Single-cell actinomycetes conidia | (16–17) 16.5 | (4–7) 5.5 |
| CS-OK124 | Pointed from one end and blunted from other end | Falcate | Single-cell actinomycetes conidia | (15–25) 20 | (5–9) 7 |
| CS-OK126 | Pointed from two ends | Falcate | Single-cell actinomycetes conidia | (12–19) 15.5 | (6–7) 6.5 |
| CS-OK128 | Pointed from two ends | Falcate | 2 celled actinomycetes conidia | (15–22) 18.5 | (3–6) 4.5 |
| CS-OK130 | Pointed from two ends | Hyaline | 2 celled actinomycetes conidia | (14–23) 13.5 | (2–7) 4.5 |
| CS-FSD139 | Pointed from one end and blunted from other end | Hyaline | 2 celled actinomycetes conidia | (16–25) 20.5 | (3–10) 6.5 |
| CS-FSD149 | Pointed from two ends | Fusiform | 2 celled actinomycetes conidia | (19–20) 19.5 | (6–7) 6.5 |
| CS-FSD0149 | Pointed from one end | Fusiform | 2 celled actinomycetes conidia | (20–22) 21 | (2–6) 4 |
| CS-FSD143 | Pointed from one end | Fusiform | Single-cell actinomycetes conidia | (15–18) 16.5 | (7–7) 7 |
| CS-FSD146 | Pointed from one end | Hyaline | Single-cell actinomycetes conidia | (16–17) 16.5 | (5–7) 6 |
| S. No. | Variety | Percent Scab Incidence | Percent Scab Index | Reaction |
|---|---|---|---|---|
| 1 | Cardinal | 37.46 | 15.91 | Medium susceptible |
| 2 | Santee | 49.63 | 20.5 | Susceptible |
| 3 | Lady Rosetta | 17.55 | 5.90 | Less susceptible |
| 4 | Kuroda | 56.35 | 20.65 | Susceptible |
| 5 | Tourag | 46.25 | 18.77 | Medium susceptible |
| 6 | Asterix | 2.90 | 1.47 | Resistant |
| 7 | Diamant | 41.55 | 11.38 | Less susceptible |
| 8 | Faisalabad White | 22.53 | 1.08 | Resistant |
| 9 | Faisalabad red | 75.24 | 25.18 | Highly susceptible |
| 10 | Sadaf | 33.38 | 7.09 | Less susceptible |
| 11 | Control | 12.01 | 6.00 | Resistant |
| Varieties | Percent Disease Incidence | Percent Scab Index | No. of Tubers |
|---|---|---|---|
| Kuroda | 56.48 a | 20.71 ab | 6.01 a |
| Santee | 50.61 b | 21.16 a | 5.66 a |
| Faisalabad red | 47.75 c | 20.64 ab | 5.01 a |
| Tourag | 45.91 c | 18.56 bc | 5.66 a |
| Diamant | 41.38 d | 11.13 d | 6.01 a |
| Cardinal | 38.11 e | 16.51 c | 4.66 a |
| Sadaf | 33.83 f | 7.09 e | 5.66 a |
| Asterix | 2.94 g | 1.55 f | 5.33 a |
| Faisalabad white | 2.81 g | 1.25 f | 5.66 a |
| Lady Rosetta | 2.35 g | 1.05 f | 5.01 a |
| Varieties | Root Weight (g) | Shoot Weight (g) | Root Length (cm) | Shoot Length (cm) |
|---|---|---|---|---|
| Kuroda | 3.64 bc | 9.76 bc | 20.66 ab | 30.66 c |
| Santee | 3.24 bc | 9.64 bc | 19.66 ab | 39.66 ab |
| Faisalabad red | 2.22 c | 6.83 d | 19.66 ab | 41.01 ab |
| Tourag | 4.24 abc | 10.44 bc | 21.33 a | 41.33 ab |
| Diamant | 4.81 ab | 11.54 ab | 19.01 ab | 34.01 bc |
| Cardinal | 5.85 a | 14.78 a | 16.01 b | 40.33 ab |
| Sadaf | 3.56 bc | 10.61 bc | 16.33 ab | 40.01 ab |
| Asterix | 4.96 ab | 12.37 ab | 20.33 ab | 26.66 c |
| Faisalabad white | 2.59 c | 7.36 cd | 18.33 ab | 30.66 c |
| Lady Rosetta | 3.81 ab | 10.63 bc | 21.33 a | 46.01 a |
| Isolates | Identified Species | Similarity% | Accession Number |
|---|---|---|---|
| CS-TAX01 | Streptomyces scabies | 100% | M57297.1 |
| CS-TAX002 | Streptomyces scabies | 98% | AF031232.1 |
| CS-TAX004 | Streptomyces scabies | 100% | AM293590.1 |
| CS-TAX08 | Streptomyces scabies | 99% | HM018077.1 |
| CS-TAX13 | Streptomyces griseoflavus | 96% | JX284407.1 |
| CS-HAZ14 | Streptomyces europaeiscabiei | 90% | HQ441817.1 |
| CS-HAZ62 | Streptomyces europaeiscabiei | 89% | AY207595.1 |
| CS-SIAL060, | Streptomyces scabies | 99% | AY207602.1 |
| CS-SIAL70 | Streptomyces scabies | 98% | AB301479.1 |
| CS-SIAL72 | Streptomyces scabies | 99% | Y15497.1 |
| S. No | Variety Name | Location/Source |
|---|---|---|
| 1 | Cardinal | National agricultural research institute (NARC), Islamabad |
| 2 | Santee | National agricultural research institute (NARC), Islamabad |
| 3 | Lady Rosetta | National agricultural research institute (NARC), Islamabad |
| 4 | Kuroda | National agricultural research institute (NARC), Islamabad |
| 5 | Tourag | National agricultural research institute (NARC), Islamabad |
| 6 | Asterix | Potato Research Institute (PRI), Sahiwal |
| 7 | Diamant | Potato Research Institute (PRI), Sahiwal |
| 8 | Faisalabad white | Potato Research Institute (PRI), Sahiwal |
| 9 | Faisalabad red | Potato Research Institute (PRI), Sahiwal |
| 10 | Sadaf | Potato Research Institute (PRI), Sahiwal |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, S.; Jiang, B.; Nasimi, Z.; Inam-ul-Haq, M.; Yamamoto, N.; Danso Ofori, A.; Khan, N.; Arshad, M.; Abbas, K.; Zheng, A. Investigation of Streptomyces scabies Causing Potato Scab by Various Detection Techniques, Its Pathogenicity and Determination of Host-Disease Resistance in Potato Germplasm. Pathogens 2020, 9, 760. https://doi.org/10.3390/pathogens9090760
Ismail S, Jiang B, Nasimi Z, Inam-ul-Haq M, Yamamoto N, Danso Ofori A, Khan N, Arshad M, Abbas K, Zheng A. Investigation of Streptomyces scabies Causing Potato Scab by Various Detection Techniques, Its Pathogenicity and Determination of Host-Disease Resistance in Potato Germplasm. Pathogens. 2020; 9(9):760. https://doi.org/10.3390/pathogens9090760
Chicago/Turabian StyleIsmail, Sohaib, Bo Jiang, Zohreh Nasimi, M. Inam-ul-Haq, Naoki Yamamoto, Andrews Danso Ofori, Nawab Khan, Muhammad Arshad, Kumail Abbas, and Aiping Zheng. 2020. "Investigation of Streptomyces scabies Causing Potato Scab by Various Detection Techniques, Its Pathogenicity and Determination of Host-Disease Resistance in Potato Germplasm" Pathogens 9, no. 9: 760. https://doi.org/10.3390/pathogens9090760