Teixobactin Provides Protection against Inhalation Anthrax in the Rabbit Model
Abstract
:1. Introduction
2. Results
2.1. Aerosol Infection Parameters
2.2. Detection of Protective Antigen
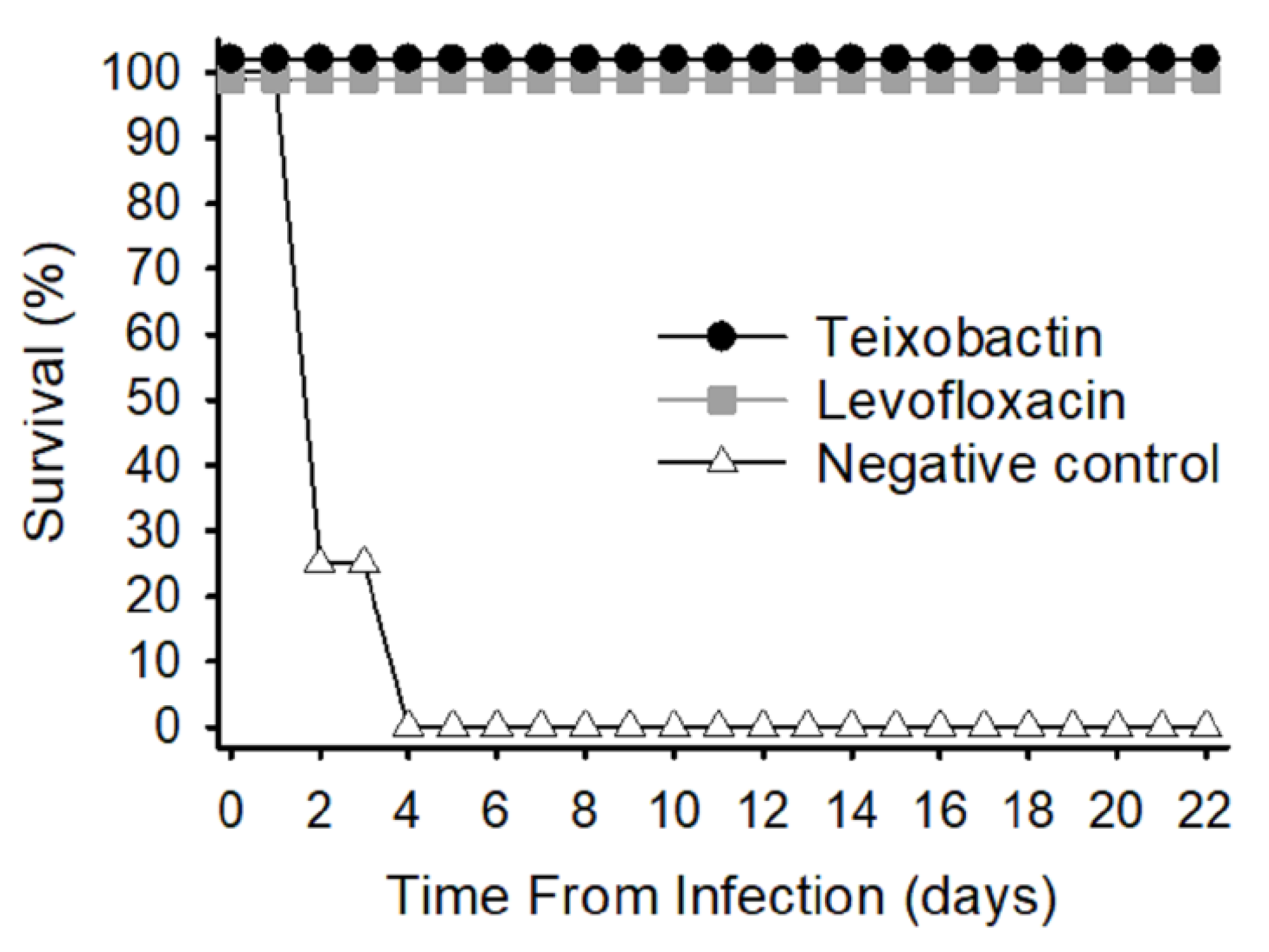
2.3. Survival Rate
2.4. Temperature Response
2.5. Level of Bacteremia
2.6. Bacterial Load in Tissues
2.7. Anti-PA Antibody Response
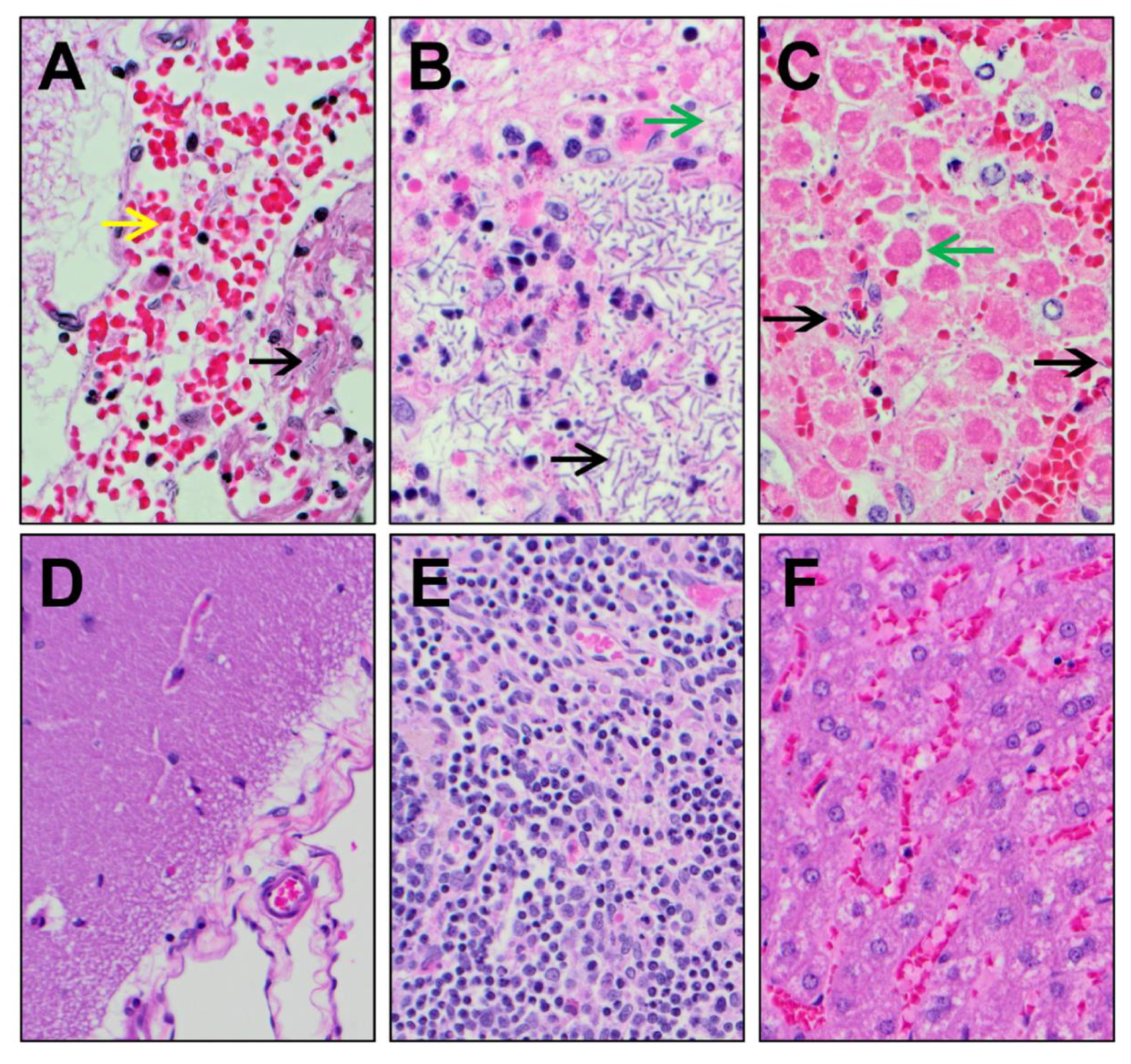
2.8. Necropsy and Histopathology
3. Discussion
4. Materials and Methods
4.1. Bacteria
4.2. Animal Experiments
4.3. Protective Antigen Detection
4.4. Assessment of Bacteremia and Bacterial Load
4.5. Quantitation of Serum Anti-PA Antibody
4.6. Necropsy and Histopathology
4.7. Statistics
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borio, L.; Frank, D.; Mani, V.; Chiriboga, C.; Pollanen, M.; Ripple, M.; Ali, S.; DiAngelo, C.; Lee, J.; Arden, J.; et al. Death due to bioterrorism-related inhalational anthrax: Report of 2 patients. J. Am. Med. Assoc. 2001, 286, 2554–2559. [Google Scholar] [CrossRef] [Green Version]
- Jernigan, J.A.; Stephens, D.S.; Ashford, D.A.; Omenaca, C.; Topiel, M.S.; Galbraith, M.; Tapper, M.; Fisk, T.L.; Zaki, S.; Popovic, T.; et al. Bioterrorism-related inhalational anthrax: The first 10 cases reported in the United States. Emerg. Infect. Dis. 2001, 7, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Savransky, V.; Ionin, B.; Reece, J. Current status and trends in prophylaxis and management of anthrax disease. Pathogens 2020, 9, 370. [Google Scholar] [CrossRef]
- Brossier, F.; Mock, M. Toxins of Bacillus anthracis. Toxicon 2001, 39, 1747–1755. [Google Scholar] [CrossRef]
- Duesbery, N.S.; Woude, G.F.V. Anthrax toxins. Cell. Mol. Life Sci. 1999, 55, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Mock, M.; Fouet, A. Anthrax. Annu. Rev. Microbiol. 2001, 55, 647–671. [Google Scholar] [CrossRef]
- Agrawal, A.; Pulendran, B. Anthrax lethal toxin: A weapon of multisystem destruction. Cell. Mol. Life. Sci. 2004, 61, 2859–2865. [Google Scholar] [CrossRef]
- Tournier, J.N.; Paccani, S.R.; Quesnel-Hellman, A.; Baldari, C.T. Anthrax toxins: A weapon to systematically dismantle the host immune defenses. Mol. Aspects Med. 2009, 30, 456–466. [Google Scholar] [CrossRef]
- Greenfield, R.A.; Bronze, M.S. Prevention and treatment of bacterial diseases caused by bacterial bioterrorism threat agents. Drug Discov. Today 2003, 8, 881–888. [Google Scholar] [CrossRef]
- Beierlein, J.M.; Anderson, A.C. New developments in vaccines, inhibitors of anthrax toxins, and antibiotic therapeutics for Bacillus anthracis. Curr. Med. Chem. 2011, 18, 5083–5094. [Google Scholar] [CrossRef]
- Brouillard, J.E.; Terriff, C.M.; Tofan, A.; Garrison, M.W. Antibiotic selection and resistance issues with fluoroquinolones and doxycycline against bioterrorism agents. Pharmacother. J. Hum. Phapyrmacol. Drug Ther. 2006, 26, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schaberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.W. Teixobactin: A novel anti-infective agent. Expert Rev. Anti. Infect. Ther. 2019, 17, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J.V. Teixobactin, the first of a new class of antibiotics discovered by iChip technology? J. Antimicrob. Chemother. 2015, 70, 2679–2680. [Google Scholar] [CrossRef]
- Sherpa, R.T.; Reese, C.J.; Aliabadi, H.M. Application of iChip to grow “uncultivable” microorganisms and its impact on antibiotic discovery. J. Pharm. Pharm. Sci. 2015, 18, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Y.; Chan-Park, M.B.; Mu, Y. Binding modes of Teixobactin to lipid II: Molecular dynamics study. Sci. Rep. 2017, 7, 17197. [Google Scholar] [CrossRef]
- Homma, T.; Nuxoll, A.; Gandt, A.B.; Ebner, P.; Engels, I.; Schneider, T.; Gotz, F.; Lewis, K.; Conlon, B.P. Dual targeting of cell wall precursors by Teixobactin leads to cell lysis. Antimicrob. Agents Chemother. 2016, 60, 6510–6517. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Mandalapu, D.; Ji, X.; Gao, J.; Zhang, Q. Chemistry and biology of Teixobactin. Chem. Eur. J. 2018, 24, 5406–5422. [Google Scholar] [CrossRef]
- Van Harten, R.M.; Willems, R.J.L.; Martin, N.I.; Hendrickx, A.P.A. Multidrug-resistant enterococcal infections: New compounds, novel antimicrobial therapies? . Trends Microbiol. 2017, 25, 467–479. [Google Scholar] [CrossRef]
- Van Heijenoort, J. Lipid intermediates in the biosynthesis of bacterial peptidoglycan. Microbiol. Mol. Biol. Rev. 2007, 71, 620–635. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.; Santa Maria, J.P., Jr.; Walker, S. Wall teichoic acids of gram-positive bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percy, M.G.; Gründling, A. Lipoteichoic acid synthesis and function in gram-positive bacteria. Annu. Rev. Microbiol. 2014, 68, 81–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swoboda, J.G.; Campbell, J.; Meredith, T.C.; Walker, S. Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem 2010, 11, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilligan, P.H. Therapeutic challenges posed by bacterial bioterrorism threats. Curr. Opin. Microbiol. 2002, 5, 489–495. [Google Scholar] [CrossRef]
- Goel, A.K. Anthrax: A disease of biowarfare and public health importance. World, J. Clin. Cases 2015, 3, 20–33. [Google Scholar] [CrossRef]
- Heine, H.S.; Shadomy, S.V.; Boyer, A.E.; Chuvala, L.; Riggins, R.; Kesterson, A.; Myrick, J.; Craig, J.; Candela, M.G.; Barr, J.R.; et al. Evaluation of combination drug therapy for treatment of antibiotic-resistant inhalation anthrax in a murine model. Antimicrob. Agents Chemother. 2017, 61, e00788-17. [Google Scholar] [CrossRef] [Green Version]
- Riedel, S. Anthrax: A continuing concern in the era of bioterrorism. Proc. Bayl. Univ. Med. Cent. 2005, 18, 234–243. [Google Scholar] [CrossRef]
- Products Approved for Anthrax. Available online: https://www.fda.gov/drugs/bioterrorism-and-drug-preparedness/products-approved-anthrax. (accessed on 10 August 2020).
- National Preparedness: DHS and HHS Can Further Strengthen Coordination for Chemical, Biological, Radiological, and Nuclear Risk Assessments. Available online: https://www.gao.gov/new.items/d11606.pdf. (accessed on 10 August 2020).
- Heine, H.S.; Bassett, J.; Miller, L.; Hartings, J.M.; Ivins, B.E.; Pitt, M.L.; Fritz, D.; Norris, S.L.; Byrne, W.R. Determination of antibiotic efficacy against Bacillus anthracis in a mouse aerosol challenge model. Antimicrob. Agents Chemother. 2007, 51, 1373–1379. [Google Scholar] [CrossRef] [Green Version]
- Henderson, D.W.; Peacock, S.; Belton, F.C. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. Epidemiol. Infect. 1956, 54, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Loving, C.L.; Kennett, M.; Lee, G.M.; Grippe, V.K.; Merkel, T.J. Murine aerosol challenge model of anthrax. Infect. Immun. 2007, 75, 2689–2698. [Google Scholar] [CrossRef] [Green Version]
- Friedlander, A.M.; Welkos, S.L.; Pitt, M.L.M.; Ezzell, J.W.; Worsham, P.L.; Rose, K.J.; Ivins, B.E.; Lowe, J.R.; Howe, G.B.; Mikesell, P.; et al. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 1993, 167, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Bower, W.A.; Schiffer, J.; Atmar, R.L.; Keitel, W.A.; Friedlander, A.M.; Liu, L.; Yu, Y.; Stephens, D.S.; Quinn, C.P.; Hendricks, K. Use of Anthrax Vaccine in the United States: Recommendations of the Advisory Committee on Immunization Practices, 2019. MMWR Recomm. Rep. 2019, 68, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeCubellis, J.; Graham, J. Gastrointestinal disease in guinea pigs and rabbits. Vet. Clin. North Am. Exot. Anim. Pract. 2013, 16, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Borriello, S.P. Clostridial disease of the gut. Clin. Infect. Dis. 1995, 20, S242–S250. [Google Scholar] [CrossRef]



| Treatment Group | Animal Identification Number | Time of PA Detection (Time Post-Infection) | Concentration of PA (ng/mL) |
|---|---|---|---|
| Teixobactin | 4490 | 18 h | 0.230 |
| 4493 | 24 h | 1.000 | |
| 4497 | 36 h | 6.009 | |
| 4498 | 24 h | 0.302 | |
| 44873 | 18 h | 0.566 | |
| 44881 | 18 h | 1.180 | |
| 44882 | 24 h | 0.170 | |
| Levofloxacin | 4499 | 24 h | 0.312 |
| 44874 | 18 h | 0.419 | |
| Negative control | 4494 | 12 h | 0.099 |
| 4496 | 30 h | 0.360 | |
| 44872 | 24 h | 0.817 | |
| 44879 | 18 h | 0.920 |
| Treatment Group | Animal No. | Bacteremia Level (cfu/mL) | |||
|---|---|---|---|---|---|
| 24 h PI | 24 h PT * | 48 h PT * | Terminal | ||
| Teixobactin | 4490 | 0 | 0 | 0 | 0 |
| 4493 | 1.27 × 102 | 0 | 0 | 0 | |
| 4497 | 0 | 0 | 0 | 0 | |
| 4498 | 0 | 2.00 × 101 | 0 | 0 | |
| 44873 | 5.12 × 104 | 0 | 0 | 0 | |
| 44881 | 5.23 × 104 | 0 | 0 | 0 | |
| 44882 | 0 | 0 | 0 | 0 | |
| Levofloxacin | 4499 | 0 | 0 | 0 | 0 |
| 44874 | 1.74 × 104 | 0 | 0 | 0 | |
| Negative control | 4494 | 2.63 × 104 | 1.03 × 106 | euthanized | 1.09 × 108 |
| 4496 | 0 | 6.90 × 102 | 2.15 × 104 | 4.12 × 107 | |
| 44872 | 0 | 8.00 × 103 | euthanized | 3.23 × 103 # | |
| 44879 | 7.35 × 104 | 8.57 × 106 | euthanized | 7.30 × 107 | |
| Animal No. | Bacterial Load (cfu/g) | |||
|---|---|---|---|---|
| Lung | Brain | Lymph Node | Spleen | |
| 4494 | 6.96 × 104 | 3.54 × 105 | 1.16 × 106 | 9.35 × 106 |
| 4496 | 7.90 × 103 | 4.80 × 104 | 2.56 × 107 | 1.49 × 104 |
| 44872 | 2.26 × 104 | 6.83 × 106 | 2.94 × 106 | 1.42 × 103 |
| 44879 | 9.40 × 106 | 5.46 × 106 | 2.44 × 107 | 1.84 × 106 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrence, W.S.; Peel, J.E.; Sivasubramani, S.K.; Baze, W.B.; Whorton, E.B.; Beasley, D.W.C.; Comer, J.E.; Hughes, D.E.; Ling, L.L.; Peterson, J.W. Teixobactin Provides Protection against Inhalation Anthrax in the Rabbit Model. Pathogens 2020, 9, 773. https://doi.org/10.3390/pathogens9090773
Lawrence WS, Peel JE, Sivasubramani SK, Baze WB, Whorton EB, Beasley DWC, Comer JE, Hughes DE, Ling LL, Peterson JW. Teixobactin Provides Protection against Inhalation Anthrax in the Rabbit Model. Pathogens. 2020; 9(9):773. https://doi.org/10.3390/pathogens9090773
Chicago/Turabian StyleLawrence, William S., Jennifer E. Peel, Satheesh K. Sivasubramani, Wallace B. Baze, Elbert B. Whorton, David W. C. Beasley, Jason E. Comer, Dallas E. Hughes, Losee L. Ling, and Johnny W. Peterson. 2020. "Teixobactin Provides Protection against Inhalation Anthrax in the Rabbit Model" Pathogens 9, no. 9: 773. https://doi.org/10.3390/pathogens9090773
APA StyleLawrence, W. S., Peel, J. E., Sivasubramani, S. K., Baze, W. B., Whorton, E. B., Beasley, D. W. C., Comer, J. E., Hughes, D. E., Ling, L. L., & Peterson, J. W. (2020). Teixobactin Provides Protection against Inhalation Anthrax in the Rabbit Model. Pathogens, 9(9), 773. https://doi.org/10.3390/pathogens9090773





