Effect of Ewe Diet on Milk and Muscle Fatty Acid Composition of Suckling Lambs of the Protected Geographical Origin Abbacchio Romano
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Diets, and Feeding Routine
- (1)
- P—pasture group: ewes had daily access to pasture for 22 h/day without supplementation (average stocking rate: 15 ewes/ha). The pasture primarily consisted of Sulla (Hedysarum coronarium) as well as oats (Avena sativa) and clover (Trifolium incarnatum) seeded the previous fall. This composition was typical for pastures in the Latium region.
- (2)
- F—farm group (un-supplemented TMR): ewes had no access to pasture but were housed in straw-bedded pens and received a winter farm ration, which was the same as that normally practised in Central Italy. The ingredients of the total mix ration (TMR) were grass hay at 1.100 and 0.800 kg/day of a concentrate-based meal (oat, barley, and soybean)
- (3)
- L—linseed-enriched group: ewes had no access to pasture but were housed in straw-bedded pens and received the same winter ration as group F. The ingredients of the TMR were grass hay at 1100 and 800 g/day of a concentrate-based meal with 0.190 kg of extruded linseed added. Linseed, ground to pass through a 4-mm screen, was extruded in a single screw extruder with a throughput of 1600 kg/h (barrel length: 3.2 m; die diameter: 7 mm; screw speed: 300 rpm; temperature at the end of the barrel: 130–138 °C; duration: 1 min). After extrusion, the product was dried in a counter flow cooler for 12 min.
2.2. Milk Sampling and Composition
2.3. Slaughter Procedure and Carcass and Meat Measurements
2.4. Fatty Acid Composition of Meat
2.5. Statistical Analysis
3. Results
3.1. Milk
3.2. Lamb Performance and Carcass Characteristics
3.3. Meat Quality of Suckling Lambs
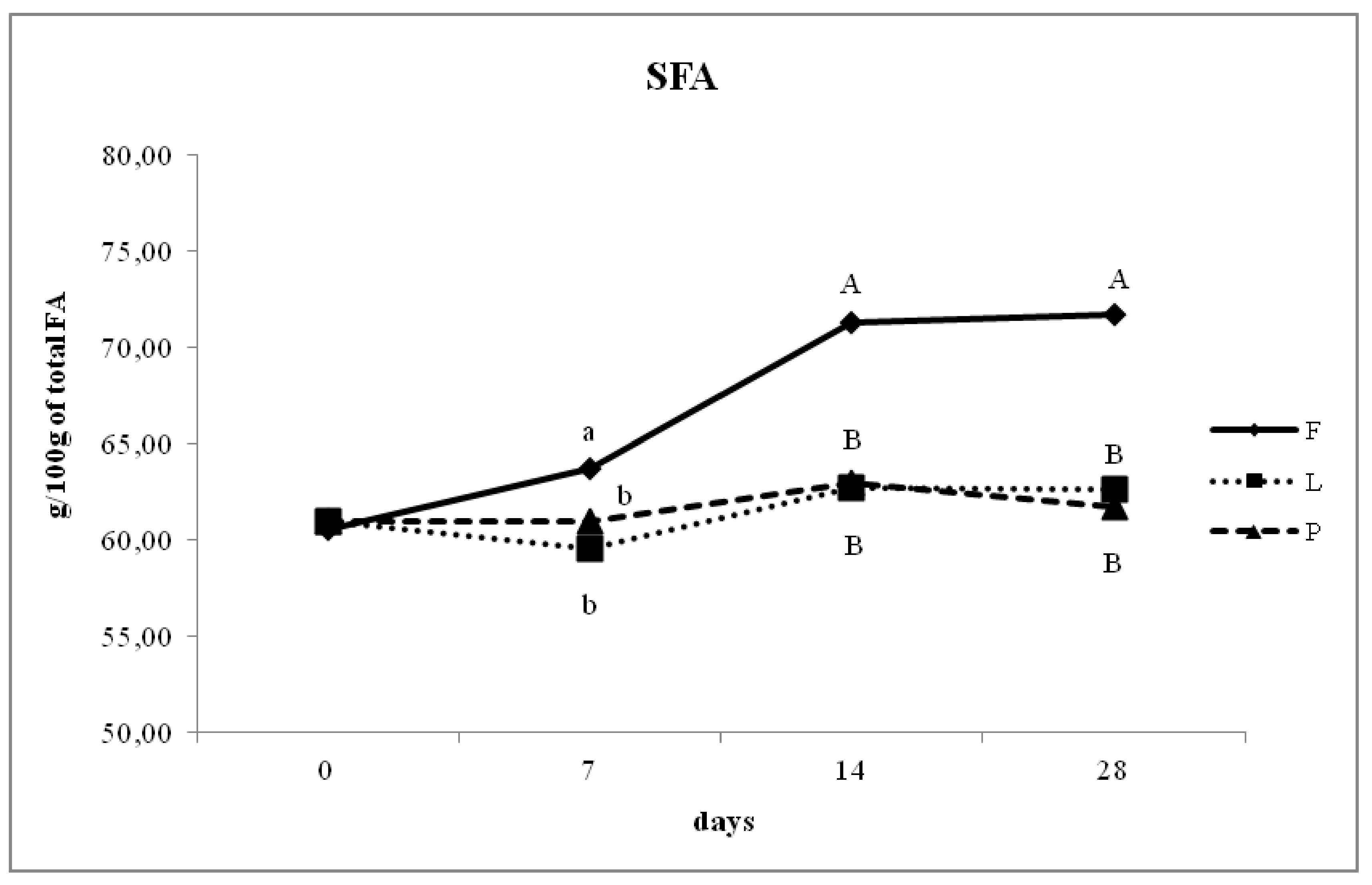
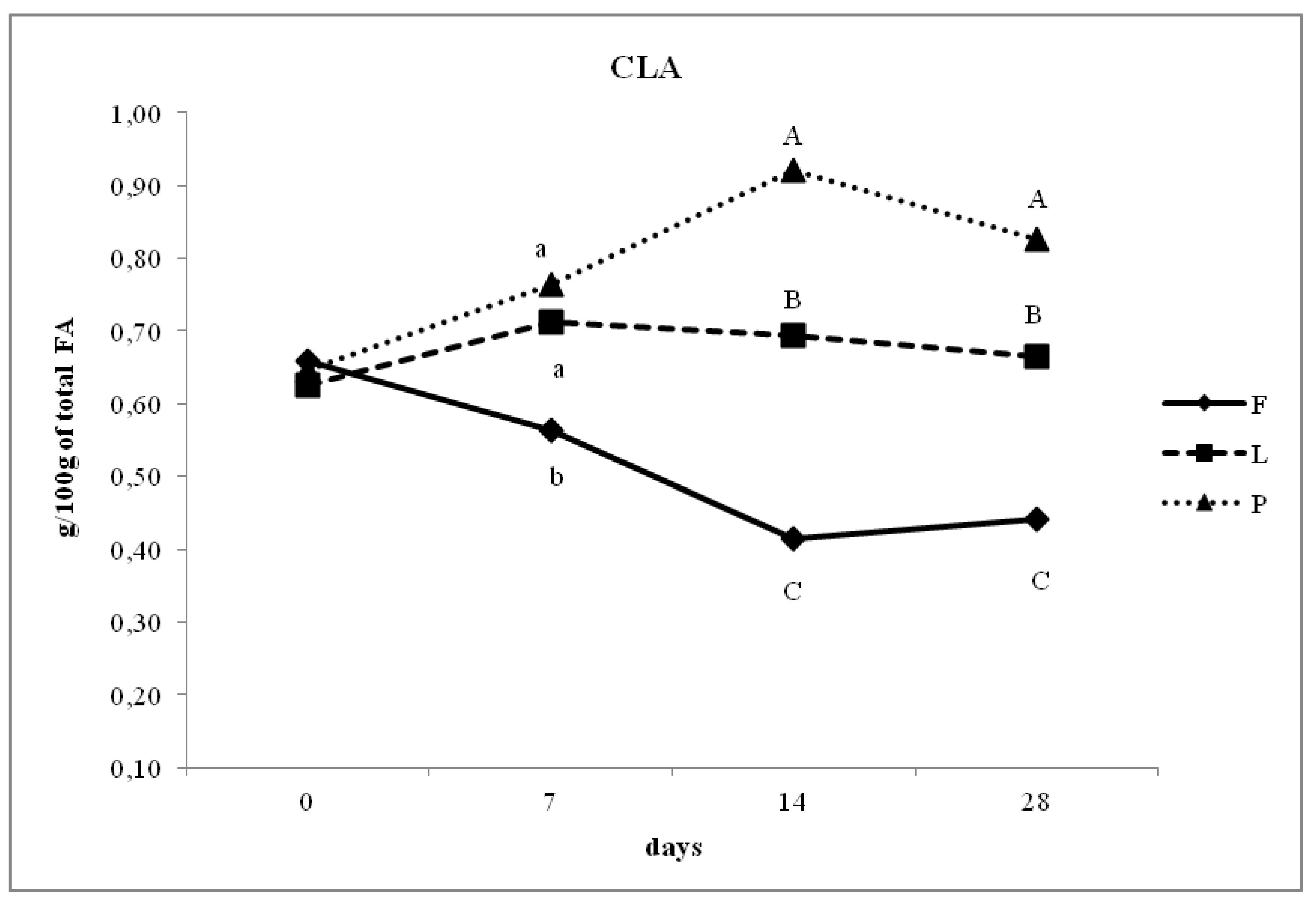
3.4. Fatty Acid Composition of Intramuscular Fat in Relation to Different Dam Dietary Treatments
4. Discussion
4.1. Milk Composition
4.2. Milk Fatty Acid Composition
4.3. Suckling Lamb Performance
4.4. Intramuscular Fatty Acid Composition
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Napolitano, F.; Cifuni, G.F.; Pacelli, C.; Riviezzi, A.M.; Girolami, A. Effect of artificial rearing on lambs welfare and meat quality. Meat Sci. 2002, 60, 307–315. [Google Scholar] [CrossRef]
- Valvo, M.A.; Lanza, M.; Bella, M.; Fasone, V.; Scerra, M.; Biondi, L.; Priolo, A. Effect of ewe feeling system (grass v. concentrate) on intramuscular fatty acids of lambs raised exclusively on maternal milk. Anim. Sci. 2005, 81, 431–436. [Google Scholar] [CrossRef]
- Lanza, M.; Bella, M.; Priolo, A.; Barbagallo, D.; Galofaro, V.; Landi, C.; Pennisi, P. Lamb meat quality as affect a natural or artificial milk feeling regime. Meat Sci. 2006, 73, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Manso, T.; Bodas, R.; Vieira, C.; Mantecón, A.R.; Castro, T. Feeding vegetable oils to lactating ewes modifies the fatty acid profile of suckling lambs. Animal 2011, 5, 1659–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Cortés, P.; Frutos, P.; Mantecón, A.R.; Juárez, M.; De la Fuente, M.A.; Hervás, G. Effect of supplementation on grazing dairy ewes with a cereal concentrate on animal performance and milk fatty acid profile. J. Dairy Sci. 2009, 92, 3964–3972. [Google Scholar]
- Scerra, M.; Caparra, P.; Foti, F.; Galofaro, V.; Sinatra, M.C.; Scerra, V. Influence of ewe feeding systems on fatty acid composition of suckling lambs. Meat Sci. 2007, 76, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, I.; Giammarco, M.; Chincarini, M.; Odintsov Vaintrub, M.; Formigoni, A.; Mammi, L.M.E.; Vignola, G. Fatty acids, health indices and sensory properties of Ricotta cheese from sheep fed three different diets. Inter. J. Dairy Tech. 2019, 72, 427–434. [Google Scholar] [CrossRef]
- Mele, M.; Contarini, G.; Cercaci, L.; Serra, A.; Buccioni, A.; Povolo, M.; Conte, G.; Funaro, A.; Banni, S.; Lercker, G.; et al. Enrichment of Pecorino cheese with conjugated linoleic acid by feeding dairy ewes with extruded linseed: Effect on fatty acid and triglycerides composition and on oxidative stability. Int. Dairy. J. 2011, 21, 365–372. [Google Scholar] [CrossRef]
- Vannice, G.; Rasmussen, H. Position of the Academy of Nutrition and Dietetics: Dietary fatty acids for healthy adults. J. Acad. Nutr. Diet. 2014, 114, 136–153. [Google Scholar] [CrossRef] [Green Version]
- Fattore, E.; Massa, E. Dietary fats and cardiovascular health: A summary of the scientific evidence and current debate. Int. J. Food Sci. Nutr. 2018, 69, 916–927. [Google Scholar] [CrossRef]
- Mateo, J.; Caro, I.; Carballo, D.E.; Gutierrez-Mendez, N.; Arranz, J.J.; Gutierrez-Gil, B. Carcass and meat quality characteristics of churra and assaf suckling lambs. Animal 2018, 12, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Shingfield, K.J.; Bonnet, M.; Scollan, N.D. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal 2013, 7, 132–162. [Google Scholar] [CrossRef] [PubMed]
- Nudda, A.; Battacone, G.; Bee, G.; Boe, R.; Castanares, N.; Lovicu, M.; Pulina, G. Effect of linseed supplementation of the gestation and lactation diets of dairy ewes on the growth performance and the intramuscular fatty acid composition of their lambs. Animal 2015, 9, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Nudda, A.; Battacone, G.; Boe, R.; Manca, M.G.; Rassu, S.P.G.; Pulina, G. Influence of outdoor and indoor rearing system of suckling lambs on fatty acid profile and lipid oxidation of raw and cooked meat. Ital. J Anim. Sci. 2013, 12, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Nudda, A.; Battacone, G.; Boaventura, N.O.; Cannas, A.; Francesconi, A.H.D.; Atzori, A.S.; Pulina, G. Feeding strategies to design the fatty acid profile of sheep milk and cheese. R. Bras. Zootec. 2014, 43, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, B.; Manca, M.G.; Mantecon, A.R.; Nudda, A.; Manso, T. Effects of linseed oil and natural or synthetic vitamin E supplementation in lactating ewes’ diets on meat fatty acid profile and lipid oxidation from their milk fed lambs. Meat Sci. 2015, 102, 79–89. [Google Scholar] [CrossRef] [PubMed]
- European Union, Directive 2010/63/Eu Of The European Parliament And Of The Council of 22 September 2010 on the Protection of Animals used for Scientific Purposes. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 19 December 2019).
- Council Directive 93/119/EC of 22 December 1993 on the protection of animals at the time of slaughter or killing. Official Journal L 340, 31/12/1993. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A31993L0119 (accessed on 19 December 2019).
- AOAC International. International Official Methods of Analysis AOAC, 17th ed.; USDA: Washington, DC, USA, 2000. [Google Scholar]
- ASPA (Scientific Association of Animal Production). Methods for the Assessment of Quality Characteristics of Meat; Università degli Studi di Perugia: Perugia, Italy, 1996; p. 107. [Google Scholar]
- Normand, J.; Theriez, M.; Bas, P.; Aurousseau, M.; Sauvant, D. Effect of energy source, cereals vs. sugar beet pulp, on growth performance and carcass quality of intensively reared lambs. Anim. Res. 1999, 48, 367–380. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Slogane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Fraga, N.; Lerker, G. Rapid methods for the quality control of food oils. Riv. Ital. Sostanze. Gr. 1984, 61, 385–391. [Google Scholar]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Harris, W.S.; Assad, B.; Poston, C. Tissue omega-6/omega-3 fatty acid ratio and risk for coronary heart disease. Am. J. Cardiol. 2006, 98, 191–261. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Dołegowska, B.; Chlubek, D.; Wesolowska, T.; Ciechanowski, K.; Gutowski, P.; Szumiłowicz, H.; Turowski, R. Dietary trans fatty acids and composition of human atheromatous plaques. Eur. J. Nutr. 2004, 43, 313–318. [Google Scholar] [CrossRef] [PubMed]
- SPSS, Version 13; IBM Inc.: Armonk, NY, USA, 2006.
- Luna, P.; Fontecha, J.; Juárez, M.; de la Fuente, M.A. Changes in the milk and cheese fat composition of ewes fed commercial supplements containing linseed with special reference to the CLA content and isomer composition. Lipids 2005, 40, 445–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Cortés, P.; Bach, A.; Luna, P.; Juárez, M.; de la Fuente, M.A. Effects of extruded linseed supplementation on n-3 fatty acids and conjugated linoleic acid in milk and cheese from ewes. J. Dairy. Sci. 2009, 92, 4122–4134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonthier, C.; Mustafa, A.F.; Ouellet, D.R.; Chouinard, P.Y.; Berthiaume, R.; Petit, H.V. Feeding micronized and extruded flaxseed to dairy cows: Effects on blood parameters and milk fatty acid composition. J. Dairy. Sci. 2005, 88, 748–756. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Cortés, P.; Frutos, P.; Mantecón, A.R.; Juárez, M.; de la Fuente, M.A.; Hervás, G. Milk production, conjugated linoleic acid content, and in vitro ruminal fermentation in response to high levels of soybean oil in dairy ewe diet. J. Dairy Sci. 2008, 91, 1560–1569. [Google Scholar] [CrossRef]
- Cannas, A. Feeding of lactating ewes. In Dairy Sheep Nutrition; Pulina, G., Ed.; CAB International: Wallingford, Oxfordshire, UK, 2004; pp. 79–108. [Google Scholar]
- Martinez Marin, A.L.; Gómez-Cortés, P.; Gomez Castro, A.G.; Juarez, M.; Perez Alba, L.M.; Perez Hernandez, M.; de la Fuente, M.A. Animal performance and milk fatty acid profile of dairy goats fed diets with different unsaturated plant oils. J. Dairy. Sci. 2011, 94, 5359–5368. [Google Scholar] [CrossRef] [Green Version]
- Jouany, J.P.; Lassalas, B.; Doreau, M.; Glasser, F. Dynamic features of the rumen metabolism of linoleic acid, linolenic acid and linseed oil measured in vitro. Lipids 2007, 42, 351–360. [Google Scholar] [CrossRef]
- Klieve, A.V.; Hennessy, D.; Ouwerkerk, D.; Forster, R.J.; Mackie, R.I.; Attwood, GT. Establishing populations of Megasphaera elsdenii YE 34 and Butyrivibrio fibrisolvens YE 44 in the rumen of cattle fed high grain diets. J. Appl. Microbiol. 2003, 95, 621–630. [Google Scholar] [CrossRef]
- Cabiddu, A.; Addis, M.; Fiori, M.; Spada, S.; Decandia, M.; Molle, G. Pros and cons of the supplementation with oilseed enriched concentrates on milk fatty acid profile of dairy sheep grazing mediterranean pastures. Small Rumin. Res. 2017, 147, 63–72. [Google Scholar] [CrossRef]
- Berthelot, V.; Bas, P.; Pottier, E.; Normand, J. The effect of maternal linseed supplementation and/or lamb linseed supplementation on muscle and subcutaneous adipose tissue fatty acid composition of indoor lambs. Meat Sci. 2012, 90, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Wachira, A.M.; Sinclair, L.A.; Wilkinson, R.G.; Wood, J.D.; Enser, M. Effects of dietary n-3 polyunsaturated fatty acids, breed and dietary vitamin E on the fatty acids of lamb muscle, liver and adipose tissue. Brit. J. Nutr. 2004, 91, 551–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radunz, A.E.; Wickersham, L.A.; Loerch, S.C.; Fluharty, F.L.; Reynolds, C.K.; Zerby, H.N. Effects of dietary polyunsaturated fatty acid supplementation on fatty acid composition in muscle and subcutaneous adipose tissue of lambs. J. Anim. Sci. 2009, 87, 4082–4091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, A.; Sañudo, C.; Campo, M.M.; Olleta, J.L.; Muela, E.; Macedo, R.M.G.; Macedo, F.A.F. Effect of linseed supplementation level and feeding duration on performance, carcass and meat quality of cull ewes. Small Rumin. Res. 2018, 167, 70–77. [Google Scholar] [CrossRef]
- Borys, A.; Borys, J.; Pająk, J. The fatty acid profile of meat of suckling lambs from ewes fed rapeseed and linseed. J. Anim. Feed Sci. 2005, 14, 223–226. [Google Scholar] [CrossRef]
- Khliji, S.; Van de Ven, R.; Lamb, T.A.; Lanza, M.; Hopkins, D.L. Relationship between consumer ranking of lamb colour and objective measures of colour. Meat Sci. 2010, 85, 224–229. [Google Scholar] [CrossRef]
- Priolo, A.; Micol, D.; Agabriel, J. Effects of grass feeding systems on ruminant meat colour and flavour. A review. Anim. Res. 2001, 50, 185–200. [Google Scholar] [CrossRef]
- Juárez, M.; Horcada, A.; Alcalde, M.J.; Valera, M.; Mullen, A.M.; Molina, A. Estimation of factors influencing fatty acid profiles in light lambs. Meat Sci. 2008, 79, 203–210. [Google Scholar] [CrossRef]
- Nudda, A.; Palmquist, D.L.; Battacone, G.; Fancellu, S.; Rassu, S.P.G.; Pulina, G. Relationships between the contents of vaccenic acid, CLA and n-3 fatty acids of goat milk and the muscle of their suckling kids. Livest. Sci. 2008, 118, 195–203. [Google Scholar] [CrossRef]
- Derakhshande-Rishehri, S.M.; Mansourian, M.; Kelishadi, R.; Heidari-Beni, M. Association of foods enriched in conjugated linoleic acid CLA and CLA supplements with lipid profile in human studies: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 2041–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Jacome-Sosa, M.M.; Proctor, S.D. The role of ruminant trans fat as a potential nutraceutical in the prevention of cardiovascular disease. Food. Res. Int. 2012, 46, 460–468. [Google Scholar] [CrossRef]
- Cardi, E.; Corrado, G.; Cavaliere, M.; Frandina, G.; Pacchiarotti, C.; Rea, P.; Mazza, M.L.; Nardelli, F.; Agazie, E. Rezza-Cardi’s diet as dietary treatment of short bowel syndrome. Gastroenterology 1998, 114, 869. [Google Scholar] [CrossRef]
- Martino, F.; Bruno, G.; Aprigliano, D.; Agolini, D.; Guido, F.; Giardini, O.; Businco, L. Effectiveness of a home-made meat based formula (the Rezza-Cardi diet) as a diagnostic tool in children with food-induced atopic dermatitis. Pediatr. Allergy Immunol. 1998, 9, 192–196. [Google Scholar] [CrossRef]
- Cantani, A. A home-made meat-based formula for feeding atopic babies: A study in 51 children. Eur. Rev. Med. Pharmacol. Sci. 2006, 10, 61–68. [Google Scholar]
- Nudda, A.; Atzori, A.S.; Boe, R.; Francesconi, A.H.; Battacone, G.; Pulina, G. Seasonal variation in the fatty acid profile in meat of Sarda suckling lambs. Ital. J. Anim. Sci. 2018, 18, 488–497. [Google Scholar] [CrossRef] [Green Version]
- Palmquist, D.L.; Lock, A.L.; Shingfield, K.J.; Bauman, D.E. Biosynthesis of conjugated linoleic acid in ruminants and humans. Adv. Food Nutr. Res. 2005, 50, 179–217. [Google Scholar]
- Hadj Ahmed, H.A.; Kharroubi, W.; Kaoubaa, N.; Zarrouk, A.; Batbout, F.; Gamra, H.; Fadhel Najjar, M.; Lizard, G.; Hininger-Favier, I.; Hammami, M. Correlation of trans fatty acids with the severity of coronary artery disease lesions. Lipids Health Dis. 2018, 17, 52. [Google Scholar] [CrossRef] [Green Version]


| Ingredients | Dietary Treatment 1 | ||
|---|---|---|---|
| F | L | P | |
| Grass hay | 56.79 | 56.79 | …. |
| Oatmeal | 12.91 | 10.33 | …. |
| Barley meal | 12.91 | 10.33 | …. |
| Soybean meal | 15.85 | 11.20 | …. |
| Extruded linseed | …. | 9.81 | …. |
| Mineral and vitamin mix | 1.55 | 1.55 | …. |
| Chemical composition | |||
| DM | 89.12 | 90.56 | 16.72 |
| CP | 20.71 | 19.97 | 19.57 |
| EE | 2.86 | 4.83 | 2.50 |
| NDF | 39.24 | 40.75 | 29.77 |
| ADF | 25.48 | 25.2 | 21.91 |
| ADL | 3.68 | 3.93 | 1.41 |
| Fatty acid profile (% of total FA) | |||
| C12:0 | 0.15 | 0.18 | 0.17 |
| C14:0 | 0.6 | 0.2 | 0.30 |
| C16:0 | 18.8 | 8.8 | 12.7 |
| C16:1 | 1.01 | 0.98 | 1.1 |
| C16:1c9 | 0.3 | 0.1 | 0.2 |
| C18:0 | 2.6 | 4.1 | 1.11 |
| C18:1c9 | 23.8 | 21.1 | 1.81 |
| C18:2c9, c12 | 40.0 | 13.3 | 11.5 |
| C18:3c9, c12, c15 | 11.7 | 50.6 | 70.3 |
| C18:1c11 | 1.0 | 0.6 | 0.8 |
| Item | Dietary Treatment 1 | p Value | SEM | ||
|---|---|---|---|---|---|
| F | L | P | |||
| Fat (%) | 4.89 b | 5.21 c | 4.27 a | <0.05 | 0.30 |
| Protein (%) | 4.76 B | 4.55 B | 5.37 A | <0.001 | 0.10 |
| Lactose (%) | 4.58 | 4.52 | 5.01 | 0.435 | 0.17 |
| Total solids (%) | 14.47 | 14.69 | 15.54 | 0.558 | 0.44 |
| Item | Dietary Treatment 1 | p Value | SEM | ||
|---|---|---|---|---|---|
| F | L | P | |||
| Birth weight (kg) | 4.87 | 5.05 | 4.97 | 0.23 | 0.96 |
| Pre-slaughtered weight (kg) | 10.72 | 10.92 | 10.78 | 0.86 | 0.25 |
| Age at slaughter (d) | 28.2 | 27.5 | 28.7 | 0.26 | 1.01 |
| Daily gain (g) | 221 | 246 | 229 | 0.90 | 1.12 |
| Cold carcass weight (kg) | 6.74 | 6.90 | 6.77 | 0.84 | 0.98 |
| Dressing (%) | 51.94 | 53.07 | 50.28 | 0.60 | 0.45 |
| Conformation score 2 | 8.5 | 8.9 | 8.4 | 0.21 | 1.23 |
| Fatness score 3 | 7.3 | 6.9 | 7.1 | 0.32 | 0.45 |
| Fat softness score 4 | 3.0 | 2.9 | 3.2 | 0.45 | 0.87 |
| Fat colour score 5 | 1.5 a | 1.2 a | 2.8 b | <0.05 | 0.36 |
| Item | Dietary Treatment 1 | p Value | SEM | |||
|---|---|---|---|---|---|---|
| F | L | P | ||||
| pH1 | 6.67 | 6.75 | 6.78 | 0.32 | 0.03 | |
| pHu | 5.56 | 5.49 | 5.54 | 0.42 | 0.02 | |
| Meat colour 2 | ||||||
| L* | 52.43 | 53.27 | 49.16 | 0.14 | 0.85 | |
| a* | 12.29 B | 10.70 B | 14.33 A | <0.001 | 0.26 | |
| b* | 8.08 | 6.87 | 6.39 | 0.21 | 0.38 | |
| Fat colour 2 | ||||||
| L* | 68.5 | 67.7 | 69.1 | 0.23 | 0.96 | |
| a* | 4.65 b | 5.54 b | 6.33 a | <0.05 | 0.36 | |
| b* | 7.12 | 7.56 | 8.16 | 0.45 | 0.48 | |
| Water holding capacity | ||||||
| Drip loss | % | 3.38 | 3.58 | 3.60 | 0.74 | 0.13 |
| Cooking loss | % | 19.79 | 16.91 | 20.55 | 0.37 | 1.07 |
| Chemical composition | ||||||
| Moisture | % | 74.79 | 75.43 | 75.45 | 0.72 | 0.33 |
| Protein | % | 21.70 | 21.05 | 21.02 | 0.66 | 0.31 |
| Fat | % | 2.29 | 2.36 | 2.35 | 0.98 | 0.14 |
| Ash | % | 1.22 | 1.16 | 1.18 | 0.68 | 0.02 |
| Fatty Acids | Dietary Treatment 1 | p Value | SEM | ||
|---|---|---|---|---|---|
| F | L | P | |||
| Principal FA categories | |||||
| SFA | 44.16 c | 40.95 a | 42.08 b | <0.05 | 0.12 |
| MUFA | 37.09 | 37.14 | 38.01 | 0.77 | 0.36 |
| PUFA | 18.75 a | 21.91 c | 19.90 b | <0.05 | 1.25 |
| UFA | 56.84 a | 59.05 b | 57.91 b | <0.05 | 0.89 |
| CLA | 0.47 a | 0.69 b | 0.80 c | <0.05 | 0.36 |
| n-3 | 3.09 a | 4.80 c | 3.67 b | <0.01 | 1.02 |
| n-6 | 16.19 | 16.54 | 14.43 | 0.25 | 0.77 |
| UTFA | 0.73 c | 0.49 b | 0.29 a | <0.05 | 0.37 |
| Nutritional index and ratio | |||||
| SFA/UFA | 0.77 | 0.69 | 0.72 | 0.06 | 0.45 |
| n-6/n-3 | 5.30 c | 3.65 a | 4.30 b | <0.05 | 0.69 |
| TI | 0.98 | 0.88 | 0.84 | 0.22 | 0.89 |
| AI | 0.76 | 0.66 | 0.86 | 0.24 | 1.04 |
| I-HARRIS | 1.56 a | 1.73 b | 1.49 c | <0.05 | 0.81 |
| DI C14:0 | 3.49 | 3.67 | 3.78 | 0.35 | 0.23 |
| DI C16:0 | 6.71 | 5.55 | 6.34 | 0.23 | 0.87 |
| DI C18:0 | 73.29 | 69.61 | 73.80 | 0.65 | 0.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusaro, I.; Giammarco, M.; Chincarini, M.; Odintsov Vaintrub, M.; Palmonari, A.; Mammi, L.M.E.; Formigoni, A.; Di Giuseppe, L.; Vignola, G. Effect of Ewe Diet on Milk and Muscle Fatty Acid Composition of Suckling Lambs of the Protected Geographical Origin Abbacchio Romano. Animals 2020, 10, 25. https://doi.org/10.3390/ani10010025
Fusaro I, Giammarco M, Chincarini M, Odintsov Vaintrub M, Palmonari A, Mammi LME, Formigoni A, Di Giuseppe L, Vignola G. Effect of Ewe Diet on Milk and Muscle Fatty Acid Composition of Suckling Lambs of the Protected Geographical Origin Abbacchio Romano. Animals. 2020; 10(1):25. https://doi.org/10.3390/ani10010025
Chicago/Turabian StyleFusaro, Isa, Melania Giammarco, Matteo Chincarini, Michael Odintsov Vaintrub, Alberto Palmonari, Ludovica Maria Eugenia Mammi, Andrea Formigoni, Lorella Di Giuseppe, and Giorgio Vignola. 2020. "Effect of Ewe Diet on Milk and Muscle Fatty Acid Composition of Suckling Lambs of the Protected Geographical Origin Abbacchio Romano" Animals 10, no. 1: 25. https://doi.org/10.3390/ani10010025
APA StyleFusaro, I., Giammarco, M., Chincarini, M., Odintsov Vaintrub, M., Palmonari, A., Mammi, L. M. E., Formigoni, A., Di Giuseppe, L., & Vignola, G. (2020). Effect of Ewe Diet on Milk and Muscle Fatty Acid Composition of Suckling Lambs of the Protected Geographical Origin Abbacchio Romano. Animals, 10(1), 25. https://doi.org/10.3390/ani10010025






