Enhancement of IPMC Bending Controllability Through Immobile Negative Charges and Electrochemically Reactive Substances Within IPMC Body
Abstract
:1. Introduction
2. Specimens
2.1. Meaning of Silver Surface Coating and Dehydration Treatment of IPMCs
2.2. Fabrication
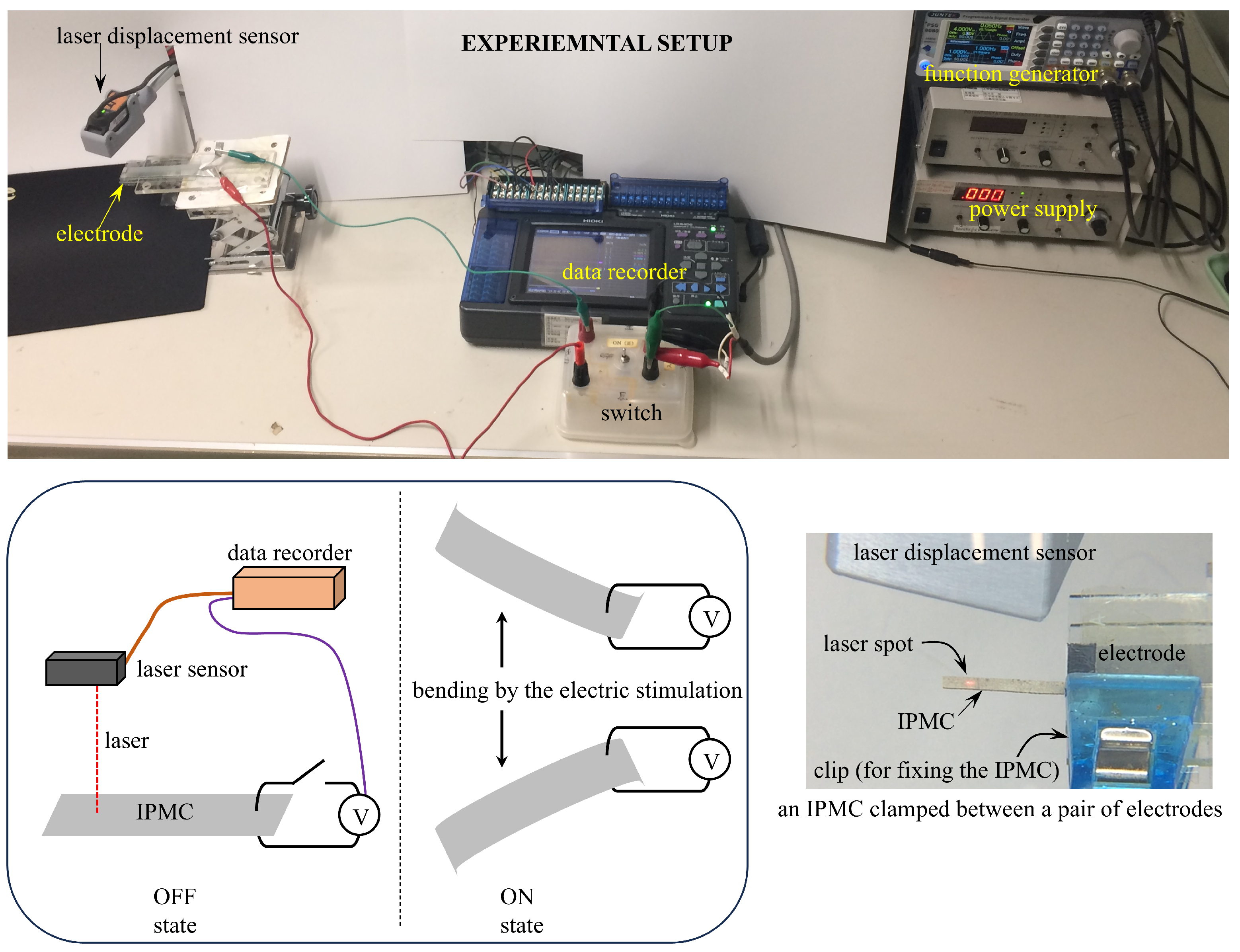
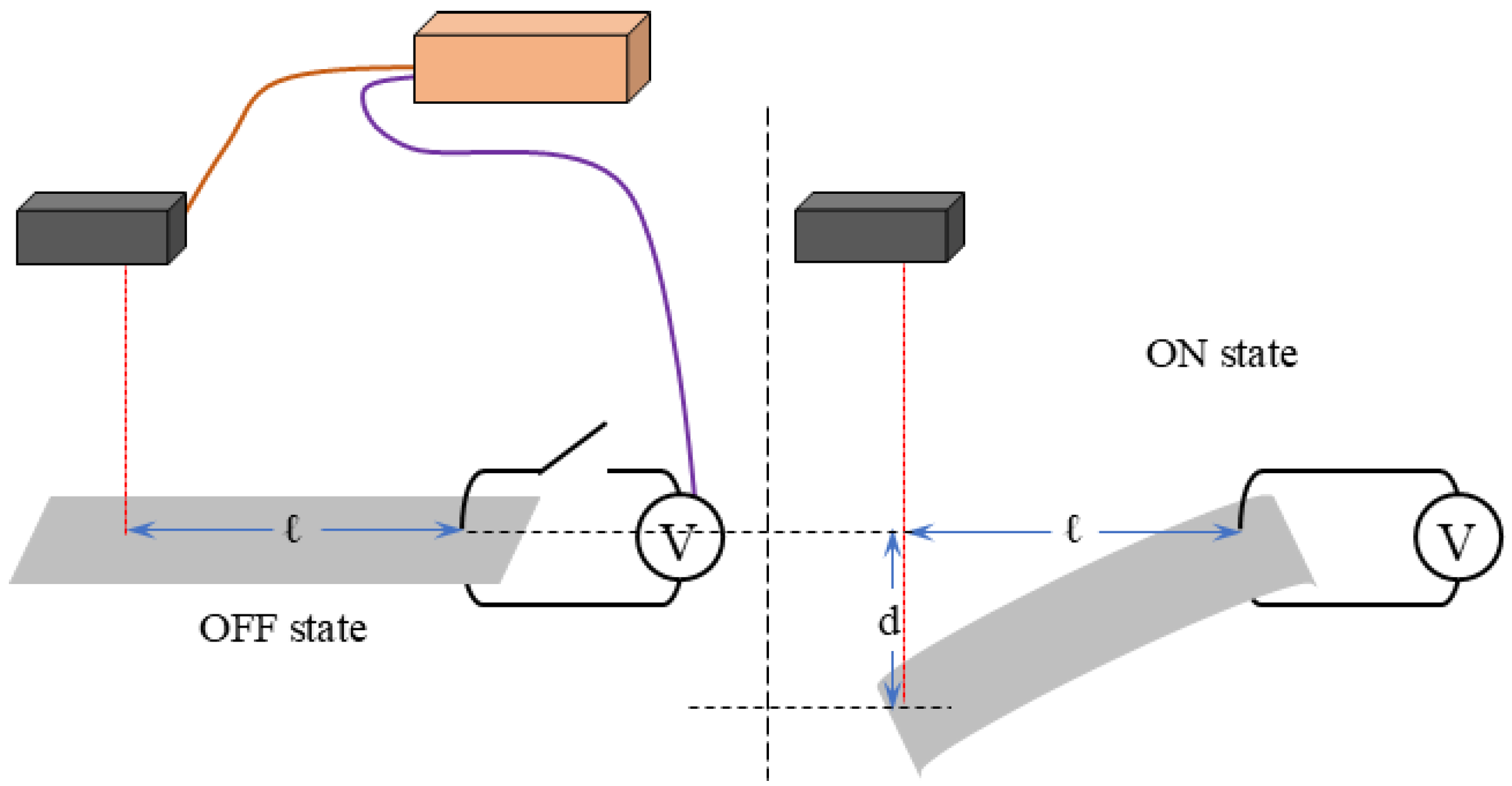
3. Experiment of Bending Tests
3.1. Bending Response Under Constant Voltage
3.2. Bending Response to Alternate Voltage
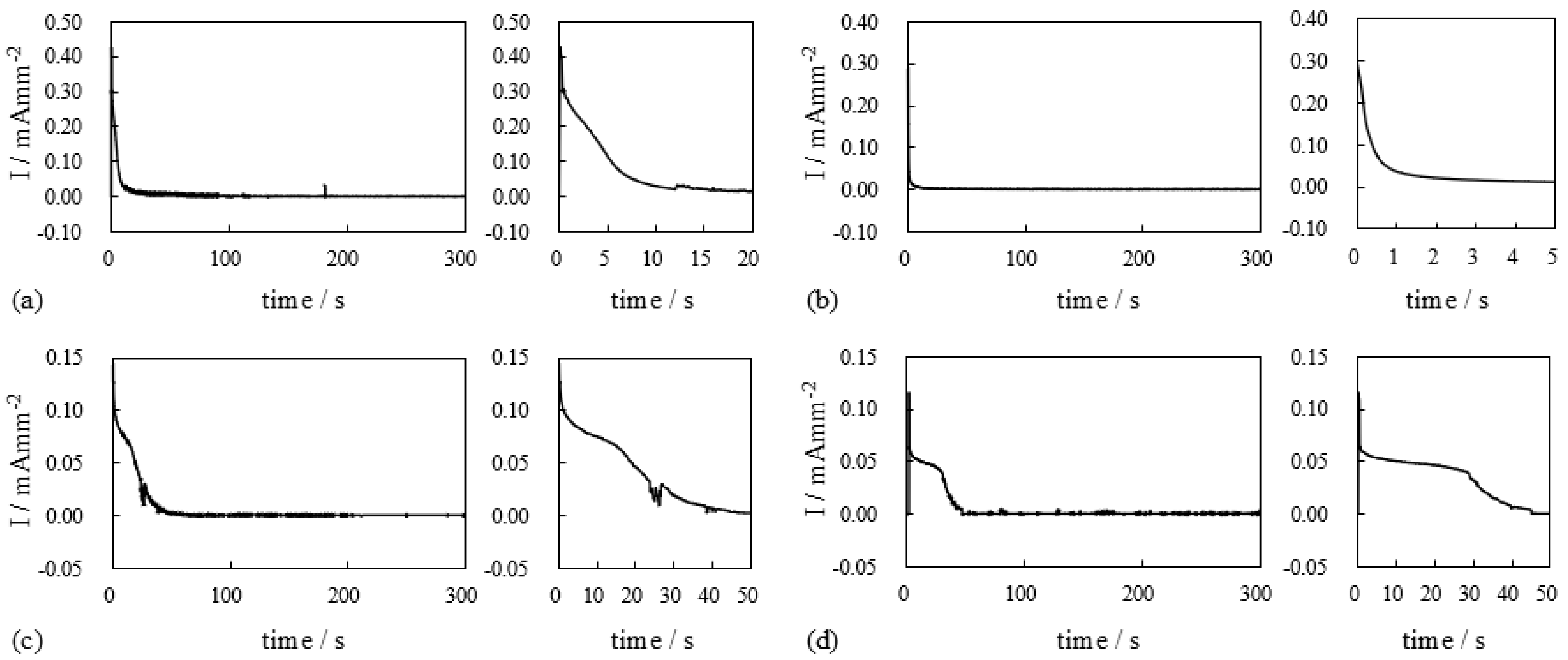
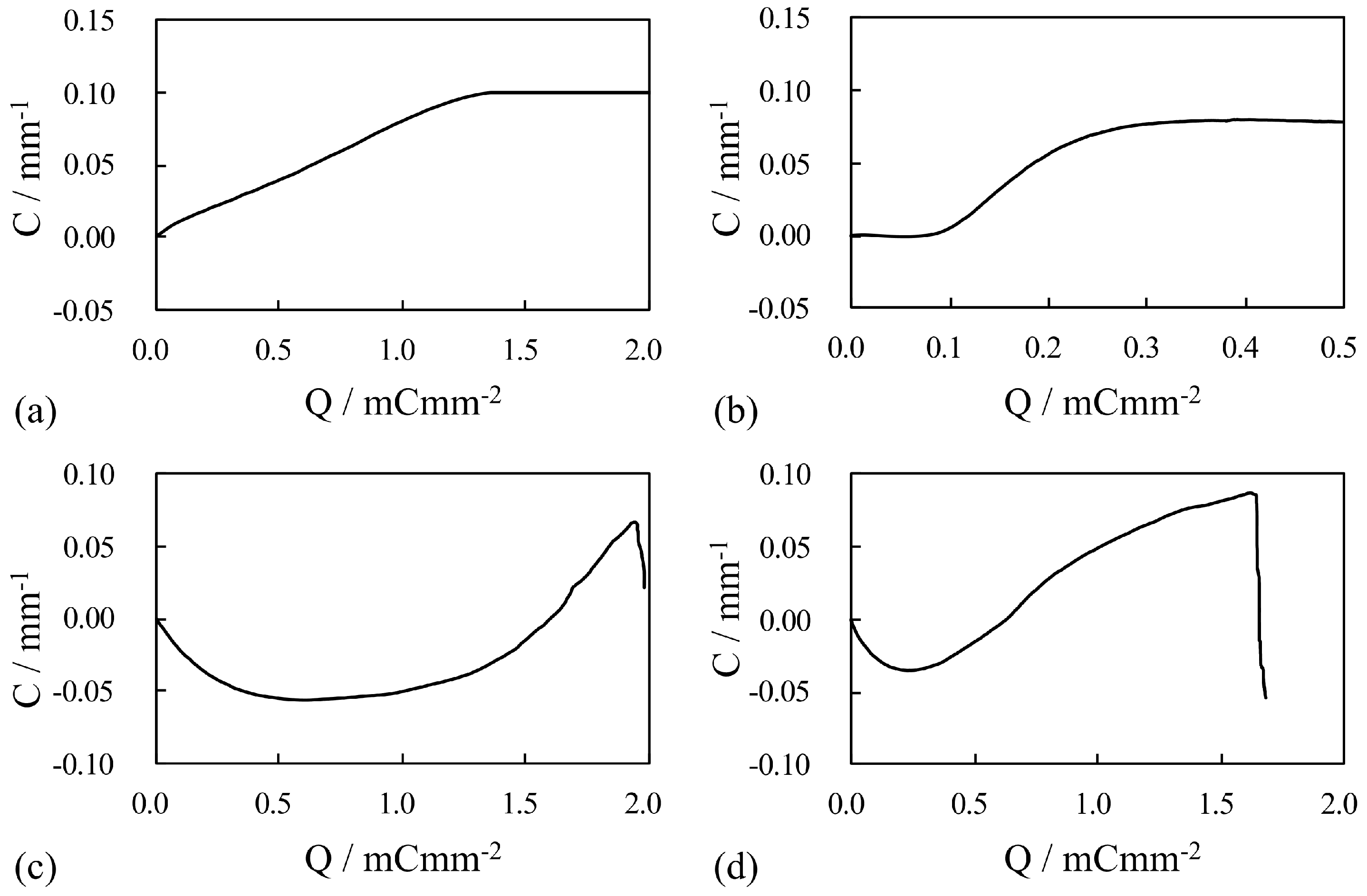
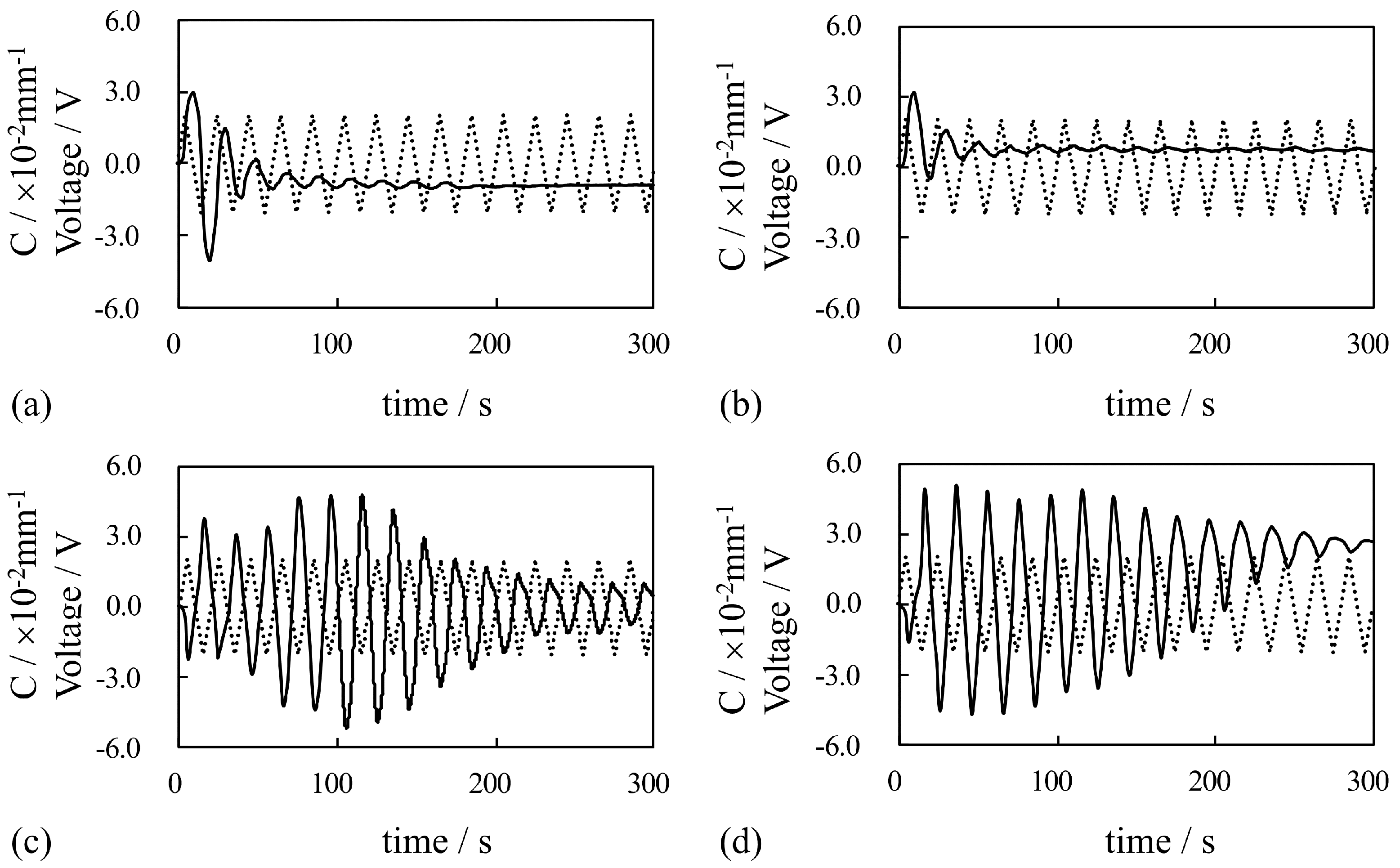
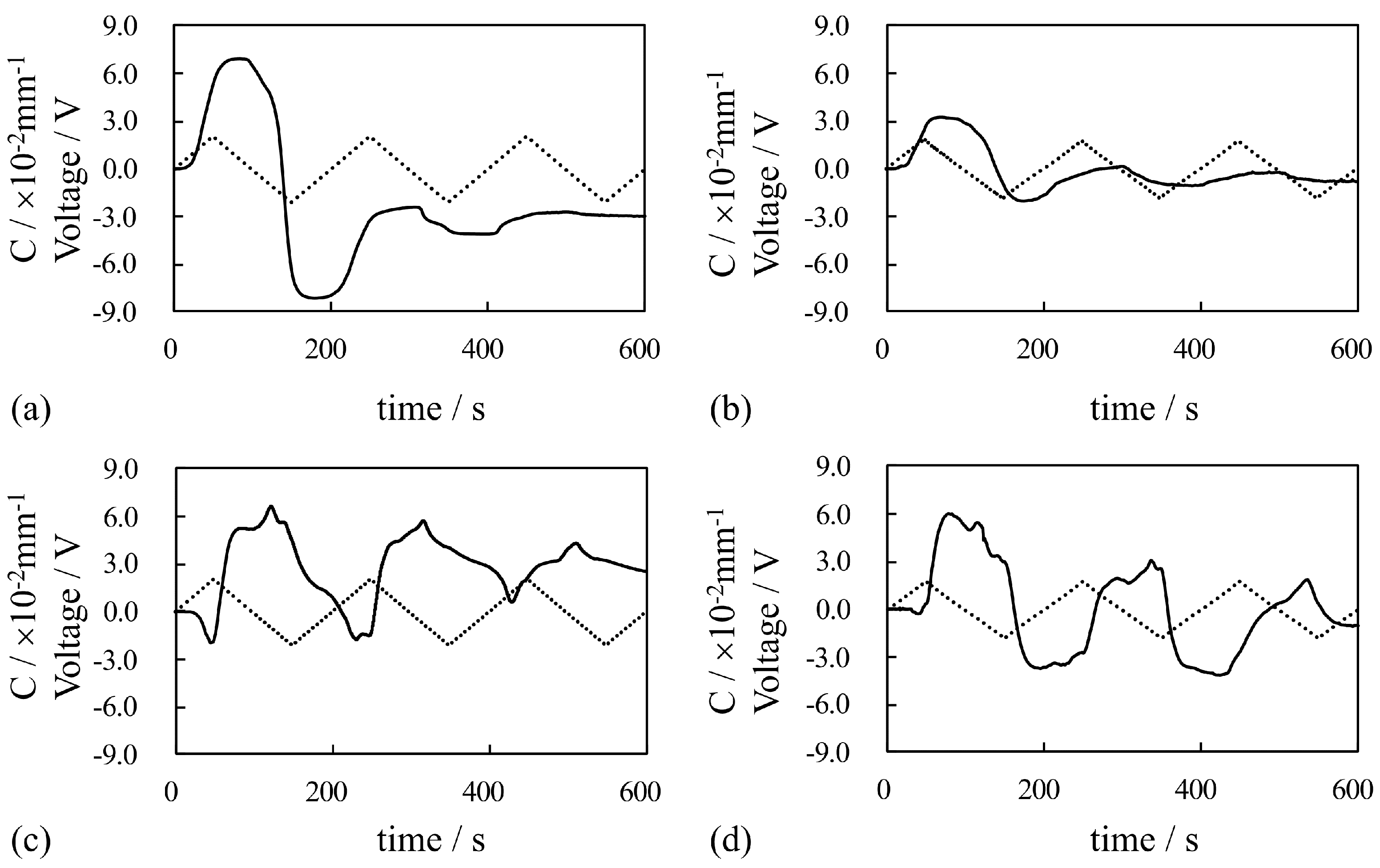
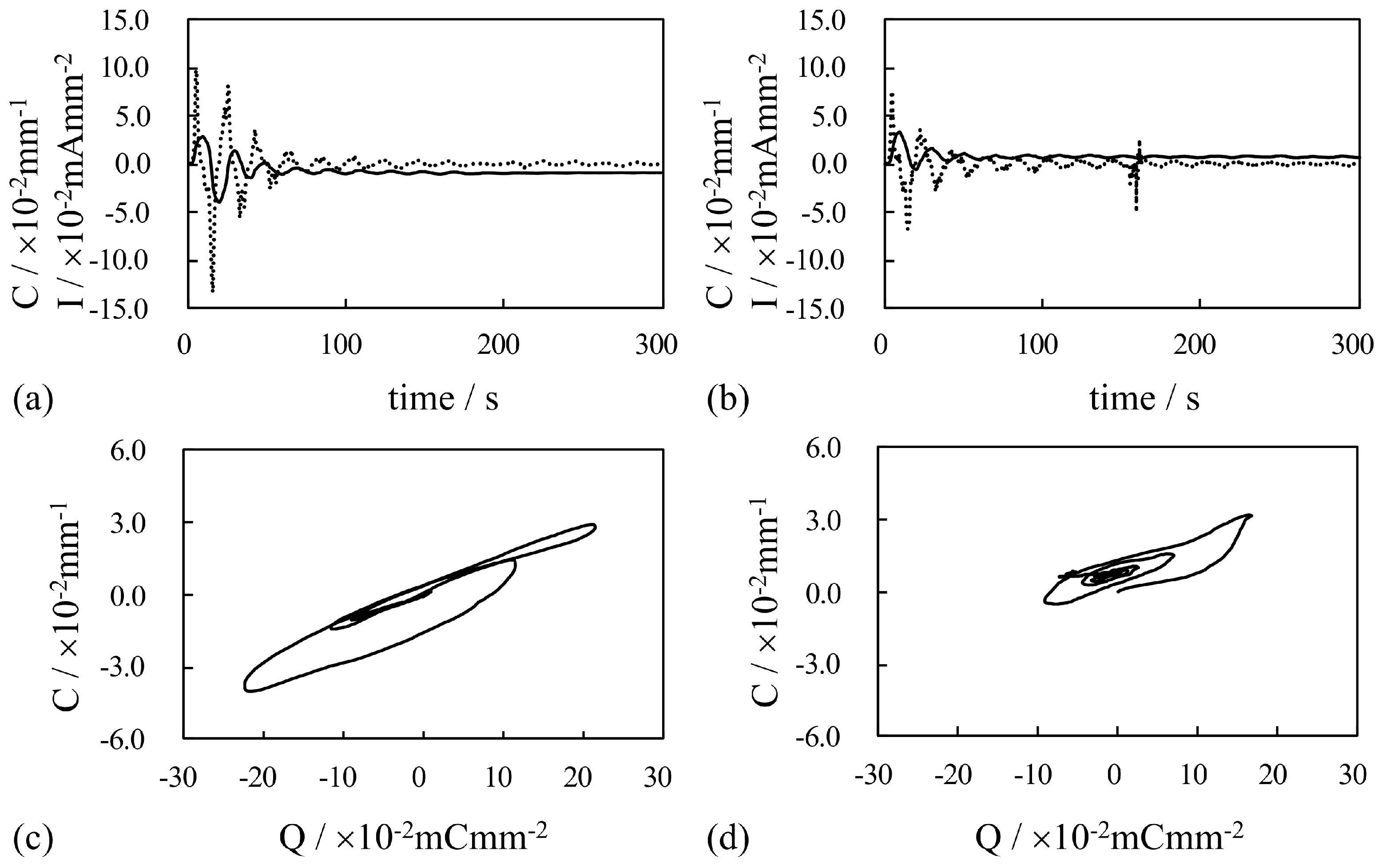
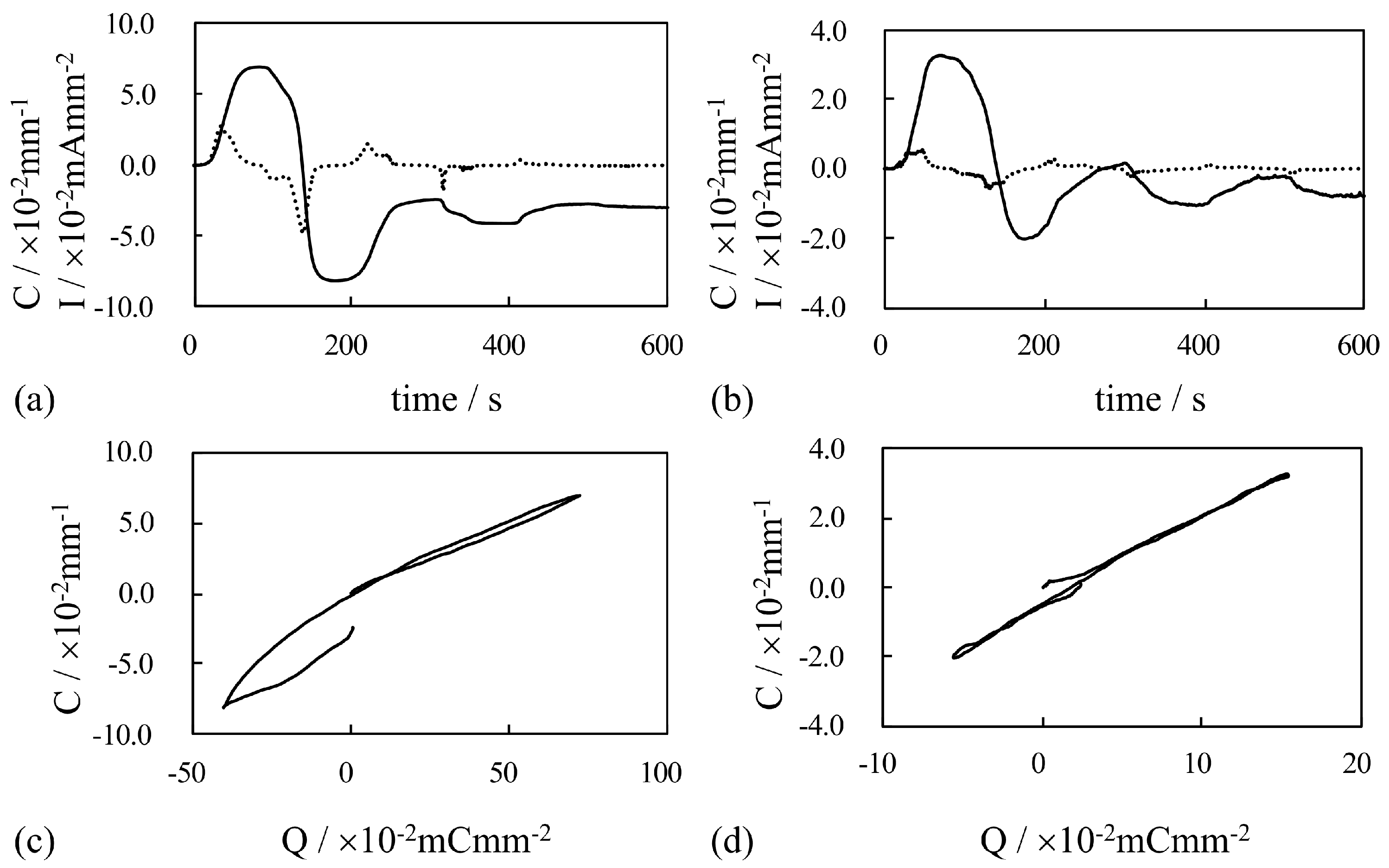
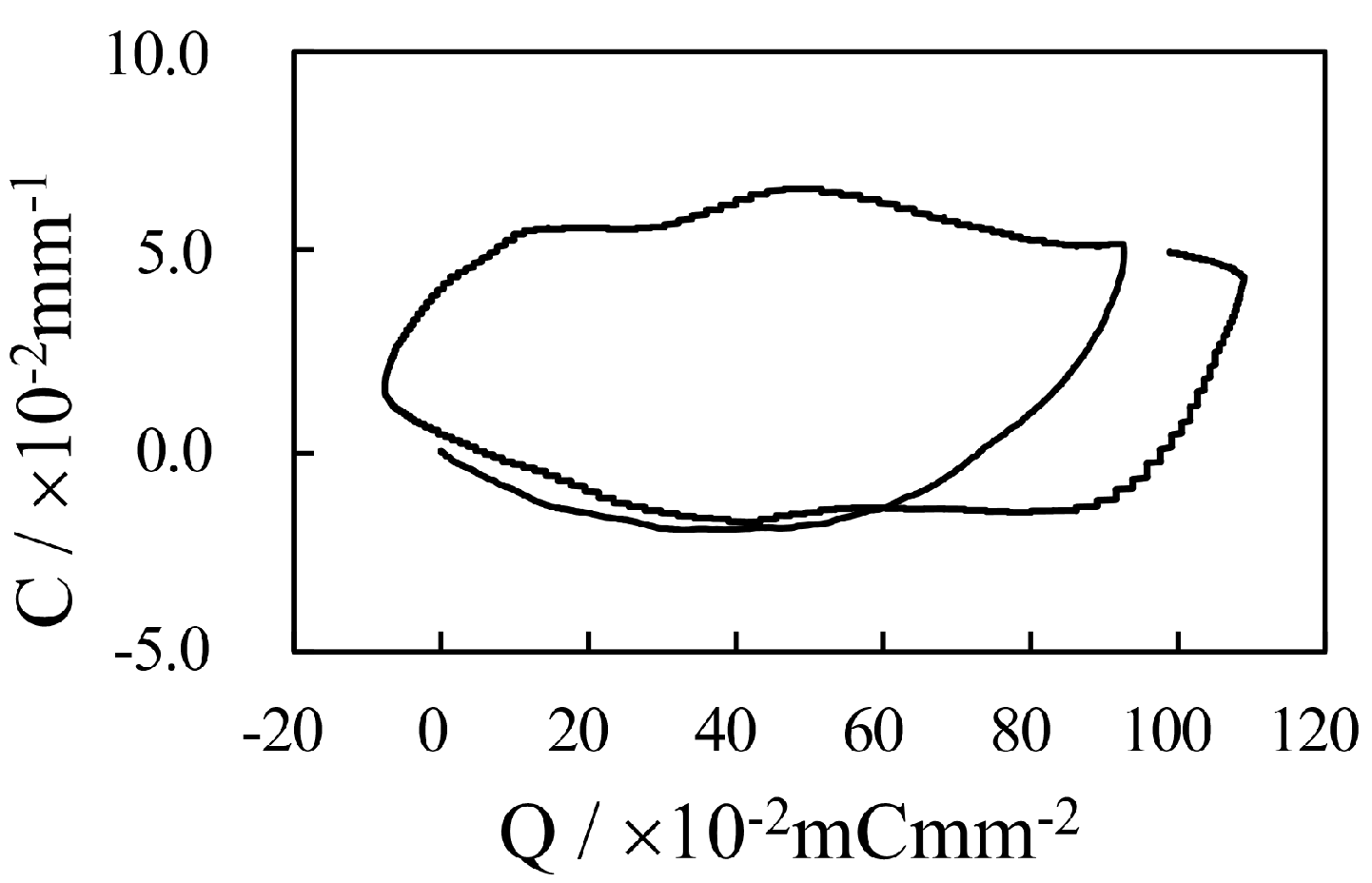
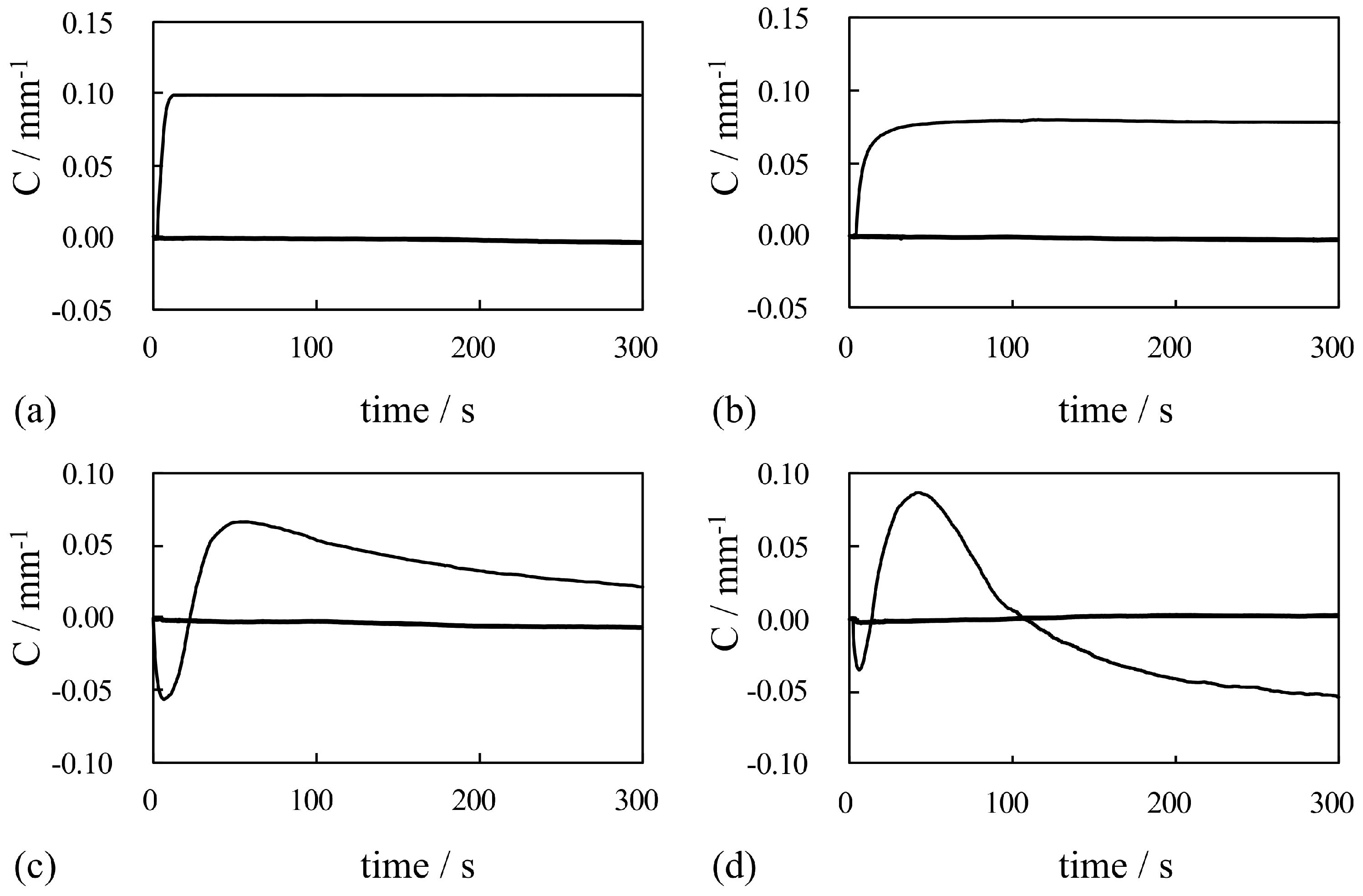
4. Results and Discussion
4.1. Silver-Coated IPMC
4.1.1. Bending Under a Constant Voltage
4.1.2. Bending Under an Alternating Voltage
4.2. Bending of Au-Coated IPMCs Under Constant Voltage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Katchalsky, A. Polyelectrolytes and Their Biological Interactions. Phys. Chem. Macromol. 1949, 4, 9–41. [Google Scholar] [CrossRef] [PubMed]
- Katchalsky, A.; Zwick, M. Mechanochemistry and ion exchange. J. Polym. Sci. 1955, 16, 221–234. [Google Scholar] [CrossRef]
- Okuzaki, H.; Osada, Y. Classification and Research Trend of Soft Actuators. JSPE 2014, 80, 709–712. [Google Scholar]
- Jiang, J.; Lin, C.; Xu, S.; Yu, Y.; Yao, L.; Huang, Z. Application-Oriented Modeling of Soft Actuator Ionic Polymer-Metal Composites: A Review. Adv. Intell. Syst. 2024, 6, 2300568. [Google Scholar] [CrossRef]
- Tanaka, T. Classification and Research Trend of Soft Actuators. Phys. Rev. Lett. 1978, 40, 820–823. [Google Scholar] [CrossRef]
- Tanaka, T. Gels. Sci. Am. 1982, 244, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Tokita, M. Phase Transition of Gels—A Review of Toyoichi Tanaka’s Research. Gels 2022, 8, 550. [Google Scholar] [CrossRef] [PubMed]
- Oguro, K.; Kawami, Y.; Takenaka, H. Bending of anion-Conducting Polymer Film-Electrode Composite by an Electric Stimulus at Low Voltage. J. Micromach. Soc. 1992, 5, 27–30. [Google Scholar]
- Kanno, R.; Tadokoro, S.; Takamori, T.; Hattori, M.; Oguro, K. Linear Approximate Dynamic Model of ICPF (Ionic Conducting Polymer Gel Film) Actuator. In Proceedings of the 1996 IEEE International Conference on Robotics and Automation, Minneapolis, MN, USA, 22–28 April 1996; pp. 219–225. [Google Scholar]
- Salehpoor, K.; Shahinpoor, M.; Mojarrad, M. Linear and Platform Type Robotic Actuators Made From Ion-Exchange Membrane-Metal Composites. In Proceedings of SPIE Conference on Smart Materials Technologies, San Diego, CA, USA, 3–4 March 1997; Volume 3040, pp. 192–197. [Google Scholar]
- Guo, S.; Fukuda, T.; Kato, N.; Oguro, K. Development of Underwater Microrobot Using ICPF Actuator. In Proceedings of the 1998 IEEE International Conference on Robotics and Automation, Leuven, Belgium, 16–20 May 1998; pp. 1829–1834. [Google Scholar]
- Bar-Cohen, Y.; Xue, T.; Shahinpoor, M.; Simpson, J.O.; Smith, J. Low-mass muscle actuators using electroactive polymers (EAP). In Proceedings of the SPIE Conference on Smart Materials Technologies, San Diego, CA, USA, 1–5 March 1998; Volume 3324, pp. 218–223. [Google Scholar]
- Shahinpoor, M.; Bar-Cohen, Y.; Xue, T.; Simpson, J.O.; Smith, J.; Hung, M.; Sheng, W. Some Experimental Results on Ionic Polymer-Metal Composites (IPMC) As Biomimetic Sensors and Actuators. In Proceedings of the SPIE Conference on Smart Materials Technologies, San Diego, CA, USA, 1–5 March 1998; Volume 3324, pp. 251–267. [Google Scholar]
- Onishi, K.; Sewa, S.; Asaka, K.; Fujiwara, N.; Oguro, K. The effects of counter ions on characterization and performance of a solid polymer electrolyte actuator. Elcctrochimica Acta 2001, 46, 1233–1241. [Google Scholar] [CrossRef]
- Yun, K.; Kim, W.-J. System identification and microposition control of ionic polymer metal composite for three-finger gripper manipulation. Proc. Inst. Mech. Eng. Part I J. Syst. Control Eng. 2005, 220, 539–551. [Google Scholar] [CrossRef]
- Hung, M.; Sheng, W. Robust Adaptive Control with Leakage Modification for a Nonlinear Model of Ionic Polymer Metal Composites (IPMC). In Proceedings of the 2008 IEEE International Conference on Robotics and Biomimetics, Bangkok, Thailand, 21–26 February 2009; pp. 1783–1788. [Google Scholar]
- He, Q.; Song, L.; Yu, M.; Dai, Z.D. Fabrication, characteristics and electrical model of an ionic polymer metal-carbon nanotube composite. Smart Mater. Struct. 2015, 24, 07S001. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.W.; Song, D.S.; Jho, J.Y. Highly Enhanced Force Generation of lonic Polymer-Metal Composite Actuators via Thickness Manipulation. ACS Appl. Mater. Interfaces 2015, 7, 16659–16667. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, M.; Lu, M.; He, Q.; Ji, K.; Liu, L. Effects of Cu2+ Counter Ions on the Actuation Performance of Flexible Ionic Polymer Metal Composite Actuators. J. Bionic Eng. 2018, 15, 1047–10567. [Google Scholar] [CrossRef]
- Zhai, Z.; Tian, A.; Zhang, X.; Du, H.; Wang, Y. Performance prediction of IPMC modified with SiO2-SGO based on backpropagation neural network. Nanotechnol. Precis. Eng. 2024, 7, 043007. [Google Scholar] [CrossRef]
- Hara, S.; Zama, T.; Takashima, W.; Kaneto, K. Artificial Muscles Based on Polypyrrole Actuators with Large Strain and Stress Induced Electrically. Polym. J. 2004, 36, 151–161. [Google Scholar] [CrossRef]
- Choi, H.-J.; Song, Y.-M.; Chung, I.; Ryu, K.-S.; Jo, N.-J. Conducting polymer actuator based on chemically deposited polypyrrole and polyurethane-based solid polymer electrolyte working in air. Smart Mater. Struct. 2009, 18, 024006. [Google Scholar] [CrossRef]
- Chiba, S.; Stanford, S.; Pelrine, R.; Kornbluh, R.; Prahlad, H. Electroactive Polymer Artificial Muscle. JRSJ 2006, 24, 466–470. [Google Scholar] [CrossRef]
- O’Brien, B.M.; Rosset, S. Ion implanted dielectric elastomer circuits. Appl. Phys. A Mater. Sci. Process. 2013, 111, 943–950. [Google Scholar] [CrossRef]
- Yip, M.C.; Niemeye, G. High-Performance Robotic Muscles from Conductive Nylon Sewing Thread. In Proceedings of the 2015 IEEE International Conference on Robotics and Automation (ICRA), Seattle, WA, USA, 26–30 May 2015. [Google Scholar]
- Wang, S.; Huang, H.; Huang, H.; Li, B.; Huang, K. A Lightweight Soft Gripper Driven by Self-Sensing Super-Coiled Polymer Actuator. IEEE Robot. Autom. Lett 2021, 6, 2775–2782. [Google Scholar] [CrossRef]
- Musavir, B.; Rajendran, P. A review on electroactive polymers development for aerospace applications. J. Intell. Mater. Syst. Struct. 2018, 18, 3681–3695. [Google Scholar] [CrossRef]
- Porfiri, M.; Leronni, A.; Bardella, L. An alternative explanation of back-relaxation in ionic polymer metal composites. Extrem. Mech. Lett. 2017, 13, 78–83. [Google Scholar] [CrossRef]
- Annabestani, M.; Sayad, M.H.; Esmaeili-Dokht, P.; Gorji, R.; Fardmanesh, M. Eliminating Back Relaxation in Large-Deformable IPMC Artificial Muscles: A Noise-Assistive Pattern-Free Electrode Approach. In Proceedings of the 27th National and 5th International Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 26–27 November 2020. [Google Scholar]
- Onouchi, Y.; Sasaki, M.; Tamagawa, H. Current-controlled Selemion bending in the controlled humidity environment. Sens. Actuators B Chem. 2009, 135, 465–471. [Google Scholar] [CrossRef]
- Sasaki, M.; Lin, W.; Tamagawa, H.; Ito, S.; Kikuchi, K. Self-Sensing Control of Nafion-Based Ionic Polymer-Metal Composite (IPMC) Actuator in the Extremely Low Humidity Environment. Actuators 2013, 2, 74–85. [Google Scholar] [CrossRef]
- Nishida, G.; Sugiura, M.; Yamakita, M.; Maschke, B.; Ikeura, R. Multi-Input Multi-Output Integrated Ionic Polymer-Metal Composite for Energy Controls. Micromachines 2012, 3, 126–136. [Google Scholar] [CrossRef]
- Tamagawa, T.; Nogata, F.; Watanabe, T.; Abe, A.; Yagasaki, K.; Jin, J.-Y. Influence of metal plating treatment on the electric response of Nafion. J. Mater. Sci. 2003, 38, 1039–1044. [Google Scholar] [CrossRef]
- Onouchi, Y.; Tamagawa, H.; Sasaki, M. Dependence of curvature of dehydrated Selemion on the total charge and environmental humidity. JSAEM 2009, 17, 59–64. [Google Scholar]
- Tamagawa, H.; Nogata, F.; Popovic, S. Roles of Ag redox reaction and water absorption inducing the Selemion bending. J. Membr. Sci. 2005, 251, 145–150. [Google Scholar] [CrossRef]
- Tamagawa, H.; Nogata, F. Atomic structural change of silver-plating layers on the surfaces of Selemion, resulting in its excellent bending controllability. Sens. Actuators B Chem. 2006, 114, 781–787. [Google Scholar] [CrossRef]
- Tamagawa, H.; Okada, K.; Mulembo, T.; Sasaki, M.; Naito, K.; Nagai, G.; Nitta, T.; Yes, K.-C.; Ikeda, K. Simultaneous Enhancement of Bending and Blocking Force of an Ionic Polymer-Metal Composite (IPMC) by the Active Use of Its Material Characteristics Change. Actuators 2019, 8, 29. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamagawa, H.; Kojima, I.; Torii, S.; Lin, W.; Sasaki, M. Enhancement of IPMC Bending Controllability Through Immobile Negative Charges and Electrochemically Reactive Substances Within IPMC Body. Actuators 2024, 13, 517. https://doi.org/10.3390/act13120517
Tamagawa H, Kojima I, Torii S, Lin W, Sasaki M. Enhancement of IPMC Bending Controllability Through Immobile Negative Charges and Electrochemically Reactive Substances Within IPMC Body. Actuators. 2024; 13(12):517. https://doi.org/10.3390/act13120517
Chicago/Turabian StyleTamagawa, Hirohisa, Iori Kojima, Sota Torii, Wenyi Lin, and Minoru Sasaki. 2024. "Enhancement of IPMC Bending Controllability Through Immobile Negative Charges and Electrochemically Reactive Substances Within IPMC Body" Actuators 13, no. 12: 517. https://doi.org/10.3390/act13120517
APA StyleTamagawa, H., Kojima, I., Torii, S., Lin, W., & Sasaki, M. (2024). Enhancement of IPMC Bending Controllability Through Immobile Negative Charges and Electrochemically Reactive Substances Within IPMC Body. Actuators, 13(12), 517. https://doi.org/10.3390/act13120517





