Investigation of an Imported Case of Rabies in a Juvenile Dog with Atypical Presentation
Abstract
:Simple Summary
This study confirms the need for vigilance and rapid diagnosis of veterinary samples to control the unexpected importation of rabies into a country, particularly when the disease signs are atypical.
Abstract
Movement of dogs between rabies-endemic and rabies-free countries carries the inherent risk of introducing the disease. In April of 2008, a juvenile dog was imported to the UK from Sri Lanka. It died shortly after transfer to a quarantine facility in the south-east of England following a short history of diarrhoea and convulsions but no overt signs of aggression. Subsequent investigation confirmed that rabies was the cause of death. Rabies virus was isolated from brain samples taken from the dog and the subsequent phylogenetic investigation confirmed that the genomic sequence from this virus shared over 99% homology with endemic rabies viruses from Sri Lanka. Histological examination of the brain demonstrated clear signs of encephalitis and rabies antigenic labeling in numerous neurons. In this particular case, Negri bodies were absent. As this case was diagnosed in a quarantine facility, the ‘rabies-free’ status of the UK was un-affected.
1. Introduction
The domestic dog is the most significant reservoir for rabies virus and dog bites are responsible for the great majority of human deaths from rabies [1]. Although rabies in dogs has been controlled and eliminated in many regions of the world, it still persists throughout Africa and Asia causing thousands of human deaths every year. Despite the availability of effective vaccination, it is only through control of dog rabies that human deaths due to rabies will be reduced [2]. The United Kingdom (UK) has been rabies free since 1922 [3]. The gradual reduction of rabies throughout western Europe, where sylvatic rabies had been endemic since the late 1960s, has further reduced the risk of rabies entering the country from mainland Europe. A similar situation now exists in much of Europe where the introduction of oral-vaccination campaigns has been highly effective at eliminating fox rabies [4]. However, the danger of importation is always present and there have been a number of well documented cases of rabies introduction, particularly through movement of dogs from Morocco into Spain and France [5]. For the UK, two methods of preventing rabies entry have been in use over the past decade. The first has been quarantine of companion animals and exotic species, particularly bats and carnivores. Figures for the numbers of dogs and cats entering quarantine between 1999 and 2005 are shown in Table 1. A decline in the number of animals entering quarantine was observed as a result of the introduction in 2000 of the second method of controlling entry of companion animals, the UK Pet Travel Scheme (PETS). This enabled immediate entry for dogs, cats and ferrets provided the animal complies with the requirements of the scheme, principally demonstration of vaccination against rabies. This scheme has proven highly popular with companion animal owners with approximately 750,000 animals entering the UK by this route between 2000 and 2010 (Table 2). Both these mechanisms of companion animal entry into the UK will be harmonized with those of other European Union member states at the start of 2012 [6].
Meldrum (1988) [7] reported 29 cases of rabies in quarantine animals up to 1988. On two occasions, cases of rabies have resulted in dogs after the six-month quarantine period [8] and as a consequence compulsory vaccination was introduced in 1974 for all dogs and cats entering quarantine. In both cases, the incursions were dealt with swiftly and no further incidence of the disease was reported. To date, the UK has never resorted to the use of vaccination to control the disease in wildlife although this contingency would be considered by the Department for Environment, Food and Rural Affairs (Defra), the government department responsible for animal disease control. Prior to 2008, the most recent rabies associated death in quarantine occurred in a dog imported from Zambia in 1990. There is a legal obligation to submit the head of any animal that dies in quarantine for rabies diagnosis. In 2008, there were 29 deaths in UK quarantine.
Two preliminary reports have previously described the clinical history of the case, rabies diagnosis [9] and the public health investigation that occurred [10]. Briefly, the dog was imported from Sri Lanka to the UK by an animal welfare charity. The dog arrived at Heathrow airport on the 17 April 2008, and spent one night at the Animal Reception Centre before being transferred to quarantine kennels in north-east London. The dog had a history of severe diarrhoea and convulsed violently before it died on the morning of the 25 April. The initial diagnosis was for infection with Hepatozoon canis for which it had been treated with doxycycline. Confirmation of rabies infection was made within 24 hours of the death of the dog. A further four dogs that had contact with the dog underwent euthanasia as a precautionary measure. Eleven individuals in the UK received post-exposure prophylaxis (PEP) following direct physical contact with the dog.
This study confirms the epidemiological link between this case of rabies and the epidemic of dog rabies in Sri Lanka. We also report the histological findings in brain sections of this animal and note the absence of Negri bodies in this particular case.
2. Materials and Methods
2.1. Rabies Virus Diagnosis
Rabies virus infection was confirmed on acetone fixed impression slides of brain samples using the Fluorescent Antibody Test [11] using FITC labeled anti-rabies monoclonal globulin (Fujirebio). This was confirmed using real-time RT-PCR [12] and virus isolation using the rabies tissue culture infection test (RTCIT) as previously described [13]. The mouse inoculation test was used following published guidelines [14]. Briefly, mice (n = 3) were inoculated with 30 μL homogenates prepared from brain samples and monitored for 28 days for the development of rabies.
2.2. Phylogenetic Analysis
RNA was extracted from brain samples using the TriZol™ method following the manufacturers protocols (Invitrogen). Total RNA was resuspended in HPLC grade water and diluted to 1 μg/μL. The primer Jw12 (5′-ATGTAACACC[C/T]CTACAATG-3′) was used to generate cDNA by reverse transcription with MMLV reverse transcriptase (Promega). The cDNA generated was used to amplify a 606 base pair (bp) fragment of the RABV nucleoprotein with primers Jw12 and Jw6dpl (5′-CAATTCGCACACATTTTGTG-3′). These primers were also used to sequence the fragment generated. Sequence analysis was conducted as described in Johnson et al. (2002) [15]. Phylogenetic analysis was undertaken on a 400 bp sequence of the RABV nucleoprotein gene (genome positions 71 to 470, based on the Pasteur virus genome, GenBank Accession number NC_001542) using the PHYLIP 3.5 package. One thousand bootstrap replicates were undertaken with values over 700 being considered significant. The consensus phylogenetic tree was visualised using the Treeview program, version 3.2.
2.3. Histopathology
Samples of the brainstem, hippocampus and cerebellum were dissected from the dog's brain and immediately fixed in formalin for five days. Fixed tissues were blocked and processed to paraffin wax. Serial 4 μm wax sections were cut and stained with hematoxylin and eosin (H & E) for histological examination or used for immunohistochemistry. Rabies virus nucleocapsid was detected using the monoclonal antibody HAM 5DF123B0 (a gift from the Swiss Rabies Centre, Switzerland). Antibody binding was detected with a biotinylated anti-mouse secondary antibody (Vector Laboratories) and amplified using an avidin-biotin-peroxidase conjugate with 3,3-diaminobenzidine (Sigma-Aldrich) for visualization, as previously described [16].
3. Results
Rabies virus infection was confirmed by the fluorescent antibody test and was corroborated by amplification of a genotype 1 lyssavirus by a differential reverse transcriptase-Polymerase Chain Reaction (RT-PCR) TaqMan assay from RNA extracted from the brain. Rabies virus was isolated from brain homogenates prepared directly from the animal and 100% of mice inoculated with this homogenate developed rabies encephalitis on day nine post-inoculation. A nested RT-PCR amplified a 606 bp fragment of the rabies nucleoprotein gene and this was used to derive 400 bp of genomic sequence from the infecting virus. Sequence alignment with a panel of isolates from Asia, and particularly from published sequences from Sri Lanka (Table 3), revealed that the RABV isolated from the dog had >99% sequence identity with RABV isolates from Sri Lanka. The phylogenetic tree (Figure 1 and inset) demonstrated that the isolated virus sequence (JN968375) clustered closely with a lineage of viruses isolated from India and the south-western provinces of Sri Lanka. This supports the evidence provided in the case history.
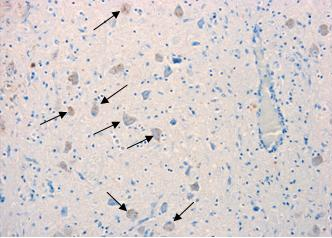
The presentation of brain sections revealed a mild non-suppurative encephalitis and leptomeningitis consistent with viral encephalitis. The characteristic changes were observed in all regions of the brain examined, including hippocampus, brainstem and cerebellum. They consisted of neuronal degeneration and necrosis, with occasional neuronal vacuolation, shrunken hyperchromatic neurons and images of central chromatolysis and nuclear margination, satellitosis, focal gliosis and lymphohistiocytic perivascular cuffing of no more than one cell of thickness [Figure 2(B)]. Inflammatory changes were more prominent in the brainstem. Negri bodies were not observed in any of the samples examined.
Immunolabelling against rabies nucleocapsid was detected in the perikaryon and neuropil of neurons in all examined regions [Figure 2(C) and Figure 3]. No staining was observed in the corresponding regions of a normal dog brain used as a control [Figure 2(A) and Figure 3(A)]. The region with a higher number of immunolabelled neurons and with a more prominent immunolabelling in their perikaryon was the brainstem (Figure 2). Immunolabelling in the hippocampus was observed in a small number of pyramidal neurons [Figure 3(D)]. Detection of viral antigen in the cerebellum was occasional in the Purkinje cell layer [Figure 3(B)] and more frequent in the cells of the granular and molecular layer.
4. Discussion
Quarantine of dogs entering the UK has been an effective measure for preventing the entry of rabies since the beginning of the 20th century. Despite the introduction of the UK and EU PETS, which caused a noticeable drop in the number of animals entering quarantine, this has been an option for those wishing to import dogs into the country with over 3,000 entries in 2005 (Table 2). Despite the introduction of vaccination either in the country of origin or upon entry into quarantine, the risk still exists that an animal may have become infected during the period prior to entering the UK and that infection has advanced to a point where vaccination becomes ineffective. A further factor that may reduce the effectiveness of vaccination is the age of the dog [24]. Two recent cases of rabies in juvenile dogs in North America have highlighted this issue [25,26]. Vaccine manufacturers do not recommend rabies vaccination of dogs before twelve weeks of age due to possible presence of maternal antibodies that may inhibit the immune response [27]. This provides a window of susceptibility for those young dogs that do not have maternal antibody protection, as is likely in the case described here. Rabies is endemic in Sri Lanka with 68 cases of human rabies and 705 cases of dog rabies reported in 2006 [28]. However, these figures may be higher due to under reporting [29]. This has been in the face of large scale dog vaccination campaigns with approximately 1 million dogs vaccinated in 2006. A phylogenetic comparison of the sequence derived from the UK case with a panel of RABV sequences previously published by studies on the phylogeny of RABV from Sri Lanka [17,18] demonstrated a clear link between the case in quarantine and RABV circulating in Sri Lanka. These earlier studies have shown that the RABVs endemic in Sri Lanka are closely related to the viruses present in southern India. The Sri Lankan RABV isolated in the UK showed almost 100% sequence homology with some members of the panel and suggests that the dog was infected in the south-western provinces of Sri Lanka.
Although the disease symptoms observed in this animal were not typical of a rabies infected dog, investigation of samples by the FAT test demonstrated that the brain was heavily infected with RABV. This was confirmed by immunohistochemistry, which revealed widespread labelling of rabies nucleoprotein in the brainstem/medulla, hippocampus and, to a lesser extent, the cerebellum. The histological changes were consistent with viral encephalitis displaying characteristic degenerative and inflammatory changes. The nature and distribution of the lesions in this case were similar to those reported in a case of rabies in a 10-week-old puppy [25]. However, in the UK case and distinct from that observed in the Canadian dog, there was a complete absence of Negri bodies in all samples examined despite this being considered a common histological sign of rabies. This reflects the observation that up to 30% of street rabies cases do not produce Negri bodies [30,31] and emphasizes the requirement for virus-specific primary diagnostic tests such as the FAT and RT-PCR in cases of viral encephalitis of undetermined origin. The relatively high detection of viral antigen in the medulla/brainstem may be a consequence of the peripheral infection, as the medulla acts as crossing of motor tracts between the spinal cord and the brain. This in turn suggests that the brainstem should also be considered as a diagnostic sample when investigating animal cases suspected of rabies.
In March 2003 Defra launched DACTARI (Dog and Cat Travel and Risk Information). This is a national voluntary scheme for the investigation of the possible occurrence of exotic diseases in dogs and cats in Great Britain such as leishmaniasis, babesiosis, ehrlichiosis and dirofilariasis (of which leishmaniasis and ehrliciosis are zoonotic). Since 2003, there have been 100 reports of exotic diseases. Sixty-seven percent of which were detected in animals imported under PETS and 25% were detected in animals within quarantine.
4. Conclusions
Rabies virus is endemic in domestic dog populations in many regions of the world and as a result of movement of companion animals between countries the risk persists of transfer of infected animals between different areas, resulting in new outbreaks. Within rabies-free areas of the world, the consequences of such introductions are varied and range from a need to control the initial outbreak to investment in surveillance to demonstrate freedom from disease. For zoonotic viruses, there is a risk to public and veterinary health, which for rabies would result in human and animal deaths. Compulsory vaccination of dogs and restrictions on dog movement would also be required. In the case described in this study, no onward transmission occurred and a potential outbreak was controlled. However, the case demonstrates the need for constant vigilance by public health bodies in detecting incursions of potentially rabies infected animals and providing a rapid response to protect human contacts from this fatal disease.



| Year | Entering by quarantine |
|---|---|
| 1999 | 6,989 * |
| 2000 | 5,296 |
| 2001 | 5,304 |
| 2002 | 3,555 |
| 2003 | 4,405 |
| 2004 | 3,514 |
| 2005 | 3,337 |
*The PETS scheme was introduced in 2000.
| Year | Cats | Dogs | Ferrets | Annual Total | Cumulative Total |
|---|---|---|---|---|---|
| 2000 | 2,062 | 12,633 | 0 | 14,695 | 14,695 |
| 2001 | 3,562 | 23,158 | 0 | 26,720 | 41,415 |
| 2002 | 4,359 | 36,410 | 0 | 40,769 | 82,184 |
| 2003 | 6,012 | 48,938 | 0 | 54,951 | 137,134 |
| 2004 | 7,314 | 57,418 | 10 | 64,742 | 201,876 |
| 2005 | 8,346 | 69,531 | 39 | 77,916 | 279,792 |
| 2006 | 8,375 | 74,403 | 32 | 82,810 | 362,602 |
| 2007 | 10,137 | 89,127 | 43 | 99,307 | 461,909 |
| 2008 | 10,287 | 93,719 | 52 | 104,058 | 565,967 |
| 2009 | 7,128 | 89,376 | 55 | 96,559 | 662,526 |
| 2010 | 7,105 | 78,076 | 56 | 85,237 | 747,736 |
| Total | 7,4687 | 672,789 | 287 | 747,763 |
| Isolate reference No. | Country of origin | Host | Genbank reference | Reference |
|---|---|---|---|---|
| RV2417 | UK (ex Sri Lanka) | Dog | JN968375 | This study |
| SRL1032 | Sri Lanka | Jackal | AB041964 | [17] |
| SRL1036 | Sri Lanka | Human | AB041965 | [17] |
| SRL1060 | Sri Lanka | Dog | AB041966 | [17] |
| SRL1077 | Sri Lanka | Mongoose | AB041967 | [17] |
| SRL1143 | Sri Lanka | Cat | AB041968 | [17] |
| SRL1145 | Sri Lanka | Water Buffalo | AB041969 | [17] |
| RV69 | Sri Lanka | Dog | AY102995 | [15] |
| 1294 | Sri Lanka | Dog | AY138549 | [18] |
| 5657 | Sri Lanka | Bovine | AY138550 | [18] |
| ? | India | AF374721 | [19] | |
| 9702INDI | India | Human | EU086191 | [20] |
| 94257SRI | Sri Lanka | Human | U22917 | [21] |
| HCM2 | Vietnam | Dog | AB299033 | Unpublished |
| HCM10 | Vietnam | Dog | AB299039 | Unpublished |
| HCM1 | Vietnam | Dog | AB299032 | Unpublished |
| VN52 | Vietnam | Dog | AB116580 | Unpublished |
| 8677MAL | Malaysia | ? | U22916 | [21] |
| Guizhou A148 | China | Dog | DQ666291 | [22] |
| Henan Sq9 | China | Dog | DQ666299 | [22] |
| Guizhou Qx2 | China | Dog | DQ666295 | [22] |
| Guizhou A103 | China | Dog | DQ666290 | [22] |
| RV277 | Pakistan | Goat | AY062069 | [15] |
| Pasteur virus | - | - | M13215 | [23] |
Acknowledgments
This work was funded by grants SV3500, SE0528 and SEO423 from the Department for Environment Food and Rural Affairs (Defra), UK.
Conflict of Interest
The authors declare no conflict of interest.
Declaration
© 2011 Crown Copyright. This is under the terms of the Open Government Licence which governs the use and publication of all Crown copyright material. For more information on the terms of the Licence and how the contribution should be credited please follow this link http://www.nationalarchives.gov.uk/information-management/uk-gov-licensing-framework.htm.
References
- Hampson, K.; Dushoff, J.; Cleaveland, S; Haydon, D.T.; Kaare, M.; Packer, C.; Dobson, A. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009, 7. [Google Scholar] [CrossRef]
- Lembo, T.; Attlan, M.; Bourhy, H.; Cleaveland, S.; Costa, P.; de Balogh, K.; Dodet, B.; Fooks, A.R.; Hiby, E.; Leanes, F.; et al. Renewed global partnerships and redesigned roadmaps for rabies prevention and control. Vet. Med. Int. 2011, 2011. [Google Scholar] [CrossRef]
- Fooks, A.R.; Roberts, D.H.; Lynch, M.; Hersteinsson, P.; Runolfsson, H. Rabies in the United Kingdom, Ireland and Iceland. In Historical Perspectives of Rabies in Europe and the Mediterranean Basin; King, A.A., Fooks, A.R., Aubert, M., Wandeler, A.I., Eds.; OIE Publications: Paris, France, 2004; pp. 25–32. [Google Scholar]
- Cliquet, F.; Aubert, M. Elimination of terrestrial rabies in Western European countries. Dev. Biol. 2004, 119, 185–204. [Google Scholar]
- Johnson, N.; Freuling, C.; Horton, D.; Müller, T.; Fooks, A.R. Imported rabies, European Union and Switzerland, 2001–2010. Emerg. Infect. Dis. 2011, 17, 753–754. [Google Scholar]
- Fooks, A.R.; Horton, D.L.; Johnson, N.; Toth, B.; Roberts, H.L. Changes to pet travel rules: Rabies, ticks and tapeworms. Vet. Rec. 2011, 169, 97–98. [Google Scholar]
- Meldrum, K.C. Rabies contingency plans in the UK. Parassitologia 1988, 30, 97–103. [Google Scholar]
- McIntosh, K.S. Rabies in Great Britain. Aust. Vet. J. 1971, 47, 20–22. [Google Scholar]
- Fooks, A.R.; Harkess, G.; Goddard, T.; Marston, D.A.; McElhinney, L.M.; Brown, K.; Morgan, D.; Paul, R.; Thomas, P.J.; Smith, B. Rabies in a dog imported to the UK from Sri Lanka. Vet. Rec. 2008, 162. [Google Scholar] [CrossRef]
- Catchpole, M.; Thomas, L.; Morgan, D.; Brown, K.; Turbitt, D.; Kirkbride, H. Imported rabies in a quarantine centre in the United Kingdom. Euro Surveill. 2008, 13, p. 18868. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18868 (accessed on 15 November 2011). [Google Scholar]
- Dean, D.J.; Abelseth, M.K.; Atanasiu, P. The Fluorescent Antibody Test. In Laboratory Techniques in Rabies, 4th ed.; Meslin, F.-X., Kaplan, M.M., Koprowski, H., Eds.; World Health Organisation: Geneva, Switzerland, 1996; pp. 88–95. [Google Scholar]
- Wakeley, P.R.; Johnson, N.; McElhinney, L.M.; Marston, D.A.; Sawyer, J.; Fooks, A.R. Development of a real-time, Taqman RT-PCR assay for detection and differentiation of lyssavirus genotypes 1, 5 and 6. J. Clin. Microbiol. 2005, 43, 2786–2792. [Google Scholar]
- Webster, W.A. A tissue-culture infection test in routine diagnosis. Can. J. Vet. Res. 1986, 51, 367–369. [Google Scholar]
- Koprowski, H. The Mouse Inoculation Test. In Laboratory Techniques in Rabies, 4th ed.; Meslin, F.-X., Kaplan, M.M., Koprowski, H., Eds.; World Health Organisation: Geneva, Switzerland, 1996; pp. 80–87. [Google Scholar]
- Johnson, N.; McElhinney, L.M.; Smith, J.; Lowings, P.; Fooks, A.R. Phylogenetic analysis of the genus Lyssavirus using distal coding sequences of the glycoprotein and nucleoprotein genes. Arch. Virol. 2002, 147, 2111–2123. [Google Scholar]
- Hicks, D.J.; Healy, D.M.; Nunez, A.; Brookes, S.M.; Johnson, N.; Fooks, A.R. Comparative pathological study of the murine brain after experimental infection with classical rabies virus and European bat lyssaviruses. J. Comp. Pathol. 2009, 140, 113–126. [Google Scholar]
- Arai, Y.T.; Takahashi, H.; Kameoka, Y.; Shiino, T.; Wimararatne, O.; Lodmell, D.L. Characterization of Sri Lanka rabies virus isolates using nucleotide sequence analysis of nucleoprotein gene. Acta Virol. 2001, 45, 327–333. [Google Scholar]
- Nanayakkara, S.; Smith, J.S.; Rupprecht, C.E. Rabies in Sri Lanka: Splendid isolation. Emerg. Infectous Dis. 2003, 9, 368–371. [Google Scholar]
- Jayakumar, R.; Tirumurugaan, K.G.; Ganga, G.; Kumanan, K.; Mahalinga Nainar, A. Characterization of nucleoprotein gene sequence of an Indian isolate of rabies virus. Acta Virol. 2004, 48, 47–50. [Google Scholar]
- Bourhy, H.; Reynes, J.M.; Dunham, E.J.; Dacheux, L.; Larrous, F.; Huong, V.T.; Xu, G.; Yan, J.; Miranda, M.E.; Holmes, E.C. The origin and phylogeography of dog rabies virus. J. Gen. Virol. 2008, 89, 2673–2681. [Google Scholar]
- Kissi, B.; Tordo, N.; Bourhy, H. Genetic polymorphism in the rabies virus nucleoprotein. Virology 1995, 209, 526–537. [Google Scholar]
- Zhang, Y.Z.; Xiong, C.L.; Zou, Y.; Wang, D.M.; Jiang, R.J.; Xiao, Q.Y.; Hao, Z.Y.; Zhang, L.Z.; Yu, Y.X.; Fu, Z.F. Molecular characterization of rabies virus isolates in China during 2004. Virus Res. 2006, 121, 179–188. [Google Scholar]
- Tordo, N.; Poch, E.; Ermine, A.; Keith, G.; Rougeon, F. Completion of the rabies virus genome sequence determination: Highly conserved domains among the L (polymerase) proteins of unsegmented negative-strand RNA viruses. Virology 1998, 165, 565–576. [Google Scholar]
- Mansfield, K.L.; Burr, P.D.; Snodgrass, D.R.; Sayers, R.; Fooks, A.R. Factors affecting the serological response of dogs and cats to rabies vaccination. Vet. Rec. 2004, 154, 423–426. [Google Scholar]
- White, J.; Taylor, S.M.; Wolfram, K.L.; O'Conner, B.P. Rabies in a 10-week-old puppy. Can. Vet. J. 2007, 48, 931–934. [Google Scholar]
- Castrodale, L.; Walker, V.; Baldwin, J.; Hafmann, J.; Hanlon, C. Rabies in a puppy imported from India to the USA, March 2007. Zoonoses Public Health 2008, 55, 427–430. [Google Scholar]
- Winters, W.D. Time dependent decreases of maternal canine virus antibodies in newborn pups. Vet. Rec. 1981, 108, 295–299. [Google Scholar]
- Fu, Z.F. The rabies situation in Far East Asia. Dev. Biol. 2008, 131, 65–72. [Google Scholar]
- Kumarapeli, V.; Awerbuch-Friedlander, T. Human rabies focusing on dog ecology—A challenge to public health in Sri Lanka. Acta Trop. 2009, 112, 33–37. [Google Scholar]
- Kristensson, K.; Dastur, D.K.; Manghani, D.K.; Tsiang, H.; Bentivoglio, M. Rabies: Interactions between neurons and viruses. A review of the history of Negri inclusion bodies. Neuropathol. Appl. Neurobiol. 1996, 22, 179–187. [Google Scholar]
- Bentivoglio, M. Intraneuronal inclusion bodies: From Negri bodies to proteosomal dysfunction. Rend. Fis. Acc. Lincei 2003, 14, 263–279. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Johnson, N.; Nunez, A.; Marston, D.A.; Harkess, G.; Voller, K.; Goddard, T.; Hicks, D.; McElhinney, L.M.; Fooks, A.R. Investigation of an Imported Case of Rabies in a Juvenile Dog with Atypical Presentation. Animals 2011, 1, 402-413. https://doi.org/10.3390/ani1040402
Johnson N, Nunez A, Marston DA, Harkess G, Voller K, Goddard T, Hicks D, McElhinney LM, Fooks AR. Investigation of an Imported Case of Rabies in a Juvenile Dog with Atypical Presentation. Animals. 2011; 1(4):402-413. https://doi.org/10.3390/ani1040402
Chicago/Turabian StyleJohnson, Nicholas, Alex Nunez, Denise A. Marston, Graeme Harkess, Katja Voller, Trudy Goddard, Daniel Hicks, Lorraine M. McElhinney, and Anthony R. Fooks. 2011. "Investigation of an Imported Case of Rabies in a Juvenile Dog with Atypical Presentation" Animals 1, no. 4: 402-413. https://doi.org/10.3390/ani1040402
APA StyleJohnson, N., Nunez, A., Marston, D. A., Harkess, G., Voller, K., Goddard, T., Hicks, D., McElhinney, L. M., & Fooks, A. R. (2011). Investigation of an Imported Case of Rabies in a Juvenile Dog with Atypical Presentation. Animals, 1(4), 402-413. https://doi.org/10.3390/ani1040402