MCI Detection Using Kernel Eigen-Relative-Power Features of EEG Signals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. EEG Recording and Data Collection
2.3. Feature Extraction
2.3.1. Relative Power (RP)
2.3.2. Optimal Relative Power Feature Selection
2.3.3. Kernel Eigen-Relative-Power (KERP) Extraction
2.4. Classification and Parameter Optimization
3. Results and Discussion
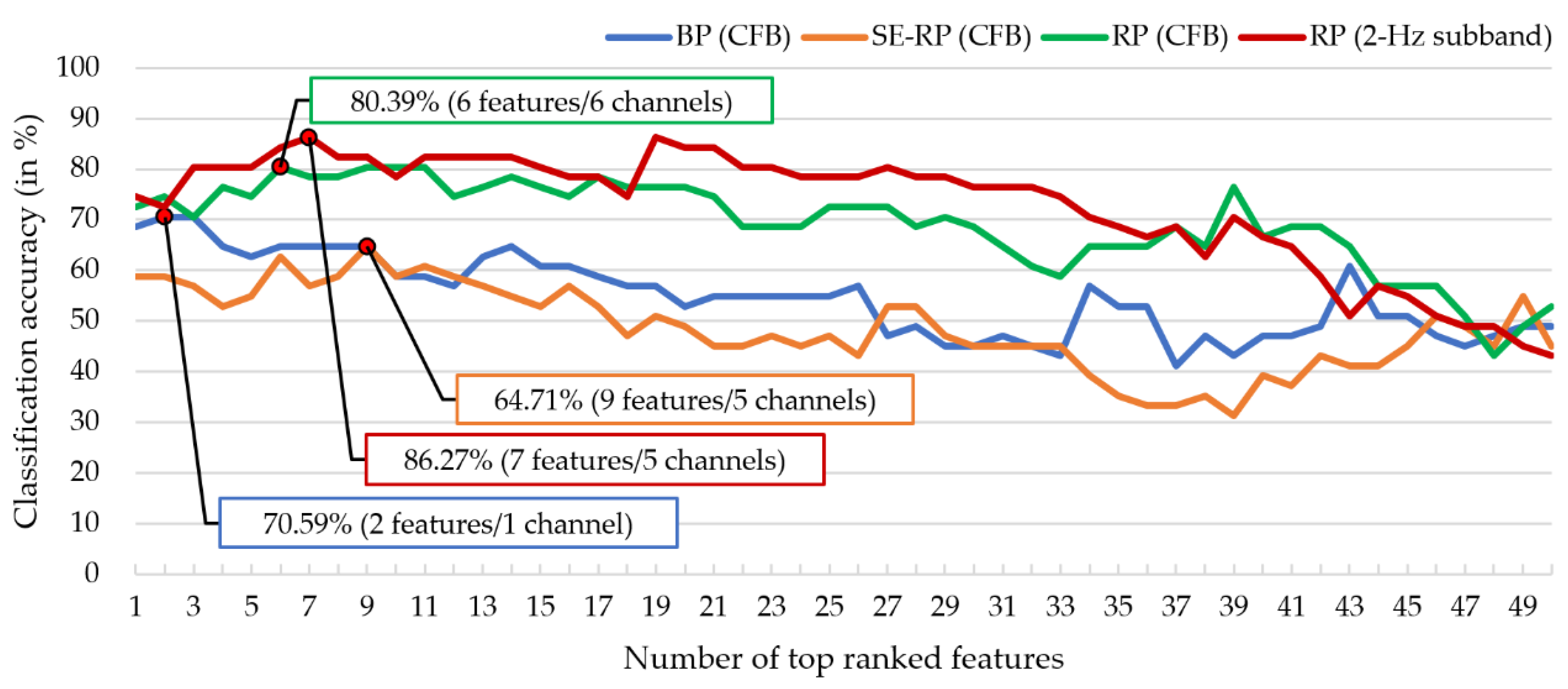
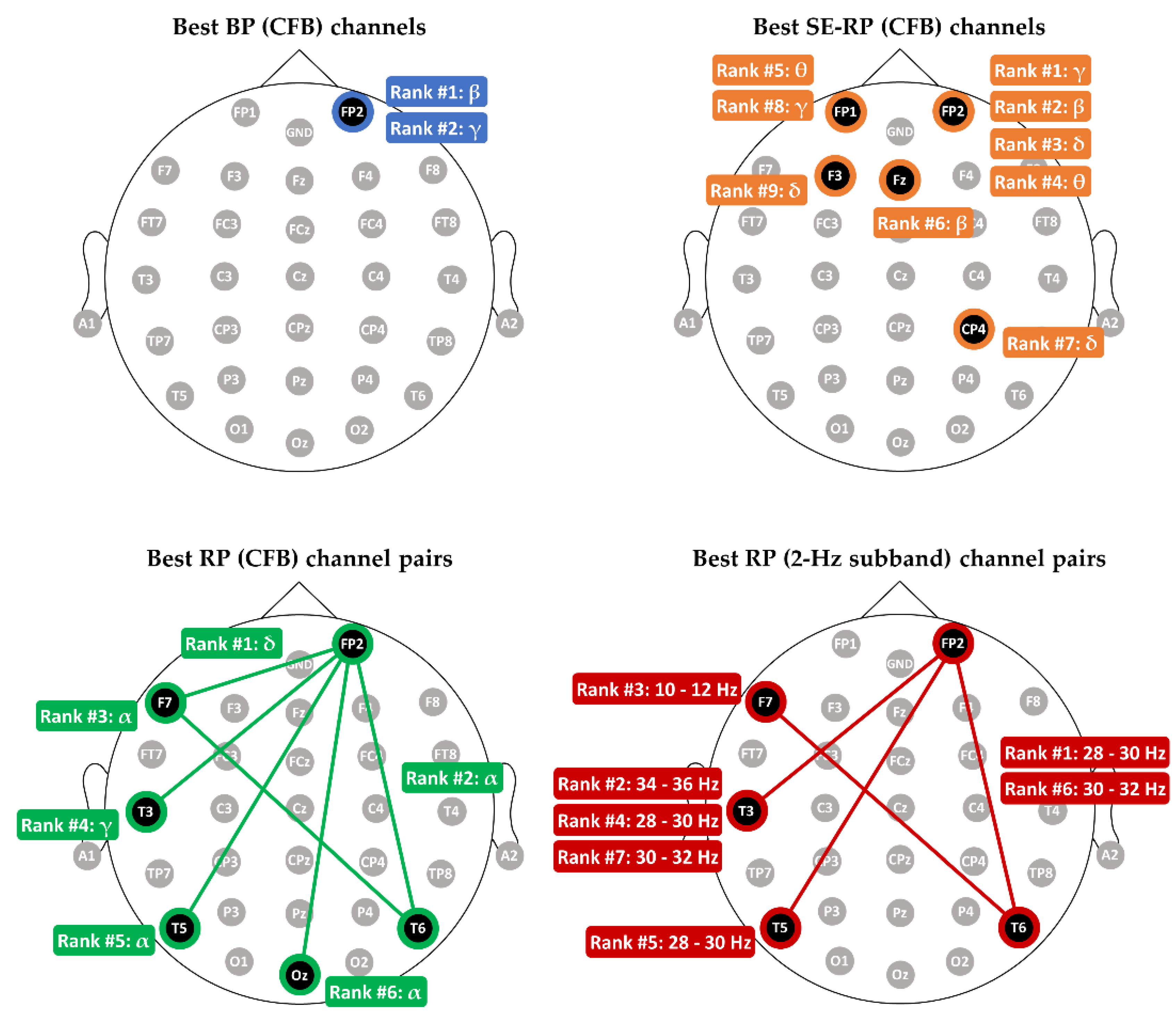
3.1. Comparing Relative Power with other Spectral Features in MCI-HC Classification
- (1)
- BP (CFB): number of features is 150 ();
- (2)
- SE-RP (CFB): (), where the 5 RPs for each electrode are -RP, -RP, -RP, -RP, and -RP, and, for example, -RP is the ratio of delta power to the total power of 1–44 Hz;
- (3)
- RP (CFB): ();
- (4)
- RP (2-Hz subband): ().
3.2. Comparing Classification Performance between Different Scalp Regions and Frequency Bands
- (1)
- For a specific frequency band and a scalp region. Take frontal BP as an example. We extracted the BP features from the seven electrodes’ EEG signals from each participant and fed the seven BPs into the LDA classifier. The classification accuracy is 54.90%. Then, we performed the feature selection task on the seven BPs. The selected optimal BPs achieved a slightly higher accuracy of 56.86%.
- (2)
- For the term “merged”. Take frontal BP as an example. We extracted the BPs of five conventional frequency bands from the EEG signals of the seven electrodes over the frontal scalp region from each participant. Then, feeding the 35 BP features into LDA classifier achieves an accuracy of 66.67%. After performing the feature selection on the 35 BP features, we again fed the selected optimal ones into LDA for classification and obtained an accuracy of 70.59%.
3.3. Comparing the Accuracies between Different Classifiers with KERP Feature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Vinters, H.V. Emerging concepts in Alzheimer’s disease. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 291–319. [Google Scholar] [CrossRef]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.D.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice guideline update summary: Mild cognitive impairment. Neurology 2017, 90, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Tardiff, S.; Dye, C.; Arrighi, H.M. Rate of Conversion from Prodromal Alzheimer’s Disease to Alzheimer’s Dementia: A Systematic Review of the Literature. Dement. Geriatr. Cogn. Disord. Extra 2013, 3, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Rodakowski, J.; Saghafi, E.; Butters, M.A.; Skidmore, E.R. Non-pharmacological interventions for adults with mild cognitive impairment and early stage dementia: An updated scoping review. Mol. Asp. Med. 2015, 43, 38–53. [Google Scholar] [CrossRef] [Green Version]
- Balietti, M.; Giuli, C.; Fattoretti, P.; Fabbietti, P.; Postacchini, D.; Conti, F. Cognitive Stimulation Modulates Platelet Total Phospholipases A2 Activity in Subjects with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2016, 50, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Sherman, D.S.; Mauser, J.; Nuno, M.; Sherzai, D. The Efficacy of Cognitive Intervention in Mild Cognitive Impairment (MCI): A Meta-Analysis of Outcomes on Neuropsychological Measures. Neuropsychol. Rev. 2017, 27, 440–484. [Google Scholar] [CrossRef] [Green Version]
- Khatun, S.; Morshed, B.I.; Bidelman, G.M. A Single-Channel EEG-Based Approach to Detect Mild Cognitive Impairment via Speech-Evoked Brain Responses. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1063–1070. [Google Scholar] [CrossRef]
- Suk, H.-I.; Lee, S.-W.; Shen, D. Latent feature representation with stacked auto-encoder for AD/MCI diagnosis. Brain Struct. Funct. 2015, 220, 841–859. [Google Scholar] [CrossRef]
- Ni, H.; Qin, J.; Zhou, L.; Zhao, Z.; Wang, J.; Hou, F. Network analysis in detection of early-stage mild cognitive impairment. Phys. A Stat. Mech. Appl. 2017, 478, 113–119. [Google Scholar] [CrossRef]
- Grunwald, M.; Busse, F.; Hensel, A.; Riedel-Heller, S.; Kruggel, F.; Arendt, T.; Wolf, H.; Gertz, H.-J. Theta-power Differences in Patients with Mild Cognitive Impairment Under Rest Condition and During Haptic Tasks. Alzheimer Dis. Assoc. Disord. 2002, 16, 40–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretti, D.V.; Miniussi, C.; Frisoni, G.; Zanetti, O.; Binetti, G.; Geroldi, C.; Galluzzi, S.; Rossini, P.M. Vascular damage and EEG markers in subjects with mild cognitive impairment. Clin. Neurophysiol. 2007, 118, 1866–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossini, P.M.; Buscema, M.; Capriotti, M.; Grossi, E.; Rodriguez, G.; Del Percio, C.; Babiloni, C. Is it possible to automatically distinguish resting EEG data of normal elderly vs. mild cognitive impairment subjects with high degree of accuracy? Clin. Neurophysiol. 2008, 119, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Prichep, L.S.; John, E.R.; Ferris, S.H.; Rausch, L.; Fang, Z.; Cancro, R.; Torossian, C.; Reisberg, B. Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiol. Aging 2006, 27, 471–481. [Google Scholar] [CrossRef]
- Yener, G.G.; Emek-Savaş, D.D.; Lizio, R.; Çavuşoğlu, B.; Carducci, F.; Ada, E.; Güntekin, B.; Babiloni, C.C.; Başar, E. Frontal delta event-related oscillations relate to frontal volume in mild cognitive impairment and healthy controls. Int. J. Psychophysiol. 2016, 103, 110–117. [Google Scholar] [CrossRef]
- Kashefpoor, M.; Rabbani, H.; Barekatain, M. Automatic diagnosis of mild cognitive impairment using electroencephalogram spectral features. J. Med. Signals Sens. 2016, 6, 25–32. [Google Scholar] [CrossRef]
- Kashefpoor, M.; Rabbani, H.; Barekatain, M. Supervised dictionary learning of EEG signals for mild cognitive impairment diagnosis. Biomed. Signal Process. Control 2019, 53, 101559. [Google Scholar] [CrossRef]
- Ruiz-Gómez, S.; Gómez, C.; Poza, J.; Gutiérrez-Tobal, G.; Tola-Arribas, M.; Cano, M.; Hornero, R. Automated Multiclass Classification of Spontaneous EEG Activity in Alzheimer’s Disease and Mild Cognitive Impairment. Entropy 2018, 20, 35. [Google Scholar] [CrossRef] [Green Version]
- Musaeus, C.S.; Engedal, K.; Høgh, P.; Jelic, V.; Mørup, M.; Naik, M.; Oeksengaard, A.-R.; Snaedal, J.; Wahlund, L.-O.; Waldemar, G.; et al. EEG Theta Power Is an Early Marker of Cognitive Decline in Dementia due to Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 1359–1371. [Google Scholar] [CrossRef]
- Farina, F.R.; Emek-Savaş, D.D.; Rueda-Delgado, L.; Boyle, R.; Kiiski, H.; Yener, G.; Whelan, R. A comparison of resting state EEG and structural MRI for classifying Alzheimer’s disease and mild cognitive impairment. Neuroimage 2020, 215, 116795. [Google Scholar] [CrossRef]
- Ieracitano, C.; Mammone, N.; Bramanti, A.; Hussain, A.; Morabito, F.C. A Convolutional Neural Network approach for classification of dementia stages based on 2D-spectral representation of EEG recordings. Neurocomputing 2019, 323, 96–107. [Google Scholar] [CrossRef]
- Abásolo, D.; Hornero, R.; Gómez, C.; García, M.; López, M. Analysis of EEG background activity in Alzheimer’s disease patients with Lempel–Ziv complexity and central tendency measure. Med. Eng. Phys. 2006, 28, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abásolo, D.; Escudero, J.; Hornero, R.; Gómez, C.; Espino, P. Approximate entropy and auto mutual information analysis of the electroencephalogram in Alzheimer’s disease patients. Med. Biol. Eng. Comput. 2008, 46, 1019–1028. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Cai, L.; Wang, J.; Wang, R.; Yu, H.; Cao, Y.; Liu, J. Characterization of complexity in the electroencephalograph activity of Alzheimer’s disease based on fuzzy entropy. Chaos Interdiscip. J. Nonlinear Sci. 2015, 25, 083116. [Google Scholar] [CrossRef] [PubMed]
- Maturana-Candelas, A.; Gómez, C.; Poza, J.; Pinto, N.; Hornero, R. EEG Characterization of the Alzheimer’s Disease Continuum by Means of Multiscale Entropies. Entropy 2019, 21, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Kolekar, M.H.; Jha, K.; Kumar, Y. EEG and Cognitive Biomarkers Based Mild Cognitive Impairment Diagnosis. IRBM 2018, 40, 113–121. [Google Scholar] [CrossRef]
- Cicalese, P.A.; Li, R.; Ahmadi, M.B.; Wang, C.; Francis, J.T.; Selvaraj, S.; Schulz, P.E.; Zhang, Y. An EEG-fNIRS hybridization technique in the four-class classification of Alzheimer’s disease. J. Neurosci. Methods 2020, 336, 108618. [Google Scholar] [CrossRef]
- Dolcos, F.; Rice, H.J.; Cabeza, R. Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neurosci. Biobehav. Rev. 2002, 26, 819–825. [Google Scholar] [CrossRef]
- Long, X.; Zhang, L.; Liao, W.; Jiang, C.; Qiu, B. Distinct laterality alterations distinguish mild cognitive impairment and Alzheimer’s disease from healthy aging: Statistical parametric mapping with high resolution MRI. Hum. Brain Mapp. 2013, 34, 3400–3410. [Google Scholar] [CrossRef]
- Cottone, C.; Tomasevic, L.; Porcaro, C.; Filligoi, G.; Tecchio, F. Physiological Aging Impacts the Hemispheric Balances of Resting State Primary Somatosensory Activities. Brain Topogr. 2013, 26, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Smits, F.M.; Porcaro, C.; Cottone, C.; Cancelli, A.; Rossini, P.M.; Tecchio, F. Electroencephalographic Fractal Dimension in Healthy Ageing and Alzheimer’s Disease. PLoS ONE 2016, 11, e0149587. [Google Scholar] [CrossRef]
- John, T.N.; Dharmapalan, P.S.; Menon, N.P. Exploration of time–frequency reassignment and homologous inter-hemispheric asymmetry analysis of MCI–AD brain activity. BMC Neurosci. 2019, 20, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Knott, V.; Mahoney, C.; Kennedy, S.; Evans, K. EEG power, frequency, asymmetry and coherence in male depression. Psychiatry Res. Neuroimaging 2001, 106, 123–140. [Google Scholar] [CrossRef]
- Segrave, R.A.; Cooper, N.R.; Thomson, R.H.; Croft, R.J.; Sheppard, D.M.; Fitzgerald, P.B. Individualized Alpha Activity and Frontal Asymmetry in Major Depression. Clin. EEG Neurosci. 2011, 42, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-T.; Dillon, D.; Hsu, H.-C.; Huang, S.; Barrick, E.; Liu, Y.-H. Depression Detection Using Relative EEG Power Induced by Emotionally Positive Images and a Conformal Kernel Support Vector Machine. Appl. Sci. 2018, 8, 1244. [Google Scholar] [CrossRef]
- Fang, L.; Zhao, H.; Wang, P.; Yu, M.; Yan, J.; Cheng, W.; Chen, P. Feature selection method based on mutual information and class separability for dimension reduction in multidimensional time series for clinical data. Biomed. Signal Process. Control 2015, 21, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-H.; Huang, S.; Huang, H.-C.; Peng, W.-H. Novel Motor Imagery-Based Brain Switch for Patients with Amyotrophic Lateral Sclerosis: A Case Study Using Two-Channel Electroencephalography. IEEE Consum. Electron. Mag. 2019, 8, 72–77. [Google Scholar] [CrossRef]
- Lin, Y.P.; Wang, C.H.; Jung, T.P.; Wu, T.L.; Jeng, S.K.; Duann, J.R.; Chen, J.H. EEG-based emotion recognition in music listening. IEEE Trans. Biomed. Eng. 2010, 57, 1798–1806. [Google Scholar] [CrossRef]
- Schölkopf, B.; Smola, A.; Müller, K.-R. Nonlinear Component Analysis as a Kernel Eigenvalue Problem. Neural Comput. 1998, 10, 1299–1319. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-H.; Wu, C.-T.; Cheng, W.-T.; Hsiao, Y.-T.; Chen, P.-M.; Teng, J.-T. Emotion Recognition from Single-Trial EEG Based on Kernel Fisher’s Emotion Pattern and Imbalanced Quasiconformal Kernel Support Vector Machine. Sensors 2014, 14, 13361–13388. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.-C.; Wu, C.-T.; Huang, H.-C.; Cheng, W.-T.; Liu, Y.-H. Major Depression Detection from EEG Signals Using Kernel Eigen-Filter-Bank Common Spatial Patterns. Sensors 2017, 17, 1385. [Google Scholar] [CrossRef] [Green Version]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.-F.; Lee, W.-J.; Wang, S.-J.; Shia, B.-C.; Nasreddine, Z.; Fuh, J.-L. Psychometrics of the Montreal Cognitive Assessment (MoCA) and its subscales: Validation of the Taiwanese version of the MoCA and an item response theory analysis. Int. Psychogeriatr. 2012, 24, 651–658. [Google Scholar] [CrossRef]
- Neuroscan FAQs. Available online: http://compumedicsneuroscan.com/wp-content/uploads/3502D-Neuroscan-FAQs.pdf (accessed on 3 July 2021).
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Mognon, A.; Jovicich, J.; Bruzzone, L.; Buiatti, M. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology 2011, 48, 229–240. [Google Scholar] [CrossRef]
- Jović, A.; Brkić, K.; Bogunović, N. A review of feature selection methods with applications. In Proceedings of the 2015 38th International Conference on Information and Communication Technology, Electronics and Microelectronics, Opatija, Croatia, 25–29 May 2015. [Google Scholar]
- You, W.; Yang, Z.; Ji, G. Feature selection for high-dimensional multi-category data using PLS-based local recursive feature elimination. Expert Syst. Appl. 2014, 41, 1463–1475. [Google Scholar] [CrossRef]
- Yeh, S.C.; Hou, C.L.; Peng, W.H.; Wei, Z.Z.; Huang, S.; Kung, E.Y.C.; Lin, L.; Liu, Y.H. A multiplayer online car racing virtual-reality game based on internet of brains. J. Syst. Archit. 2018, 89, 30–40. [Google Scholar] [CrossRef]




| EEG Features | Frontal | Central | Temporal | Parietal | Occipital | Entire | |
|---|---|---|---|---|---|---|---|
| BP (CFB) | δ | 56.86 (54.90) | 60.78 (60.78) | 50.98 (45.10) | 50.98 (45.10) | 47.06 (43.14) | 56.86 (43.14) |
| θ | 58.82 (56.86) | 52.94 (49.02) | 58.82 (56.86) | 54.90 (49.02) | 56.86 (47.06) | 58.82 (39.22) | |
| α | 54.90 (52.94) | 56.86 (52.94) | 58.82 (54.90) | 56.86 (54.90) | 54.90 (50.98) | 54.90 (47.06) | |
| β | 68.63 (66.67) | 70.59 (68.63) | 62.75 (54.90) | 54.90 (47.06) | 43.14 (37.25) | 68.63 (47.06) | |
| γ | 68.63 (64.71) | 58.82 (50.98) | 66.67 (54.90) | 58.82 (50.98) | 47.06 (45.10) | 68.63 (45.10) | |
| SE-RP (CFB) | δ | 68.63 (68.63) | 56.86 (49.02) | 54.90 (52.94) | 64.71 (49.02) | 45.10 (39.22) | 60.78 (37.25) |
| θ | 60.78 (58.82) | 62.75 (52.94) | 45.10 (37.25) | 62.75 (58.82) | 49.02 (33.33) | 64.71 (33.33) | |
| α | 56.86 (49.02) | 52.94 (50.98) | 54.90 (50.98) | 62.75 (58.82) | 58.82 (50.98) | 72.55 (68.63) | |
| β | 70.59 (70.59) | 52.94 (39.22) | 54.90 (52.94) | 50.98 (47.06) | 45.10 (45.10) | 68.63 (64.71) | |
| γ | 70.59 (70.59) | 54.90 (52.94) | 62.75 (62.75) | 54.90 (47.06) | 49.02 (35.29) | 70.59 (56.86) | |
| RP (CFB) | δ | 76.47 (60.78) | 62.75 (41.18) | 68.63 (47.06) | 64.71 (60.78) | 52.94 (52.94) | 78.43 (56.86) |
| θ | 70.59 (47.06) | 72.55 (62.75) | 62.75 (41.18) | 64.71 (62.75) | 50.98 (45.10) | 64.71 (45.10) | |
| α | 66.67 (56.86) | 60.78 (49.02) | 72.55 (58.82) | 66.67 (66.67) | 50.98 (43.14) | 76.47 (64.71) | |
| β | 70.59 (50.98) | 50.98 (33.33) | 60.78 (47.06) | 62.75 (37.25) | 50.98 (50.98) | 74.51 (50.98) | |
| γ | 70.59 (68.63) | 62.75 (58.82) | 70.59 (66.67) | 60.78 (33.33) | 50.98 (37.25) | 72.55 (50.98) | |
| BP (merged CFB) | 70.59 (66.67) | 58.82 (45.10) | 60.78 (50.98) | 54.90 (43.14) | 52.94 (45.10) | 70.59 (52.94) | |
| SE-RP (merged CFB) | 68.63 (56.86) | 52.94 (43.14) | 68.63 (56.86) | 64.71 (41.18) | 54.90 (33.33) | 64.71 (50.98) | |
| RP (merged CFB) | 76.47 (60.78) | 64.71 (43.14) | 72.55 (49.02) | 66.67 (58.82) | 60.78 (50.98) | 80.39 (56.86) | |
| RP (merged subband) | 74.51 (47.06) | 72.55 (50.98) | 82.35 (64.71) | 66.67 (39.22) | 80.39 (58.82) | 86.27 (43.14) | |
| Classifier | Accuracy | Sensitivity | Specificity |
|---|---|---|---|
| LDA | 88.24 | 91.67 | 85.19 |
| QDA | 82.35 | 79.17 | 85.19 |
| k-NN | 76.47 | 75.00 | 77.78 |
| SVM | 90.20 | 87.50 | 92.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, Y.-T.; Tsai, C.-F.; Wu, C.-T.; Trinh, T.-T.; Lee, C.-Y.; Liu, Y.-H. MCI Detection Using Kernel Eigen-Relative-Power Features of EEG Signals. Actuators 2021, 10, 152. https://doi.org/10.3390/act10070152
Hsiao Y-T, Tsai C-F, Wu C-T, Trinh T-T, Lee C-Y, Liu Y-H. MCI Detection Using Kernel Eigen-Relative-Power Features of EEG Signals. Actuators. 2021; 10(7):152. https://doi.org/10.3390/act10070152
Chicago/Turabian StyleHsiao, Yu-Tsung, Chia-Fen Tsai, Chien-Te Wu, Thanh-Tung Trinh, Chun-Ying Lee, and Yi-Hung Liu. 2021. "MCI Detection Using Kernel Eigen-Relative-Power Features of EEG Signals" Actuators 10, no. 7: 152. https://doi.org/10.3390/act10070152
APA StyleHsiao, Y.-T., Tsai, C.-F., Wu, C.-T., Trinh, T.-T., Lee, C.-Y., & Liu, Y.-H. (2021). MCI Detection Using Kernel Eigen-Relative-Power Features of EEG Signals. Actuators, 10(7), 152. https://doi.org/10.3390/act10070152







