Cellular and Molecular Adaptation of Bovine Granulosa Cells and Oocytes under Heat Stress
Simple Summary
Abstract
1. Introduction
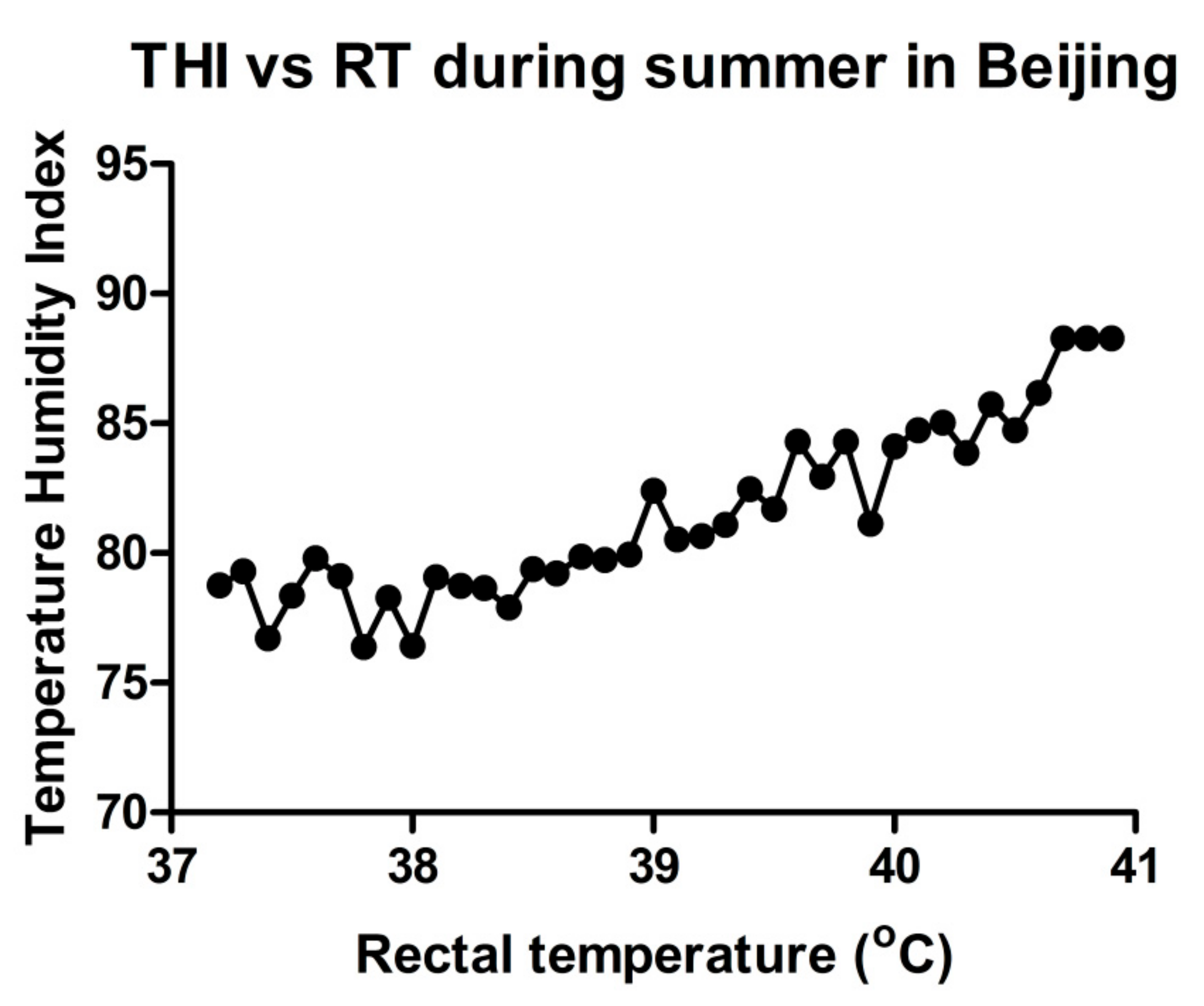
2. Heat Stress Assessment
3. Impact of Heat Stress on Cow Reproduction
Hormonal Regulation and Estrous Behavior
4. Impact of Heat Stress on Granulosa Cell Function
5. Impact of Heat Stress on Ovarian Pool of Follicles and Oocyte Quality
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hansen, P.J. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Torres-Júnior, J.R.d.S.; Pires, M.d.F.A.; de Sá, W.F.; Ferreira, A.d.M.; Viana, J.H.M.; Camargo, L.S.A.; Ramos, A.A.; Folhadella, I.M.; Polisseni, J.; de Freitas, C.; et al. Effect of maternal heat-stress on follicular growth and oocyte competence in Bos indicus cattle. Theriogenology 2008, 69, 155–166. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Wakamiya, K.; Kohka, M.; Yamamoto, Y.; Okuda, K. Summer heat stress affects prostaglandin synthesis in the bovine oviduct. Reproduction 2013, 146, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Shehab-El-Deen, M.A.M.M.; Leroy, J.L.M.R.; Fadel, M.S.; Saleh, S.Y.A.; Maes, D.; Van Soom, A. Biochemical changes in the follicular fluid of the dominant follicle of high producing dairy cows exposed to heat stress early post-partum. Anim. Reprod. Sci. 2010, 117, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Heldmaier, G.; Ortmann, S.; Elvert, R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 2004, 141, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.R.; Richardson, D.W.; Hutchison, J.S.; Germak, J.A.; Knobil, E. Hypophysiotropic signal frequency and the functioning of the pituitary-ovarian system in the rhesus monkey. Endocrinology 1983, 112, 2076–2080. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.K.; Dobson, H.; Scaramuzzi, R.J. Ovarian function in ewes made hypogonadal with GnRH antagonist and stimulated with FSH in the presence or absence of low amplitude LH pulses. J. Endocrinol. 1998, 156, 213–222. [Google Scholar] [CrossRef][Green Version]
- Voronina, E.; Lovasco, L.A.; Gyuris, A.; Baumgartner, R.A.; Parlow, A.F.; Freiman, R.N. Ovarian granulosa cell survival and proliferation requires the gonad-selective TFIID subunit TAF4b. Dev. Biol. 2007, 303, 715–726. [Google Scholar] [CrossRef]
- Su, Y.Q.; Wu, X.; O’Brien, M.J.; Pendola, F.L.; Denegre, J.N.; Matzuk, M.M.; Eppig, J.J. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: Genetic evidence for an oocyte-granulosa cell regulatory loop. Dev. Biol. 2004, 276, 64–73. [Google Scholar] [CrossRef]
- Eppig, J.J. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001, 122, 829–838. [Google Scholar] [CrossRef]
- Da Silva-Buttkus, P.; Jayasooriya, G.S.; Mora, J.M.; Mobberley, M.; Ryder, T.A.; Baithun, M.; Stark, J.; Franks, S.; Hardy, K. Effect of cell shape and packing density on granulosa cell proliferation and formation of multiple layers during early follicle development in the ovary. J. Cell Sci. 2008, 121, 3890–3900. [Google Scholar] [CrossRef]
- Sakatani, M.; Yamanaka, K.; Balboula, A.Z.; Takenouchi, N.; Takahashi, M. Heat stress during in vitro fertilization decreases fertilization success by disrupting anti-polyspermy systems of the oocytes. Mol. Reprod. Dev. 2015, 82, 36–47. [Google Scholar] [CrossRef]
- Wegner, K.; Lambertz, C.; Das, G.; Reiner, G.; Gauly, M. Effects of temperature and temperature-humidity index on the reproductive performance of sows during summer months under a temperate climate. Anim. Sci. J. 2016, 87, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J.; Aréchiga, C.F. Strategies for managing reproduction in the heat-stressed dairy cow. J. Anim. Sci. 1999, 77, 36–50. [Google Scholar] [CrossRef]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Matsuzuka, T.; Ozawa, M.; Nakamura, A.; Ushitani, A.; Hirabayashi, M.; Kanai, Y. Effects of heat stress on the redox status in the oviduct and early embryonic development in mice. J. Reprod. Dev. 2005, 51, 281–287. [Google Scholar] [CrossRef]
- Li, L.; Wu, J.; Luo, M.; Sun, Y.; Wang, G. The effect of heat stress on gene expression, synthesis of steroids, and apoptosis in bovine granulosa cells. Cell Stress Chaperones 2016, 21, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Fujita, J. Cold shock response in mammalian cells. J. Mol. Microbiol. Biotechnol. 1999, 1, 243–255. [Google Scholar] [PubMed]
- Collier, R.J.; Stiening, C.M.; Pollard, B.C.; Vanbaale, M.J.; Baumgard, L.H.; Gentry, P.C.; Coussens, P.M. Use of gene expression microarrays for evaluating environmental stress tolerance at the cellular level in cattle 1. J. Anim. Sci. 2014, 1–13. [Google Scholar] [CrossRef]
- Page, T.J.; Sikder, D.; Yang, L.; Pluta, L.; Wolfinger, R.D.; Kodadek, T.; Thomas, R.S. Genome-wide analysis of human HSF1 signaling reveals a transcriptional program linked to cellular adaptation and survival. Mol. Biosyst. 2006, 2, 627–639. [Google Scholar] [CrossRef]
- Lee, W.C.; Wen, H.C.; Chang, C.P.; Chen, M.Y.; Lin, M.T. Heat shock protein 72 overexpression protects against hyperthermia, circulatory shock, and cerebral ischemia during heatstroke. J. Appl. Physiol. 2006, 100, 2073–2082. [Google Scholar] [CrossRef]
- Dikmen, S.; Hansen, P.J. Is the temperature-humidity index the best indicator of heat stress in lactating dairy cows in a subtropical environment? J. Dairy Sci. 2009, 92, 109–116. [Google Scholar] [CrossRef] [PubMed]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Kadokawa, H.; Sakatani, M.; Hansen, P.J. Perspectives on improvement of reproduction in cattle during heat stress in a future Japan. Anim. Sci. J. 2012, 83, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.S. Environmental factors influencing heat stress in feedlot cattle 1, 2. Glob. Environ. Chang. 2006, 84, 712–719. [Google Scholar]
- Wolfenson, D.; Roth, Z.; Meidan, R. Impaired reproduction in heat-stressed cattle: Basic and applied aspects. Anim. Reprod. Sci. 2000, 60–61, 535–547. [Google Scholar] [CrossRef]
- Oseni, S.; Misztal, I.; Tsuruta, S.; Rekaya, R. Seasonality of days open in US Holsteins. J. Dairy Sci. 2003, 86, 3718–3725. [Google Scholar] [CrossRef]
- Ozawa, M.; Tabayashi, D.; Latief, T.A.; Shimizu, T.; Oshima, I.; Kanai, Y. Alterations in follicular dynamics and steroidogenic abilities induced by heat stress during follicular recruitment in goats. Reproduction 2005, 129, 621–630. [Google Scholar] [CrossRef]
- Shimizu, T.; Oshima, I.; Ozawa, M.; Takahashi, S.; Tajima, A.; Shiota, M.; Miyazaki, H.; Kanai, Y. Heat stress diminishes gonadotropin receptor expression and enhances susceptibility to apoptosis of rat granulosa cells. Reproduction 2005, 129, 463–472. [Google Scholar] [CrossRef]
- Fu, Y.; He, C.J.; Ji, P.Y.; Zhuo, Z.Y.; Tian, X.Z.; Wang, F.; Tan, D.X.; Liu, G.S. Effects of melatonin on the proliferation and apoptosis of sheep granulosa cells under thermal stress. Int. J. Mol. Sci. 2014, 15, 21090–21104. [Google Scholar] [CrossRef] [PubMed]
- Mustafi, S.B.; Chakraborty, P.K.; Dey, R.S.; Raha, S. Heat stress upregulates chaperone heat shock protein 70 and antioxidant manganese superoxide dismutase through reactive oxygen species (ROS), p38MAPK, and Akt. Cell Stress Chaperones 2009, 14, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Sunil Kumar, B.V.; Kumar, A.; Kataria, M. Effect of heat stress in tropical livestock and different strategies for its amelioration. J. Stress Physiol. Biochem. 2011, 7, 45–54. [Google Scholar]
- Vale, W.G. Effects of environment on buffalo reproduction. Ital. J. Anim. Sci. 2007, 6, 130–142. [Google Scholar] [CrossRef]
- López-Gatius, F. Is fertility declining in dairy cattle? A retrospective study in northeastern Spain. Theriogenology 2003, 60, 89–99. [Google Scholar] [CrossRef]
- Wolfenson, D.; Roth, Z. Impact of heat stress on cow reproduction and fertility. Anim. Front. 2019, 9, 32–38. [Google Scholar] [CrossRef]
- Wolfenson, D.; Lew, B.J.; Thatcher, W.W.; Graber, Y.; Meidan, R. Seasonal and acute heat stress effects on steroid production by dominant follicles in cows. Anim. Reprod. Sci. 1997, 47, 9–19. [Google Scholar] [CrossRef]
- Roth, Z.; Meiden, R.; Braw-Tal, R.; Wolfenson, D. Immediate and delayed effects of heat stress on follicular development and its association with plasma FSH and inhibin concentration in cows. J. Reprod. Fertil. 2000, 120, 83–90. [Google Scholar] [CrossRef]
- Kanai, Y.; Yagyu, N.; Shimizu, T. Hypogonadism in Heat Stressed Goats: Poor Responsiveness of the Ovary to the Pulsatile LH Stimulation Induced by Hourly Injections of a Small Dose of GnRH. J. Reprod. Dev. 1995, 41, 133–139. [Google Scholar] [CrossRef][Green Version]
- Bridges, P.J.; Brusie, M.A.; Fortune, J.E. Elevated temperature (heat stress) in vitro reduces androstenedione and estradiol and increases progesterone secretion by follicular cells from bovine dominant follicles. Domest. Anim. Endocrinol. 2005, 29, 508–522. [Google Scholar] [CrossRef]
- Howell, J.L.; Fuquay, J.W.; Smith, A.E. Corpus Luteum Growth and Function in Lactating Holstein Cows During Spring and Summer. J. Dairy Sci. 1994, 77, 735–739. [Google Scholar] [CrossRef]
- Gilad, E.; Meidan, R.; Berman, A.; Graber, Y.; Wolfenson, D. Effect of heat stress on tonic and GnRH-induced gonadotrophin secretion in relation to concentration of oestradiol in plasma of cyclic cows. J. Reprod. Fertil. 1993, 99, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.E.; Armstrong, D.V.; Huber, J.T.; Hunter, R.; Wiersma, F. Hormonal Alterations in the Lactating Dairy Cow in Response to Thermal Stress. J. Dairy Sci. 1988, 71, 2480–2485. [Google Scholar] [CrossRef]
- Chenault, J.R.; Kratzer, D.D.; Rzepkowski, R.A.; Goodwin, M.C. LH and FSH response of Holstein heifers to fertirelin acetate, gonadorelin and buserelin. Theriogenology 1990, 34, 81–98. [Google Scholar] [CrossRef]
- Wolfenson, D.; Bartol, F.F.; Badinga, L.; Barros, C.M.; Marple, D.N.; Cummins, K.; Wolfe, D.; Lucy, M.C.; Spencer, T.E.; Thatcher, W.W. Secretion of PGF2α and oxytocin during hyperthermia in cyclic and pregnant heifers. Theriogenology 1993, 39, 1129–1141. [Google Scholar] [CrossRef]
- Palta, P.; Mondal, S.; Prakash, B.S.; Madan, M.L. Peripheral inhibin levels in relation to climatic variations and stage of estrous cycle in buffalo (bubalus bubalis). Theriogenology 1997, 47, 989–995. [Google Scholar] [CrossRef]
- Thatcher, W.W.; Macmillan, K.L.; Hansen, P.J.; Drost, M. Concepts for regulation of corpus luteum function by the conceptus and ovarian follicles to improve fertility. Theriogenology 1989, 31, 149–164. [Google Scholar] [CrossRef]
- Roelofs, J.; López-Gatius, F.; Hunter, R.H.F.; van Eerdenburg, F.J.C.M.; Hanzen, C. When is a cow in estrus? Clinical and practical aspects. Theriogenology 2010, 74, 327–344. [Google Scholar] [CrossRef]
- López-Gatius, F.; Santolaria, P.; Mundet, I.; Yániz, J.L. Walking activity at estrus and subsequent fertility in dairy cows. Theriogenology 2005, 63, 1419–1429. [Google Scholar] [CrossRef]
- Sakatani, M.; Balboula, A.Z.; Yamanaka, K.; Takahashi, M. Effect of summer heat environment on body temperature, estrous cycles and blood antioxidant levels in Japanese Black cow. Anim. Sci. J. 2012, 83, 394–402. [Google Scholar] [CrossRef]
- López-Gatius, F.; Mirzaei, A.; Santolaria, P.; Bech-Sàbat, G.; Nogareda, C.; García-Ispierto, I.; Hanzen, C.; Yániz, J.L. Factors affecting the response to the specific treatment of several forms of clinical anestrus in high producing dairy cows. Theriogenology 2008, 69, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z.; Meidan, R.; Shaham-Albalancy, A.; Braw-Tal, R.; Wolfenson, D. Delayed effect of heat stress on steroid production in medium-sized and preovulatory bovine follicles. Reproduction 2001, 121, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z. Effect of Heat Stress on Reproduction in Dairy Cows: Insights into the Cellular and Molecular Responses of the Oocyte. Annu. Rev. Anim. Biosci. 2017, 5, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Edson, M.A.; Nagaraja, A.K.; Matzuk, M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009, 30, 624–712. [Google Scholar] [CrossRef]
- Richards, J.S.; Midgley, A.R. Protein Hormone Action: A Key to Understanding Ovarian Follicular and Luteal Cell Development. Biol. Reprod. 1976, 14, 82–94. [Google Scholar] [CrossRef]
- Oktay, K.; Briggs, D.; Gosden, R.G. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J. Clin. Endocrinol. Metab. 1997, 82, 3748–3751. [Google Scholar] [CrossRef]
- Albertini, D.F.; Combelles, C.M.H.; Benecchi, E.; Carabatsos, M.J. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction 2001, 121, 647–653. [Google Scholar] [CrossRef]
- Senthilkumaran, B.; Yoshikuni, M.; Nagahama, Y. A shift in steroidogenesis occurring in ovarian follicles prior to oocyte maturation. Mol. Cell. Endocrinol. 2004, 215, 11–18. [Google Scholar] [CrossRef]
- Adashi, E.Y. The IGF family and folliculogenesis. J. Reprod. Immunol. 1998, 39, 13–19. [Google Scholar] [CrossRef]
- Umer, S.; Sammad, A.; Zou, H.; Khan, A.; Weldegebriall Sahlu, B.; Hao, H.; Zhao, X.; Wang, Y.; Zhao, S.; Zhu, H. Regulation of AMH, AMHR-II, and BMPs (2,6) Genes of Bovine Granulosa Cells Treated with Exogenous FSH and Their Association with Protein Hormones. Genes. 2019, 10, 1038. [Google Scholar] [CrossRef]
- Wu, K.C.; McDonald, P.R.; Liu, J.J.; Chaguturu, R.; Klaassen, C.D. Implementation of a High-Throughput Screen for Identifying Small Molecules to Activate the Keap1-Nrf2-ARE Pathway. PLoS ONE 2012, 7, 44686. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Anderson, R.A.; Gourley, C.; Wallace, W.H.; Spears, N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 2012, 18, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, H.; Tian, Z.; Wu, Y.; Wang, Y.; Fang, Y.; Lin, L.; Han, Y.; Wu, S.; Haq, I.U.; et al. Effects of chronic heat stress on granulosa cell apoptosis and follicular atresia in mouse ovary. J. Anim. Sci. Biotechnol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Shen, M.; Wu, W.J.; Li, B.J.; Weng, Q.N.; Li, M.; Liu, H.L. Expression of PUMA in Follicular Granulosa Cells Regulated by FoxO1 Activation During Oxidative Stress. Reprod. Sci. 2015, 22, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Heads, R.J.; Yellon, D.M.; Latchman, D.S. Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J. Mol. Cell. Cardiol. 1995, 27, 1669–1678. [Google Scholar] [CrossRef]
- Iwazawa, M.; Acosta, T.J. Effect of elevated temperatures on bovine corpus luteum function: Expression of heat-shock protein 70, cell viability and production of progesterone and prostaglandins by cultured luteal cells. Anim. Prod. Sci. 2014, 54, 285–291. [Google Scholar] [CrossRef]
- Harada, T.; Koi, H.; Kubota, T.; Aso, T. Haem oxygenase augments porcine granulosa cell apoptosis in vitro. J. Endocrinol. 2004, 181, 191–205. [Google Scholar] [CrossRef]
- Agnew, L.L.; Colditz, I.G. Development of a method of measuring cellular stress in cattle and sheep. Vet. Immunol. Immunopathol. 2008, 123, 197–204. [Google Scholar] [CrossRef]
- Dangi, S.S.; Gupta, M.; Maurya, D.; Yadav, V.P.; Panda, R.P.; Singh, G.; Mohan, N.H.; Bhure, S.K.; Das, B.C.; Bag, S.; et al. Expression profile of HSP genes during different seasons in goats (Capra hircus). Trop. Anim. Health Prod. 2012, 44, 1905–1912. [Google Scholar] [CrossRef]
- Guerriero, V.; Raynes, D.A. Synthesis of heat stress proteins in lymphocytes from livestock. J. Anim. Sci. 1990, 68, 2779–2783. [Google Scholar] [CrossRef]
- Malayer, J.R.; Hansen, P.J.; Buhi, W.C. Effect of day of the oestrous cycle, side of the reproductive tract and heat shock on in-vitro protein secretion by bovine endometrium. J. Reprod. Fertil. 1988, 84, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Putney, D.J.; Malayer, J.R.; Gross, T.S.; Thatcher, W.W.; Hansen, P.J.; Drost, M. Heat Stress-Induced Alterations in the Synthesis and Secretion of Proteins and Prostaglandins by Cultured Bovine Conceptuses and Uterine Endometrium1. Biol. Reprod. 1988, 39, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Dahl, G.E.; Vanbaale, M.J. Major advances associated with environmental effects on dairy cattle. J. Dairy Sci. 2006, 89, 1244–1253. [Google Scholar] [CrossRef]
- Mishra, A.; Hooda, O.K.; Singh, G.; Meur, S.K. Influence of induced heat stress on HSP70 in buffalo lymphocytes. J. Anim. Physiol. Anim. Nutr. (Berl.) 2011, 95, 540–544. [Google Scholar] [CrossRef]
- Beck, S.C.; Paidas, C.N.; Tan, H.; Yang, J.; De Maio, A. Depressed expression of the inducible form of HSP 70 (HSP 72) in brain and heart after in vivo heat shock. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1995, 269, 608–613. [Google Scholar] [CrossRef]
- Albers, R.; Van Der Pijl, A.; Bol, M.; Seinen, W.; Pieters, R. Stress proteins (HSP) and chemical-induced autoimmunity. Toxicol. Appl. Pharmacol. 1996, 140, 70–76. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Martínez, L.; Aréchiga, C.; Bañuelos, R.; Rincón, R.M.; Urrutia, J.; Salinas, H.; Mellado, M. Circannual identification and quantification of constitutive heat shock proteins (HSP 70) in goats. J. Appl. Anim. Res. 2006, 29, 9–12. [Google Scholar] [CrossRef]
- Hayashi, Y.; Tohnai, I.; Kaneda, T.; Kobayashi, T.; Ohtsuka, K. Translocation of hsp-70 and Protein Synthesis during Continuous Heating at Mild Temperatures in HeLa Cells. Radiat. Res. 1991, 125, 80. [Google Scholar] [CrossRef]
- Kim, D.; Somji, S.; Garrett, S.H.; Sens, M.A.; Shukla, D.; Sens, D.A. Expression of hsp 27, hsp 60, hsc 70, and hsp 70 by immortalized human proximal tubule cells (HK-2) following exposure to heat shock, sodium arsenite, or cadmium chloride. J. Toxicol. Environ. Heal. Part A 2001, 63, 475–493. [Google Scholar] [CrossRef]
- Dehbi, M.; Baturcam, E.; Eldali, A.; Ahmed, M.; Kwaasi, A.; Chishti, M.A.; Bouchama, A. Hsp-72, a candidate prognostic indicator of heatstroke. Cell Stress Chaperones 2010, 15, 593–603. [Google Scholar] [CrossRef]
- Ferencz, Á.; Juhász, R.; Butnariu, M.; Deér, K.A.; Varga, I.S.; Nemcsók, J. Expression analysis of heat shock genes in the skin, spleen and blood of common carp (Cyprinus carpio) after cadmium exposure and hypothermia. Acta Biol. Hung. 2012, 63, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Givisiez, P.E.N.; Ferro, J.A.; Ferro, M.I.T.; Kronka, S.N.; Decuypere, E.; Macari, M. Hepatic concentration of heat shock protein 70 kD (Hsp70) in broilers subjected to different thermal treatments. Br. Poult. Sci. 1999, 40, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, R.; Ferro, J.A.; Gonzales, E.; Ferro, M.I.T.; Macari, M.; Bernal, F.E.M. Resistance to ascites syndrome, homoeothermic competence and levels of Hsp70 in the heart and lung of broilers. Rev. Bras. Zootec. 2002, 31, 1442–1450. [Google Scholar] [CrossRef]
- Zulkifli, I.; Che Norma, M.T.; Israf, D.A.; Omar, A.R. The effect of early-age food restriction on heat shock protein 70 response in heat-stressed female broiler chickens. Br. Poult. Sci. 2002, 43, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Edens, F.W. Stress-induced heat-shock protein synthesis in peripheral leukocytes of turkeys, Meleagris gallopavo. Comp. Biochem. Physiol. Part B Biochem. 1993, 106, 621–628. [Google Scholar] [CrossRef]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Meriin, A.B.; Sherman, M.Y.; Morimoto, R.I.; Massie, B. The Chaperone Function of hsp70 Is Required for Protection against Stress-Induced Apoptosis. Mol. Cell. Biol. 2000, 20, 7146–7159. [Google Scholar] [CrossRef]
- Stankiewicz, A.R.; Lachapelle, G.; Foo, C.P.Z.; Radicioni, S.M.; Mosser, D.D. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J. Biol. Chem. 2005, 280, 38729–38739. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, J.M.; Kim, K.R.; Im, H.; Kim, Y.H. Zinc preconditioning protects against neuronal apoptosis through the mitogen-activated protein kinase-mediated induction of heat shock protein 70. Biochem. Biophys. Res. Commun. 2015, 459, 220–226. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Kikusato, M.; Sudo, S.; Amo, T.; Toyomizu, M. Time course of ROS production in skeletal muscle mitochondria from chronic heat-exposed broiler chicken. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2010, 157, 266–271. [Google Scholar] [CrossRef]
- Gu, Z.T.; Li, L.; Wu, F.; Zhao, P.; Yang, H.; Liu, Y.S.; Geng, Y.; Zhao, M.; Su, L. Heat stress induced apoptosis is triggered by transcription-independent p53, Ca2+ dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci. Rep. 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Alemu, T.W.; Pandey, H.O.; Wondim, D.S.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Oxidative and endoplasmic reticulum stress defense mechanisms of bovine granulosa cells exposed to heat stress. Theriogenology 2018, 110, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, R.R.; Gao, S.; Zhai, P.; Zablocki, D.; Holle, E.; Yu, X.; Tian, B.; Wagner, T.; Vatner, S.F.; Sadoshima, J. Sirt1 Regulates Aging and Resistance to Oxidative Stress in the Heart. Circ. Res. 2007, 100, 1512–1521. [Google Scholar] [CrossRef]
- Dilantha Fernando, W.G.; Ramarathnama, R.; Akkanas, S.K.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar]
- Del Vesco, A.P.; Gasparino, E. Production of reactive oxygen species, gene expression, and enzymatic activity in quail subjected to acute heat stress. J. Anim. Sci. 2013, 91, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, C.R.; Jiang, B.; Shelton, J.M.; Richardson, J.A.; Hinshelwood, M.M. Transcriptional regulation of aromatase in placenta and ovary. J. Steroid Biochem. Mol. Biol. 2005, 95, 25–33. [Google Scholar] [CrossRef]
- Mosa, A.; Neunzig, J.; Gerber, A.; Zapp, J.; Hannemann, F.; Pilak, P.; Bernhardt, R. 2β- and 16β-hydroxylase activity of CYP11A1 and direct stimulatory effect of estrogens on pregnenolone formation. J. Steroid Biochem. Mol. Biol. 2015, 150, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wu, Y.; Zhao, S.; Liu, Z.X.; Zeng, S.M.; Zhang, G.X. Lysosomes are involved in induction of steroidogenic acute regulatory protein (StAR) gene expression and progesterone synthesis through low-density lipoprotein in cultured bovine granulosa cells. Theriogenology 2015, 84, 811–817. [Google Scholar] [CrossRef]
- Rekawiecki, R.; Nowik, M.; Kotwica, J. Stimulatory effect of LH, PGE2 and progesterone on StAR protein, cytochrome P450 cholesterol side chain cleavage and 3β hydroxysteroid dehydrogenase gene expression in bovine luteal cells. Prostaglandins Other Lipid Mediat. 2005, 78, 169–184. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Bauer, M. Heat shock proteins in porcine ovary: Synthesis, accumulation and regulation by stress and hormones. Cell Stress Chaperones 2011, 16, 379–387. [Google Scholar] [CrossRef]
- Pierson, R.A.; Ginther, O.J. Ultrasonic imaging of the ovaries and uterus in cattle. Theriogenology 1988, 29, 21–37. [Google Scholar] [CrossRef]
- Fortune, J.E.; Sirois, J. Ovarian Follicular Dynamics during the Estrous Cycle in Heifers Monitored by Real-Time Ultrasonography. Biol. Reprod. 1988, 39, 308–317. [Google Scholar]
- Adams, G.P.; Matteri, R.L.; Kastelic, J.P.; Ko, J.C.H.; Ginther, O.J. Association between surges of follicle-stimulating hormone and the emergence of follicular waves in heifers. J. Reprod. Fertil. 1992, 94, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J.; Bergfelt, D.R.; Kulick, L.J.; Kot, K. Pulsatility of systemic FSH and LH concentrations during follicular-wave development in cattle. Theriogenology 1998, 50, 507–519. [Google Scholar] [CrossRef]
- Mihm, M.; Good, T.E.M.; Ireland, J.L.H.; Ireland, J.J.; Knight, P.G.; Roche, J.F. Decline in Serum Follicle-Stimulating Hormone Concentrations Alters Key Intrafollicular Growth Factors Involved in Selection of the Dominant Follicle in Heifers1. Biol. Reprod. 1997, 57, 1328–1337. [Google Scholar] [CrossRef][Green Version]
- Kulick, L.J.; Kot, K.; Wiltbank, M.C.; Ginther, O.J. Follicular and hormonal dynamics during the first follicular wave in heifers. Theriogenology 1999, 52, 913–921. [Google Scholar] [CrossRef]
- Kulick, L.J.; Bergfelt, D.R.; Kot, K.; Ginther, O.J. Follicle Selection in Cattle: Follicle Deviation and Codominance Within Sequential Waves1. Biol. Reprod. 2001, 65, 839–846. [Google Scholar] [CrossRef]
- Beg, M.A.; Bergfelt, D.R.; Kot, K.; Wiltbank, M.C.; Ginther, O.J. Follicular-Fluid Factors and Granulosa-Cell Gene Expression Associated with Follicle Deviation in Cattle1. Biol. Reprod. 2001, 64, 432–441. [Google Scholar] [CrossRef]
- Xu, Z.; Allen Garverick, H.; Smith, G.W.; Smith, M.F.; Hamilton, S.A.; Youngquist, R.S. Expression of Follicle-Stimulating Hormone and Luteinizing Hormone Receptor Messenger Ribonucleic Acids in Bovine Follicles during the First Follicular Wave1. Biol. Reprod. 1995, 53, 951–957. [Google Scholar] [CrossRef]
- Ginther, O.J.; Bergfelt, D.R.; Kulick, L.J.; Kot, K. Selection of the Dominant Follicle in Cattle: Role of Estradiol1. Biol. Reprod. 2000, 63, 383–389. [Google Scholar] [CrossRef]
- Lussier, J.G.; Matton, P.; Dufour, J.J. Growth rates of follicles in the ovary of the cow. J. Reprod. Fertil. 1987, 81, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Orisaka, M.; Tajima, K.; Tsang, B.K.; Kotsuji, F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J. Ovarian Res. 2009, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.K. The endocrine and local control of ovarian follicle development in the ewe. Anim. Reprod. 2009, 6, 159–171. [Google Scholar]
- Nilsson, E.E.; Skinner, M.K. Progesterone regulation of primordial follicle assembly in bovine fetal ovaries. Mol. Cell. Endocrinol. 2009, 313, 9–16. [Google Scholar] [CrossRef]
- Kim, J.Y. Control of ovarian primordial follicle activation. Clin. Exp. Reprod. Med. 2012, 39, 10–14. [Google Scholar] [CrossRef]
- Gendelman, M.; Roth, Z. Incorporation of Coenzyme Q10 into Bovine Oocytes Improves Mitochondrial Features and Alleviates Effects of Summer Thermal Stress on Developmental Competence. Biol. Reprod. 2012, 87, 115. [Google Scholar] [CrossRef]
- Roth, Z.; Arav, A.; Bor, A.; Zeron, Y.; Braw-Tal, R.; Wolfenson, D. Improvement of quality of oocytes collected in the autumn by enhanced removal of impaired follicles from previously heat-stressed cows. Reproduction 2001, 122, 737–744. [Google Scholar] [CrossRef]
- Ozawa, M.; Hirabayashi, M.; Kanai, Y. Developmental competence and oxidative state of mouse zygotes heat-stressed maternally or in vitro. Reproduction 2002, 124, 683–689. [Google Scholar] [CrossRef]
- Wang, J.Z.; Sui, H.S.; Miao, D.Q.; Liu, N.; Zhou, P.; Ge, L.; Tan, J.H. Effects of heat stress during in vitro maturation on cytoplasmic versus nuclear components of mouse oocytes. Reproduction 2009, 137, 181–189. [Google Scholar] [CrossRef]
- Yuan, Y.; Hao, Z.D.; Liu, J.; Wu, Y.; Yang, L.; Liu, G.S.; Tian, J.H.; Zhu, S.E.; Zeng, S.M. Heat shock at the germinal vesicle breakdown stage induces apoptosis in surrounding cumulus cells and reduces maturation rates of porcine oocytes in vitro. Theriogenology 2008, 70, 168–178. [Google Scholar] [CrossRef]
- Payton, R.R.; Romar, R.; Coy, P.; Saxton, A.M.; Lawrence, J.L.; Edwards, J.L. Susceptibility of Bovine Germinal Vesicle-Stage Oocytes from Antral Follicles to Direct Effects of Heat Stress In Vitro1. Biol. Reprod. 2004, 71, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Pablos, M.I.; Agapito, M.T.; Gutierrez, R.; Recio, J.M.; Reiter, R.J.; Barlow-Walden, L.; Acuña-Castroviejo, D.; Menendez-Pelaez, A. Melatonin stimulates the activity of the detoxifying enzyme glutathione peroxidase in several tissues of chicks. J. Pineal Res. 1995, 19, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, J.; Chiosis, G. Hsp70 Molecular Chaperones: Emerging Roles in Human Disease and Identification of Small Molecule Modulators. Curr. Top. Med. Chem. 2006, 6, 1215–1225. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Khan, M.Z.; Umer, S.; Khan, I.M.; Xu, H.; Zhu, H.; Wang, Y. Cellular and Molecular Adaptation of Bovine Granulosa Cells and Oocytes under Heat Stress. Animals 2020, 10, 110. https://doi.org/10.3390/ani10010110
Khan A, Khan MZ, Umer S, Khan IM, Xu H, Zhu H, Wang Y. Cellular and Molecular Adaptation of Bovine Granulosa Cells and Oocytes under Heat Stress. Animals. 2020; 10(1):110. https://doi.org/10.3390/ani10010110
Chicago/Turabian StyleKhan, Adnan, Muhammad Zahoor Khan, Saqib Umer, Ibrar Muhammad Khan, Huitao Xu, Huabin Zhu, and Yachun Wang. 2020. "Cellular and Molecular Adaptation of Bovine Granulosa Cells and Oocytes under Heat Stress" Animals 10, no. 1: 110. https://doi.org/10.3390/ani10010110
APA StyleKhan, A., Khan, M. Z., Umer, S., Khan, I. M., Xu, H., Zhu, H., & Wang, Y. (2020). Cellular and Molecular Adaptation of Bovine Granulosa Cells and Oocytes under Heat Stress. Animals, 10(1), 110. https://doi.org/10.3390/ani10010110








