Implications of Spatially Constrained Bipennate Topology on Fluidic Artificial Muscle Bundle Actuation
Abstract
:1. Introduction
2. System Formulation
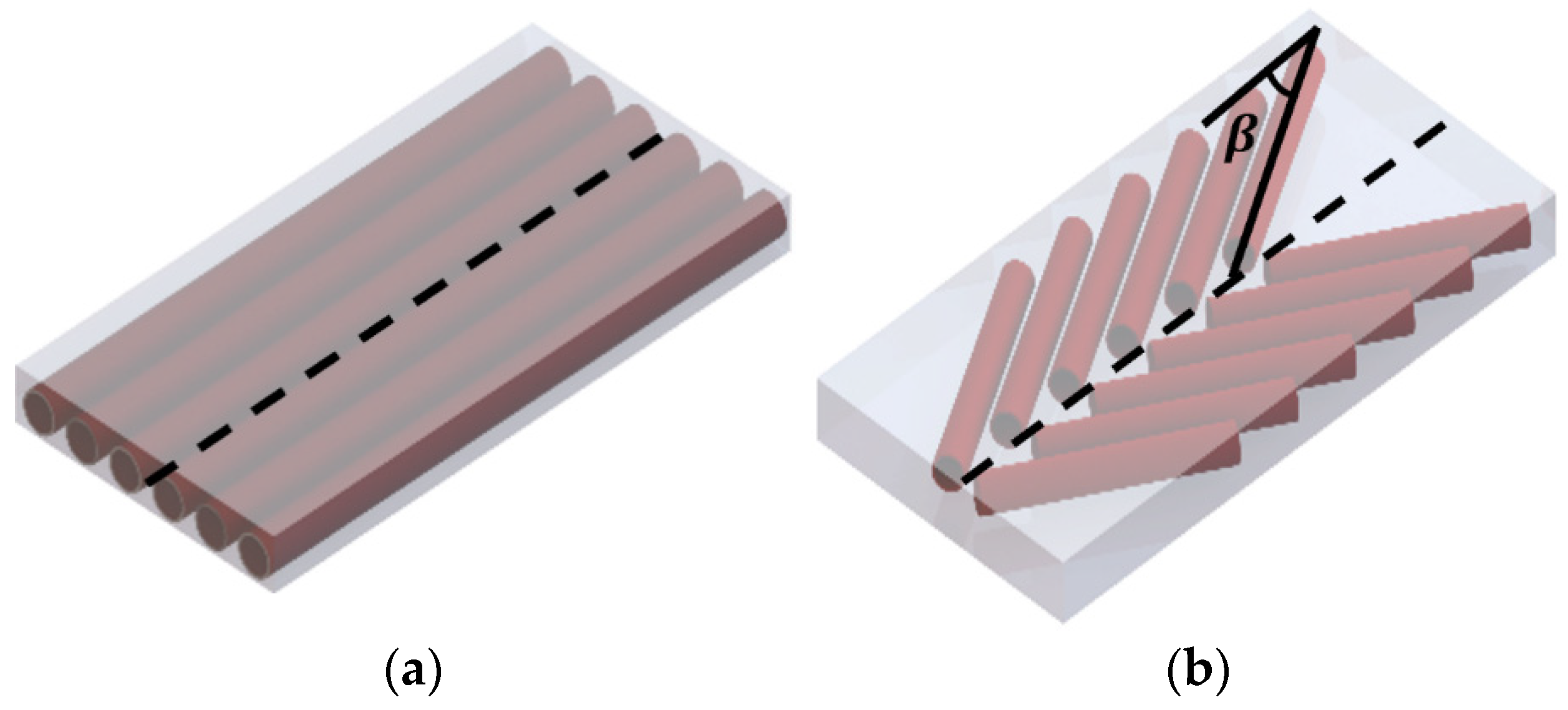
2.1. Muscle Topologies
2.2. Design Case and Fiber Boundary Conditions
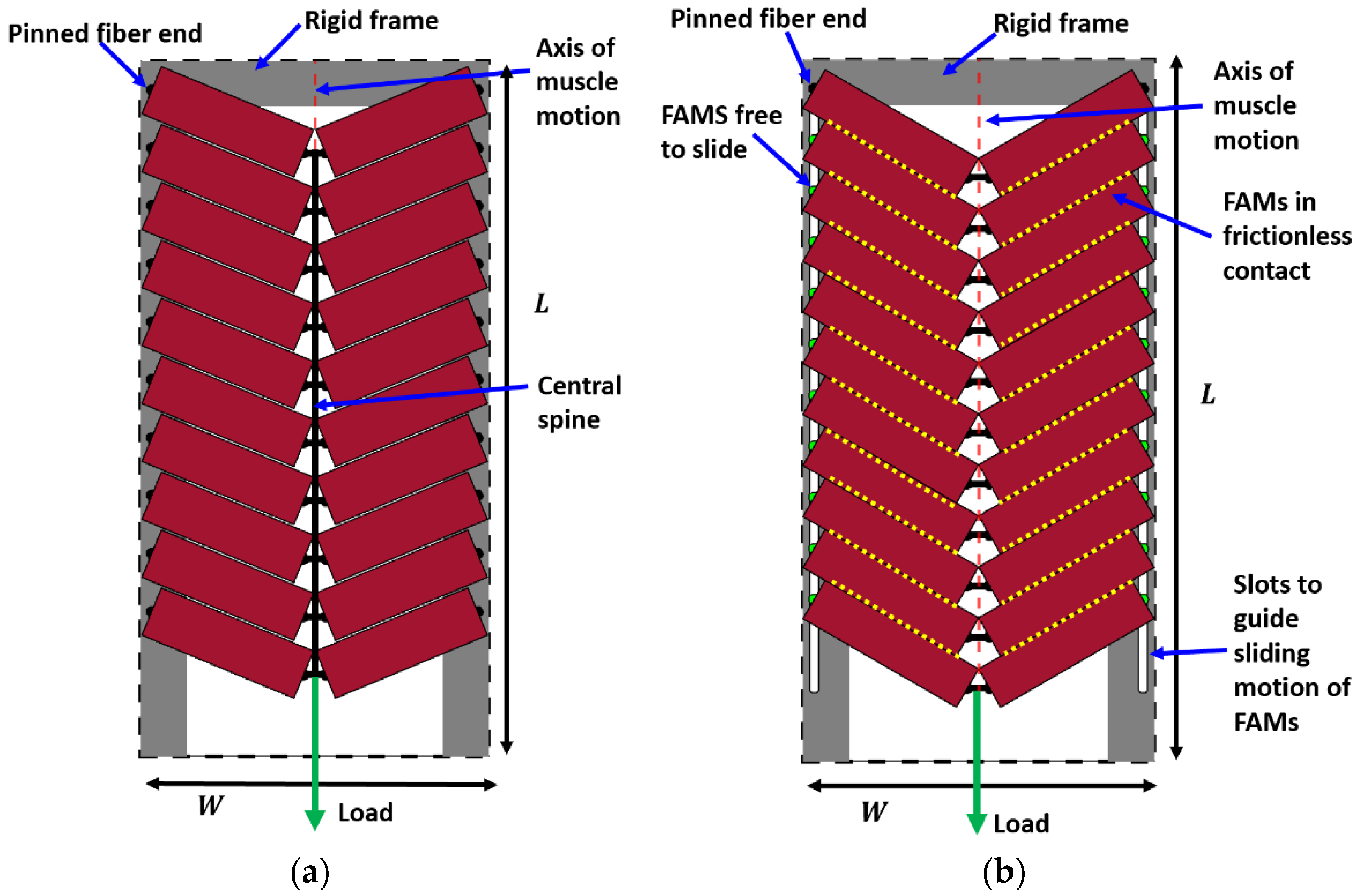
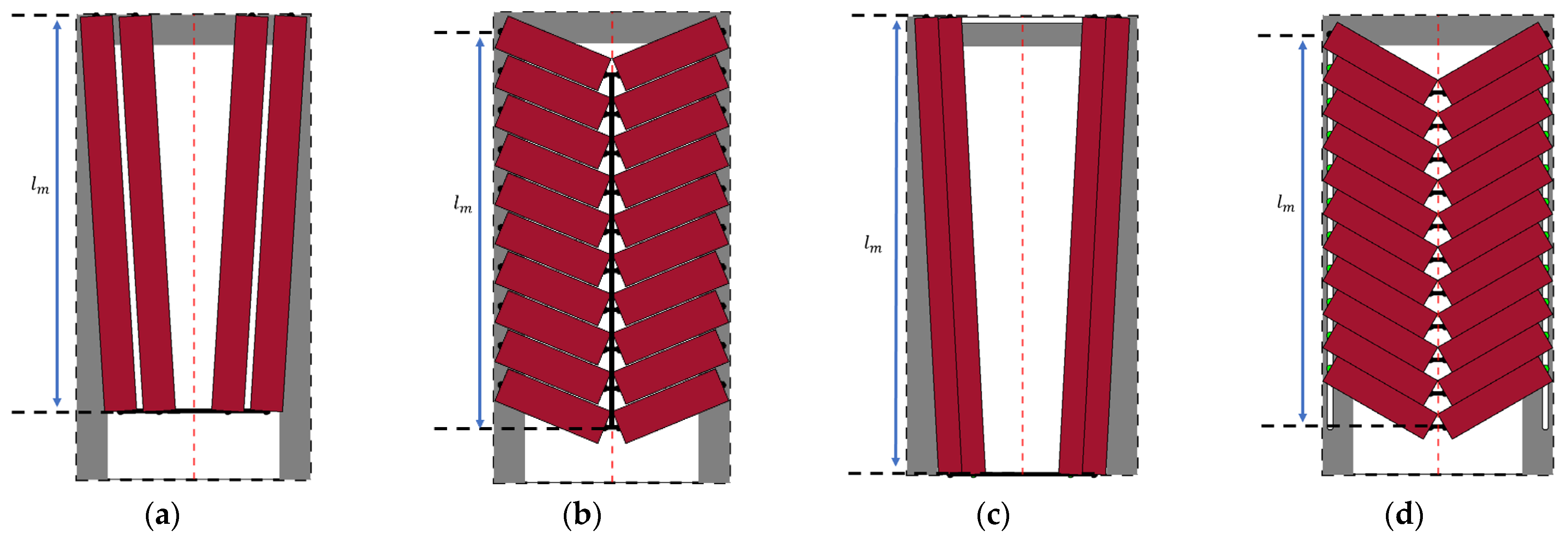
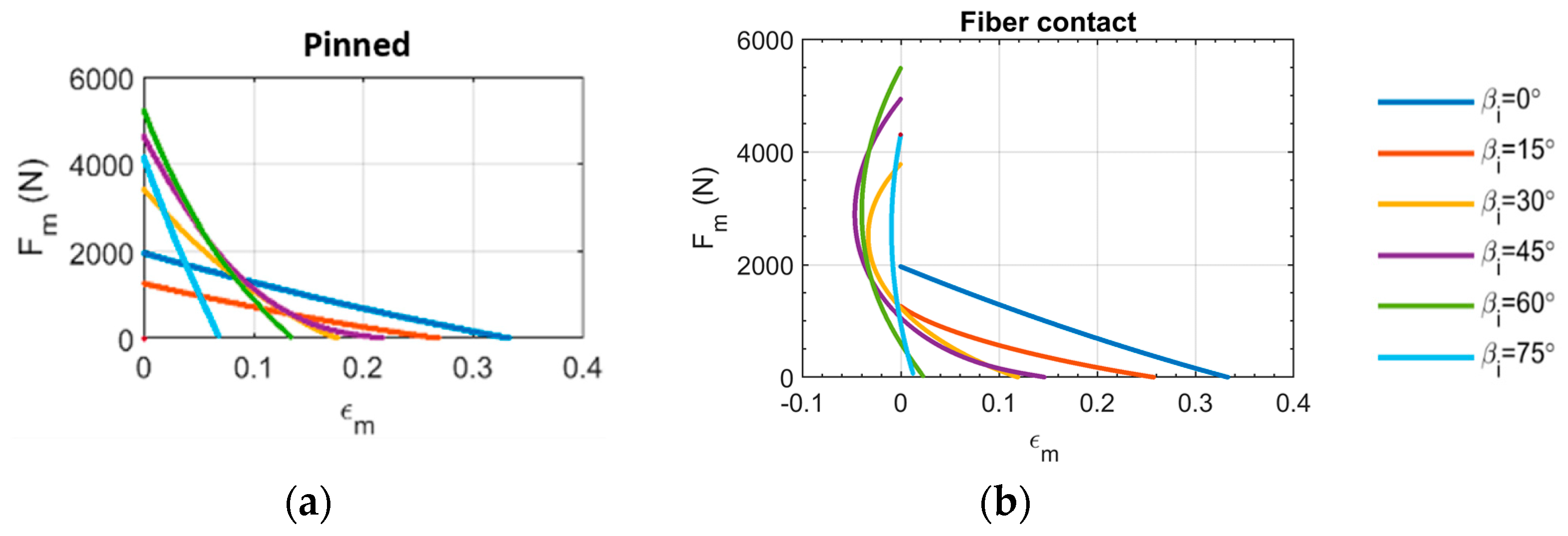
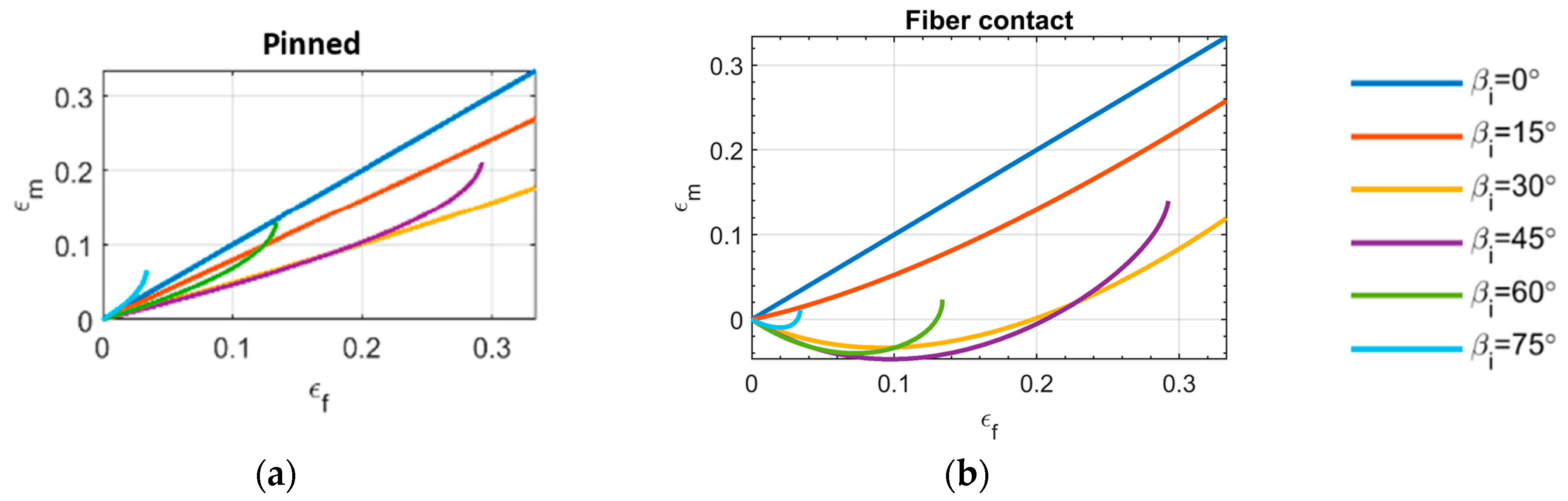
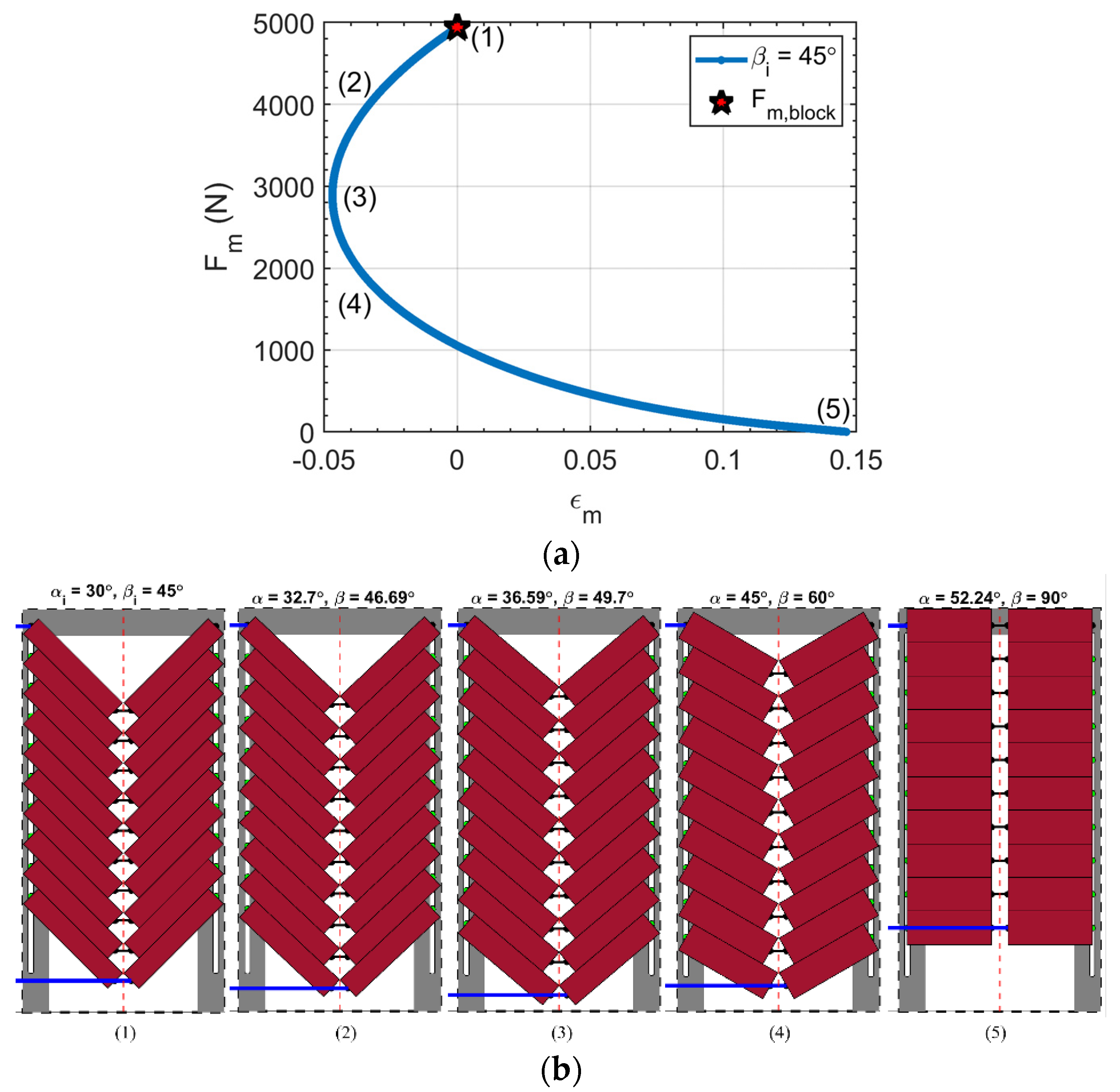
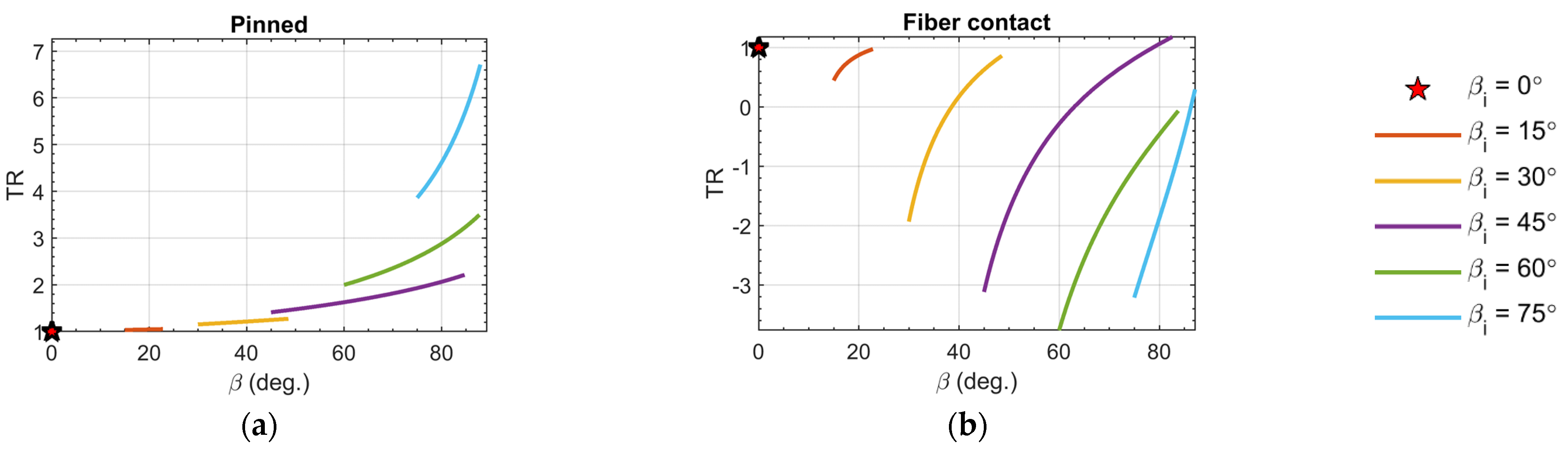
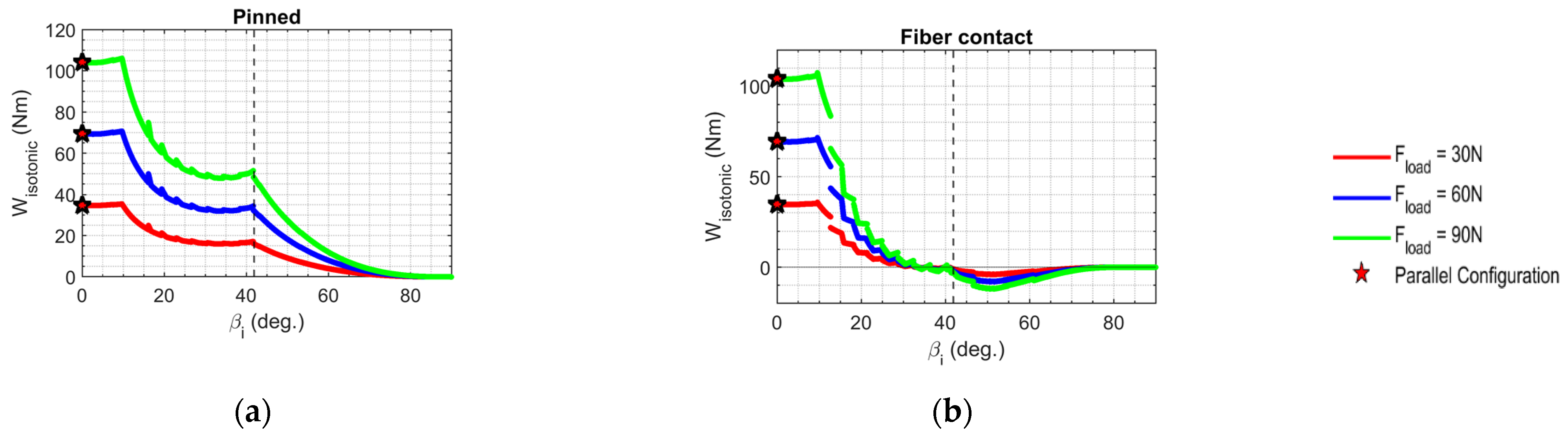
- The ‘pinned boundary condition’, illustrated in Figure 2a, defines that one end of each fiber is pinned to the rigid frame, while the other end is pinned to a central spine. Because the pin joints are fixed to rigid bodies, an initial clearance must be provided between the fibers, such that the fibers do not interfere with one another during contraction and the resulting radial expansion. We consider the case where this clearance is set, such that the fibers contact one another at exactly one contraction condition, and never interfere over the full stroke of the muscle.
- The ‘fiber contact boundary condition’ defines that contact between fibers is maintained during actuation. Fibers are assumed to remain cylindrical and are constrained to remain in frictionless, tangential contact, such that they can freely slide relative to one another, but cannot be separated. Physically, this could be implemented by pinning one end of the outer or top-most pair of fibers to a rigid external frame, while the remaining fiber ends on the rigid frame can slide freely. The opposite ends of the fibers are connected to their respective symmetric counterparts to complete the bipennate arrangement. Figure 2b illustrates a visual representation of fibers under this boundary condition. This fiber contact boundary condition represents an idealization of the function of the connective tissue in biological muscles that surrounds individual muscle fibers and enables fibers to slide relative to one another, while holding the fibers together transversally. This connective tissue, or endomysium, is deformable to adapt to volumetric changes during muscle fiber contraction, and has been shown to have a limited role in transmitting muscular force [18]. This fiber contact boundary condition thus serves as a direct analogy to biological muscle physiology.
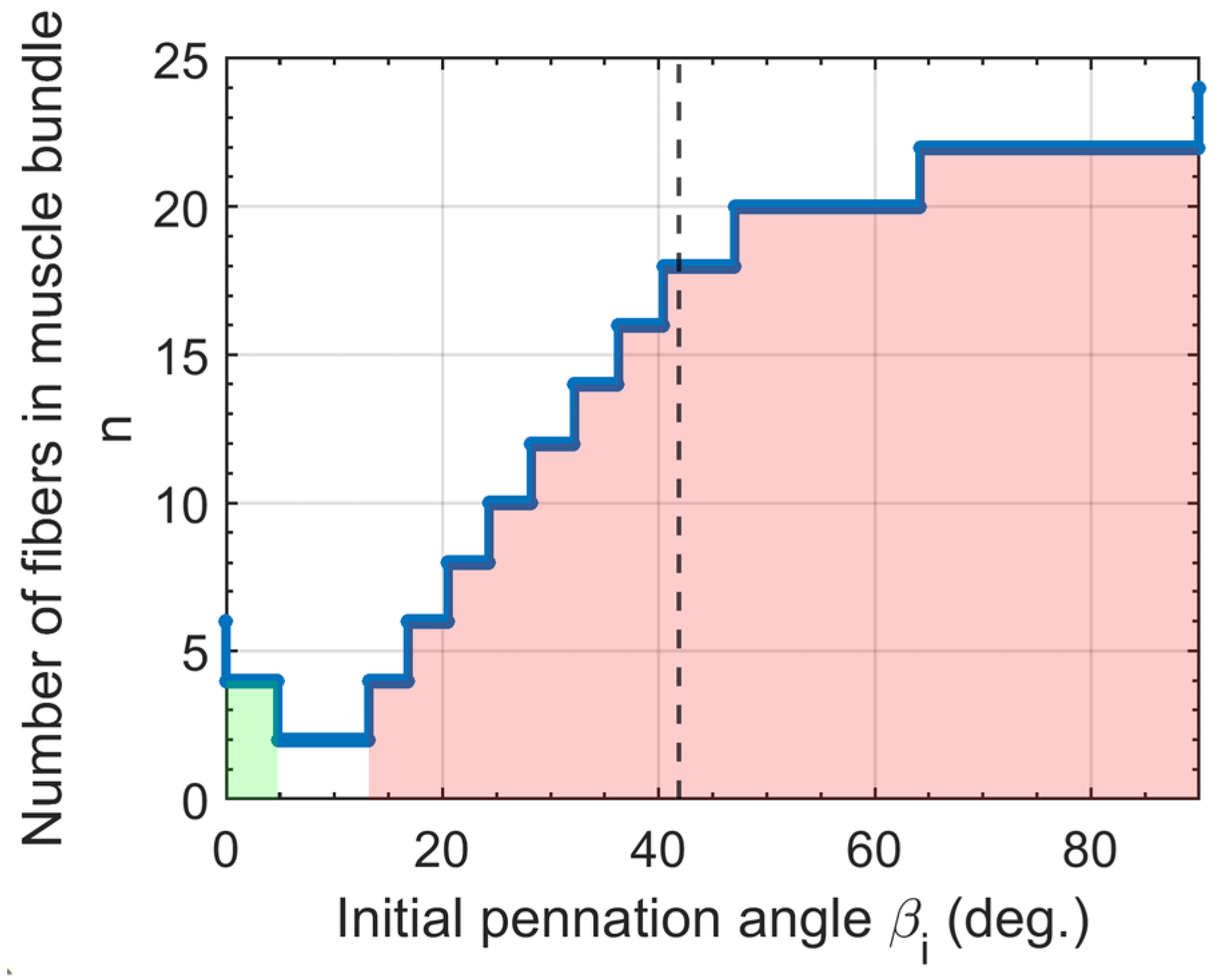
2.3. Muscle Bundle Parameterization
3. Effects of Pennation Angle on Bundle Actuator Performance
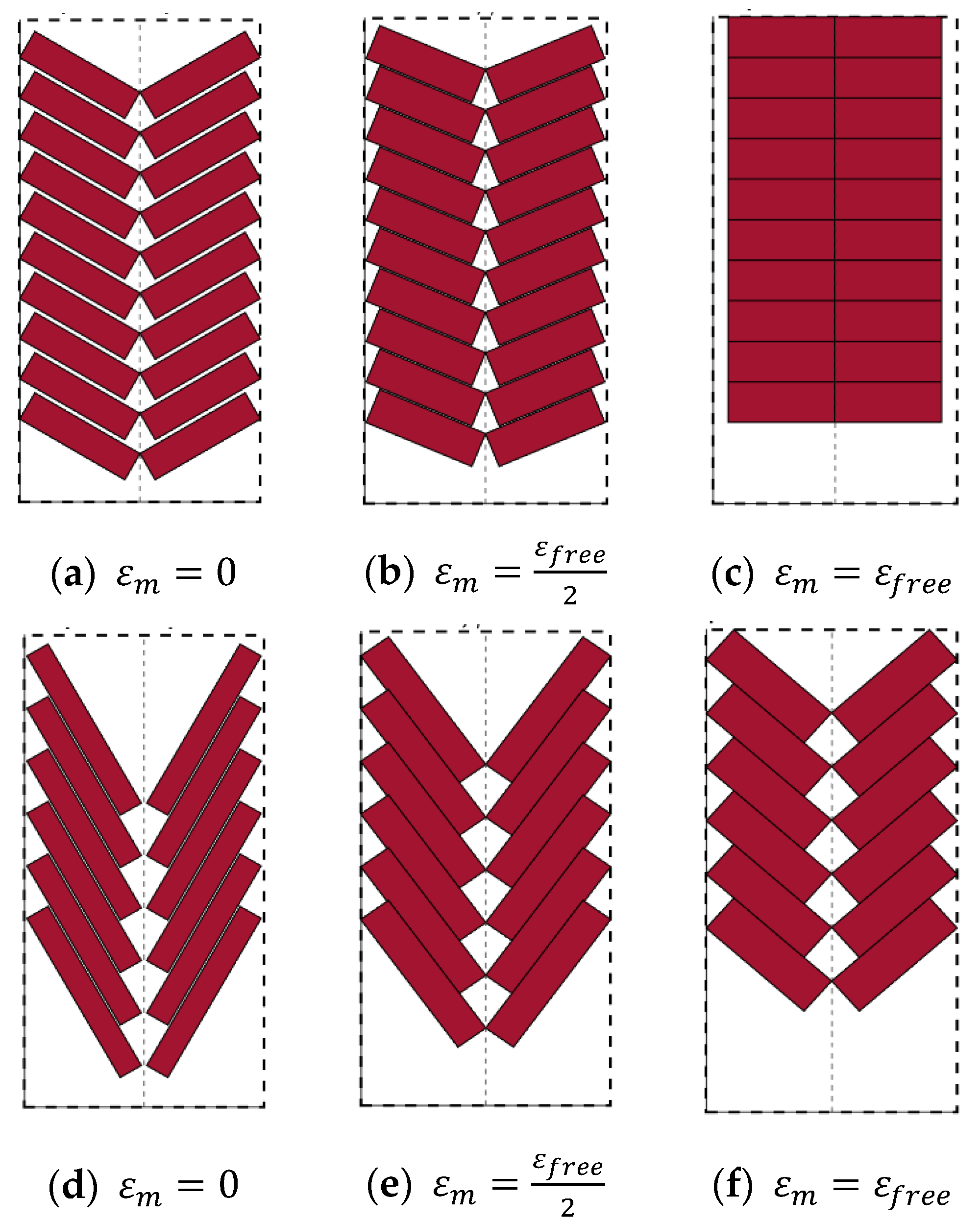
3.1. Muscle Force-Strain Behavior
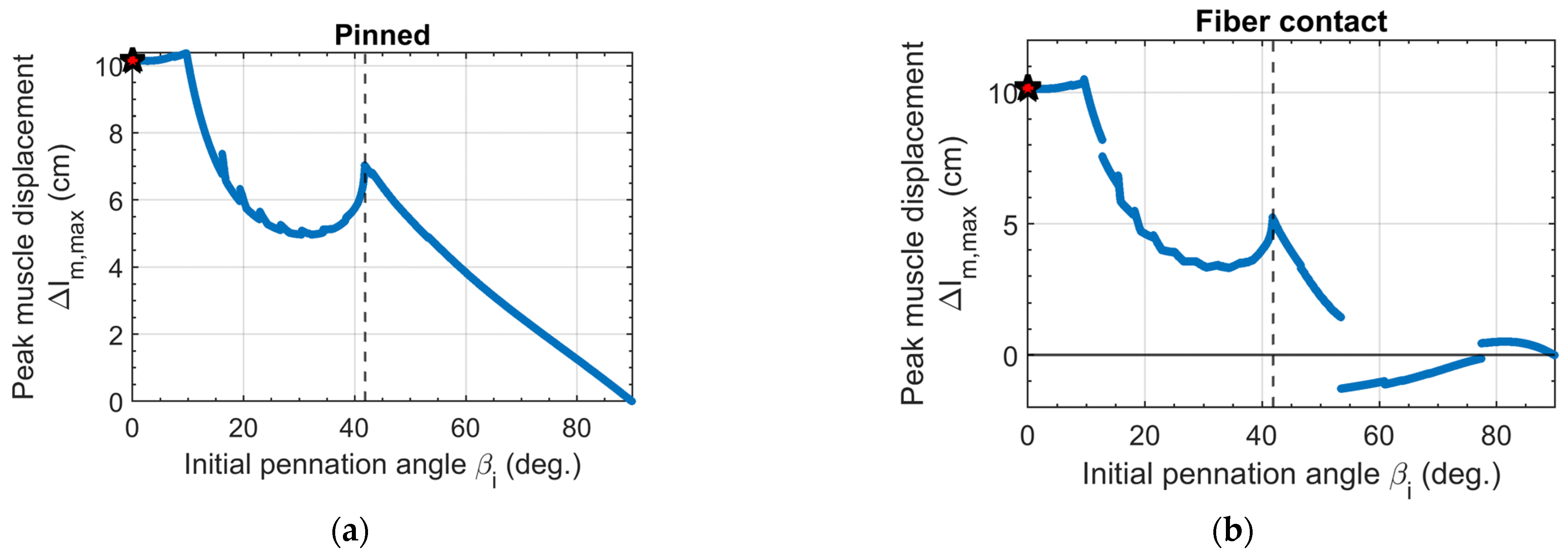
3.2. Peak Muscle Displacement
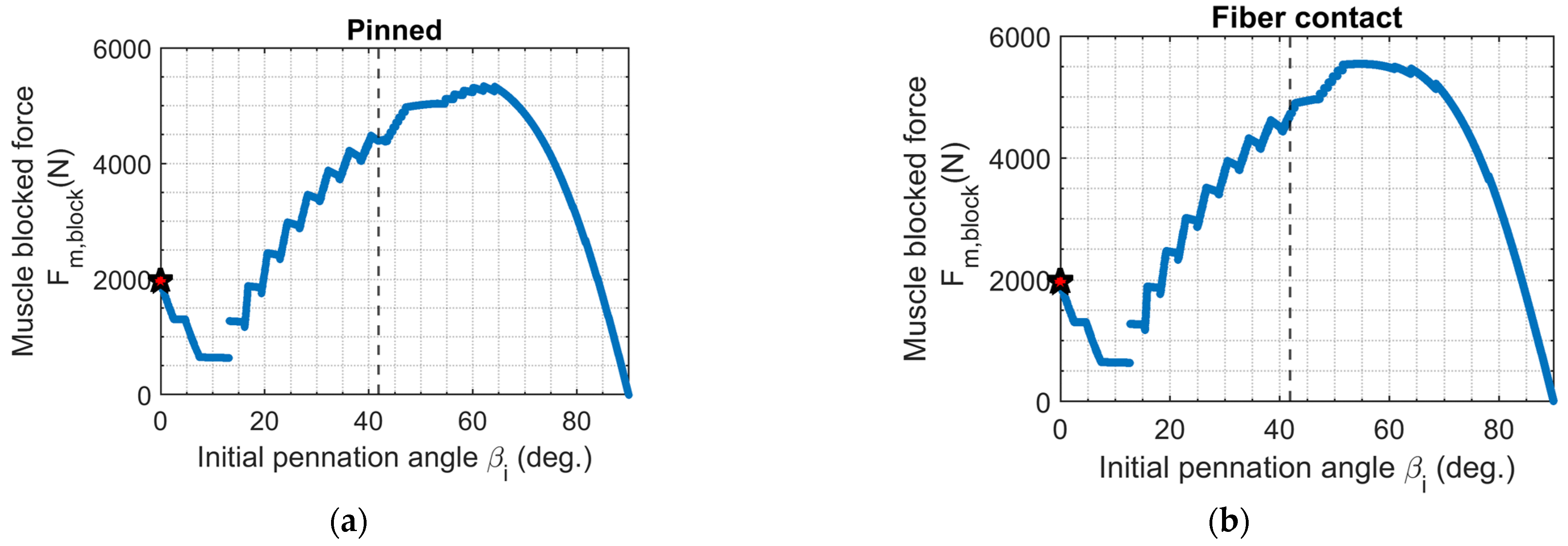
3.3. Muscle Blocked Force
3.4. Muscle Stiffness
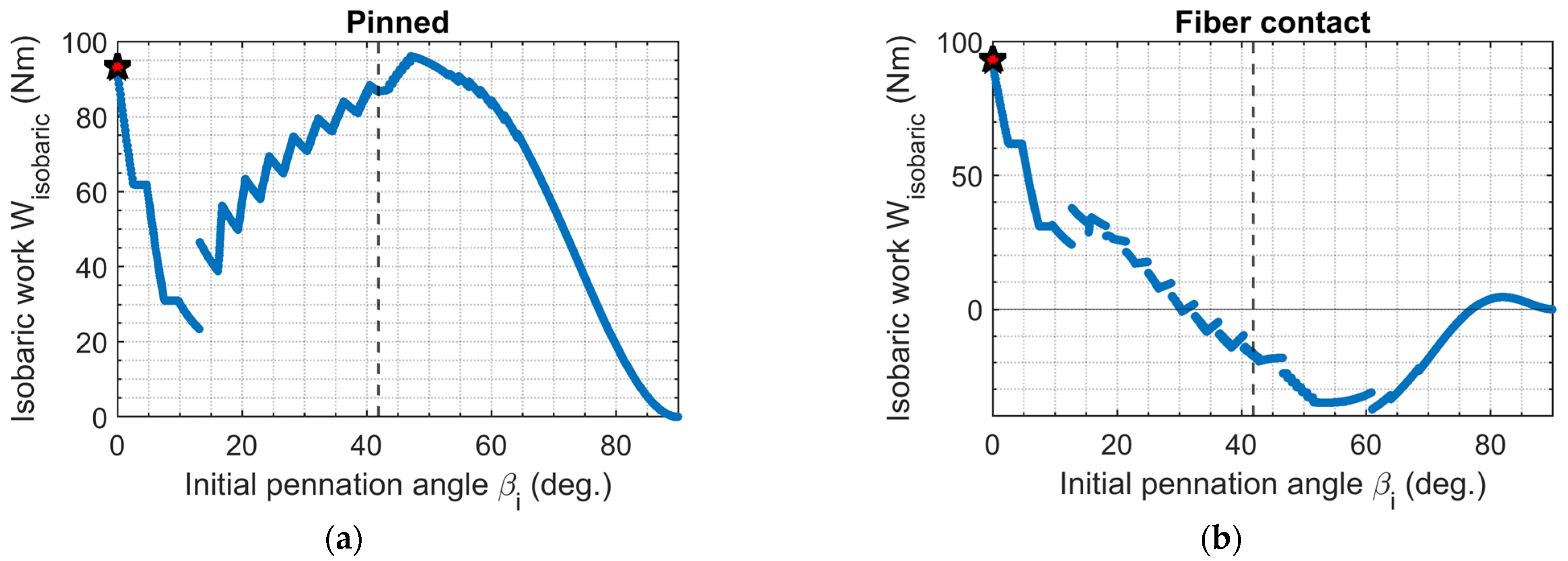
3.5. Isobaric Work Output
3.6. Isotonic Work Output
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ueda, J.; Schultz, A. Structure of cellular actuators. In Cellular Actuators: Modularity and Variability in Muscle-Inspired Actuation; Elsevier Science: Amsterdam, The Netherlands, 2017; pp. 1–44. [Google Scholar]
- Huston, D.; Esser, B.; Spencer, G.; Burns, D.; Kahn, E. Hierarchical actuator systems. In Proceedings of the Society of Photo-Optical Instrumentation Engineers, San Diego, CA, USA, 5 May 2005. [Google Scholar]
- Nassour, J.; Hamker, F.H.; Cheng, G. High-Performance Perpendicularly-Enfolded-Textile Actuators for Soft Wearable Robots: Design and Realization. IEEE Trans. Med. Robot. Bionics 2020, 20, 309–319. [Google Scholar] [CrossRef]
- Mathijssen, G.; Lefeber, D.; Vanderborght, B. Variable recruitment of parallel elastic elements: Series-parallel elastic actuators (SPEA) with dephased mutated gears. IEEE/ASME Trans. Mechatron. 2015, 20, 594–602. [Google Scholar] [CrossRef]
- Bryant, M.; Meller, M.; Garcia, E. Variable recruitment fluidic artificial muscles: Modeling and experiments. Smart Mater. Struct. 2014, 23, 074009. [Google Scholar] [CrossRef]
- Meller, M.; Chipka, J.; Volkov, A.; Bryant, M.; Garcia, E. Improving actuation efficiency through variable recruitment hydraulic McKibben muscles: Modeling, orderly recruitment control, and experiments. Bioinspir. Biomim. 2016, 11, 065004. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Kothera, C.; Wereley, N. Variable recruitment testing of pneumatic artificial muscles for Robotic Manipulators. IEEE/ASME Trans. Robot. Manip. 2015, 20, 1642–1652. [Google Scholar] [CrossRef]
- Azizi, E.; Roberts, T. Variable gearing in a biologically inspired pneumatic actuator array. Bioinspir. Biomim. 2013, 8, 026002. [Google Scholar] [CrossRef] [PubMed]
- Kianzad, S.; Pandit, M.; Lewis, J.; Berlingeri, A.; Haebler, K.J.; Madden, J. Variable stiffness structure using nylon actuators arranged in a pennate muscle configuration. In Proceedings of the Society of Photo-Optical Instrumentation Engineers, San Diego, CA, USA, 29 April 2015. [Google Scholar]
- Connolly, F.; Polygerinos, P.; Walsh, C.; Bertoldi, K. Mechanical Programming of Soft Actuators by Varying Fiber Angle. Soft Robot. 2015, 2, 26–32. [Google Scholar] [CrossRef]
- Betts, J.G.; Young, K.A.; Wise, J.A.; Johnson, E.; Poe, B.; Kruse, D.H.; Korol, O.; Johnson, J.E.; Womble, M.; DeSaix, P. Anatomy and Physiology; OpenStax, Rice University: Houston, TX, USA, 2013. [Google Scholar]
- Azizi, E.; Brainerd, E.; Roberts, T. Variable gearing in pennate muscles. Proc. Natl. Acad. Sci. USA 2008, 105, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.; Bryant, M. Pennate actuators: Force, contraction, and stiffness. Bioinspir. Biomim. 2020, 15, 046005. [Google Scholar] [CrossRef] [PubMed]
- Satheeshbabu, S.; Thompson, N.; Xiao, C.; Krishnan, G. Architectures of Soft Pneumatic Actuators Inspired by Muscle Fiber Arrangements. IEEE Int. Conf. Soft Robot. (RoboSoft) 2019, 2, 271–276. [Google Scholar]
- Jenkins, T.; Bryant, M. Variable stiffness soft robotics using pennate muscle architecture. In Proceedings of the Society of Photo-Optical Instrumentation Engineers, Denver, CO, USA, 13 March 2019. [Google Scholar]
- Wojnicz, W.; Zagrodny, B.; Ludwicki, M.; Awrejcewica, J.; Wittbrodt, E. A two-dimensional approach for modeling of pennate muscle behavior. Biocybern. Biomed. Eng. 2017, 37, 301–315. [Google Scholar]
- Kardel, T. Niels Stensen’s geometrical theory of muscle contraction(1667): A reappraisal. J. Biomech. 1990, 23, 953–965. [Google Scholar] [CrossRef]
- Stecco, C. Functional Atlas of the Human Fascical System; Churchill Livingstone: London, UK, 2015; pp. 91–92. [Google Scholar]
- Tondu, B.; Lopez, P. Modeling, and control of McKibben artificial muscle robot actuators. IEEE Control. Syst. 2000, 20, 15–38. [Google Scholar]
- Chou, C.; Hannaford, B. Measurement and modeling of McKibben pneumatic artificial muscles. IEEE Trans. Robot. Autom. 1996, 12, 90–102. [Google Scholar] [CrossRef]
- Alexander, R. The orientation of muscle fibres in the myomeres of fishes. J. Mar. Biol. Assoc. UK 1969, 49, 263–290. [Google Scholar] [CrossRef]
- Azizi, E.; Roberts, T. Geared up to stretch: Pennate muscle behavior during active lengthening. J. Exp. Biol. 2014, 217, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, C.; Ren, L.; Ren, L. Load-dependent Variable Gearing Mechanism of Muscle-like Soft Actuator. J. Bionic Eng. 2022, 19, 29–43. [Google Scholar] [CrossRef]





| Parameter | Variable | Value |
|---|---|---|
| Initial braid angle | ||
| Bounding length | ||
| Bounding width | ||
| Bounding depth |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, E.; Bryant, M. Implications of Spatially Constrained Bipennate Topology on Fluidic Artificial Muscle Bundle Actuation. Actuators 2022, 11, 82. https://doi.org/10.3390/act11030082
Duan E, Bryant M. Implications of Spatially Constrained Bipennate Topology on Fluidic Artificial Muscle Bundle Actuation. Actuators. 2022; 11(3):82. https://doi.org/10.3390/act11030082
Chicago/Turabian StyleDuan, Emily, and Matthew Bryant. 2022. "Implications of Spatially Constrained Bipennate Topology on Fluidic Artificial Muscle Bundle Actuation" Actuators 11, no. 3: 82. https://doi.org/10.3390/act11030082
APA StyleDuan, E., & Bryant, M. (2022). Implications of Spatially Constrained Bipennate Topology on Fluidic Artificial Muscle Bundle Actuation. Actuators, 11(3), 82. https://doi.org/10.3390/act11030082





