Macro-Nutritional Adaptive Strategies of Moose (Alces alces) Related to Population Density
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Population Density
2.4. Sex and Individual Determination
2.5. Diet Composition of Moose
2.6. Nutritional Composition of Diet
2.7. Data Analyses
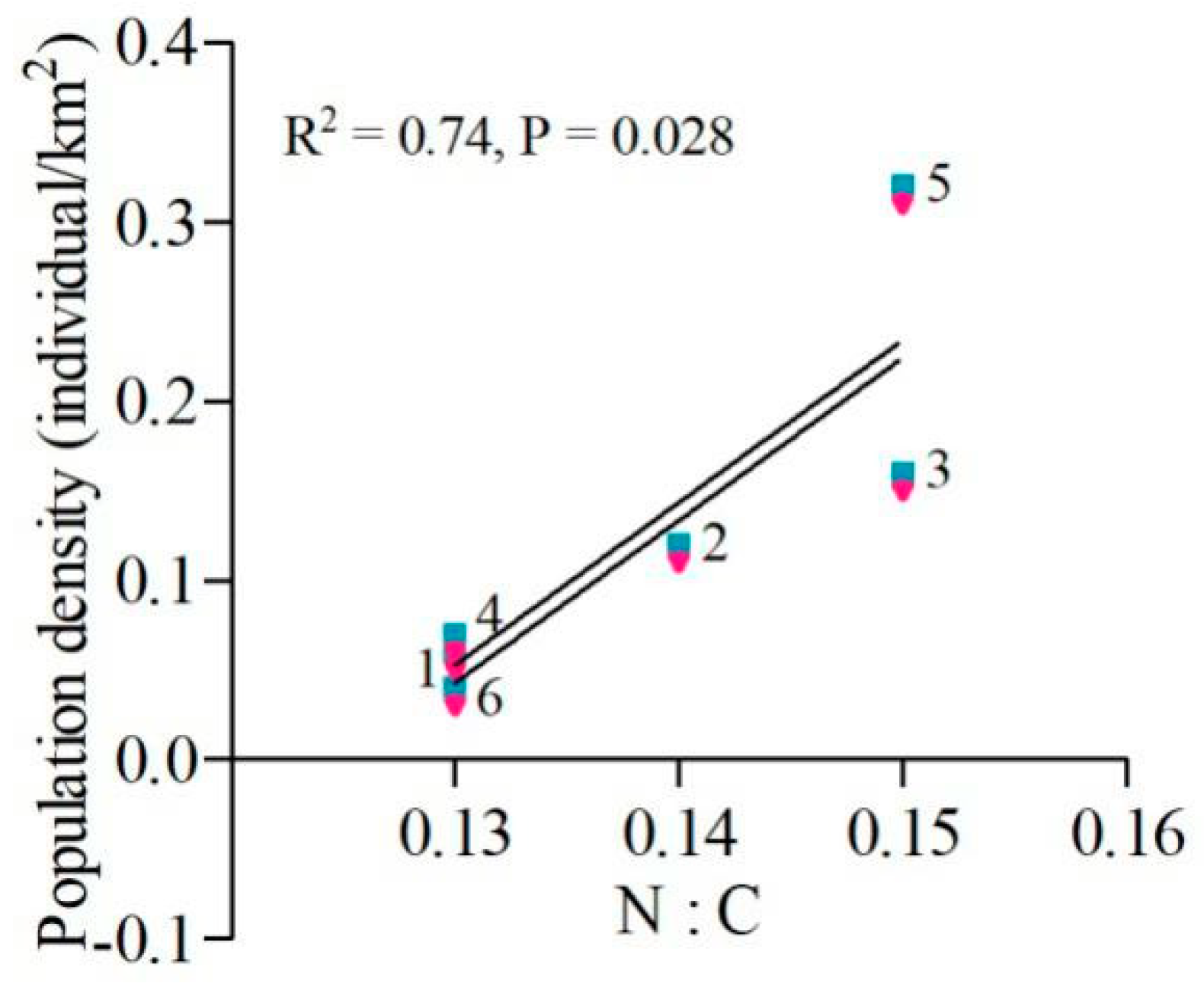
3. Results
3.1. Forage Availability and Diet Composition
3.2. Macro-Nutrient Balance of the Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

| Spieces |
|---|
| Salix spp. |
| Betula spp. |
| Corylus spp. |
| Populus spp. |
| Pinus spp. |
| Tilia amurensis |
| Quercus spp. |
| Alnus spp. |
| Picea asperata Mast. |
| Cornus alba |
| Padus asiatica |
| Lonicera spp. |
| Deutzia spp. |
| Sambucus spp. |
| Spiraea spp. |
| Schizandra chinensis |
| Sorbaria sorbifolia |
| Acanthopanx senticosus |
| Ulmus spp. |
| Viburnum sargenlii |
| Evonymus sacrosancta |
| Philadelphus schrenkii |
| Berberis amurensis |
| Acer mono |
| Vaccinium spp. |
| Ribes mandshuricum |
| Syringa amurensis |
| Rasa spp. |
| Deyeuxia angustifolia |
| Carcx spp. |
| Equisetum spp.Tilia spp. |
| Rhododendron spp. |
| Lespedeza spp. |
| Larix spp. |
| Leaf litler |
| Group 1 | Group 2 | Mean Difference | Standard Error | p | 95% Upper Limit | 95% Lower Limit |
|---|---|---|---|---|---|---|
| Mohe | Nanwenghe | −149.15 | 14.88 | 0.000 | −193.02 | −105.27 |
| Zhanhe | −192.27 | 22.07 | 0.000 | −257.30 | −127.24 | |
| Shuanghe | −67.70 | 10.70 | 0.000 | −99.38 | −36.01 | |
| Hanma | −60.47 | 9.16 | 0.000 | −87.58 | −33.36 | |
| Meitian | −154.21 | 26.99 | 0.000 | −235.58 | −72.83 | |
| Nanwenghe | Zhanhe | −43.12 | 25.78 | 0.776 | −118.92 | 32.68 |
| Shuanghe | 81.45 | 17.09 | 0.000 | 31.13 | 131.78 | |
| Hanma | 88.68 | 16.18 | 0.000 | 41.04 | 136.31 | |
| Meitian | −5.06 | 30.11 | 1.000 | −94.91 | 84.79 | |
| Zhanhe | Shuanghe | 124.57 | 23.61 | 0.000 | 55.06 | 194.08 |
| Hanma | 131.80 | 22.96 | 0.000 | 64.19 | 199.41 | |
| Meitian | 38.06 | 34.23 | 0.991 | −63.41 | 139.53 | |
| Shuanghe | Hanma | 7.23 | 12.44 | 1.000 | −29.50 | 43.96 |
| Meitian | −86.51 | 28.27 | .042 | −171.34 | −1.68 | |
| Hanma | Meitian | −93.74 | 27.73 | .016 | −177.09 | −10.39 |
References
- Milton, K. Factors Influencing Leaf Choice by Howler Monkeys: A Test of Some Hypotheses of Food Selection by Generalist Herbivores. Am. Nat. 1979, 114, 362–378. [Google Scholar] [CrossRef]
- Rosenthal, G.A.; Berenbaum, M.R. Herbivores: Their Interactions with Secondary Plant Metabolites; The Chemical Participants; Academic Press: San Diego, CA, USA, 1991; Volume 1, ISBN 978-0-12-597183-6. [Google Scholar]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E. Nitrogen Limitation and Trophic Vs. Abiotic Influences on Insect Herbivores in a Temperate Grassland. Ecology 2000, 81, 1601–1612. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesityion; Princeton University Press: Princeton, NJ, USA, 2012. [Google Scholar]
- Houston, A.I.; Higginson, A.D.; McNamara, J.M. Optimal foraging for multiple nutrients in an unpredictable environment. Ecol. Lett. 2011, 14, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.P.; Danell, K.; Sunesson, P. Response of a Herbivore Community to Increased Food Quality and Quantity: An Experiment with Nitrogen Fertilizer in a Boreal Forest. J. Appl. Ecol. 2000, 37, 247–255. [Google Scholar] [CrossRef]
- Bergstrom, R.; Danell, K. Effects of Simulated Winter Browsing by Moose on Morphology and Biomass of Two Birch Species. J. Ecol. 1987, 75, 533–544. [Google Scholar] [CrossRef]
- Palo, R.T.; Bergström, R.; Danell, K. Digestibility, Distribution of Phenols, and Fiber at Different Twig Diameters of Birch in Winter. Implication for Browsers. Oikos 1992, 65, 450–454. [Google Scholar] [CrossRef]
- Suomela, J.; Ossipov, V.; Haukioja, E. Variation among and within mountain birch trees in foliage phenols, carbohydrates, and amino acids, and in growth of Epirrita autumnata larvae. J. Chem. Ecol. 1995, 21, 1421–1446. [Google Scholar] [CrossRef]
- Karasov, W.H. Physiological Ecology: How Animals Process Energy, Nutrients, and Toxins; Princeton University Press: Princeton, NJ, USA, 2007; ISBN 978-0-691-07453-5. [Google Scholar]
- Behmer, S.T.; Joern, A. Coexisting generalist herbivores occupy unique nutritional feeding niches. Proc. Natl. Acad. Sci. USA 2008, 105, 1977–1982. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.P.; Behmer, S.T.; Simpson, S.J. Nutrient regulation in relation to diet breadth: A comparison of Heliothis sister species and a hybrid. J. Exp. Biol. 2006, 209, 2076–2084. [Google Scholar] [CrossRef] [Green Version]
- Behmer, S.T.; Joern, A. Insect Herbivore Outbreaks Viewed through a Physiological Framework: Insights from Orthoptera. In Insect Outbreaks Revisited; John Wiley & Sons, Blackwell Publishing Ltd.: Oxford, UK, 2012; pp. 1–29. ISBN 978-1-118-29520-5. [Google Scholar]
- Hawlena, D.; Schmitz, O.J. Physiological stress as a fundamental mechanism linking predation to ecosystem functioning. Am. Nat. 2010, 176, 537–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Börger, L.; Dalziel, B.D.; Fryxell, J.M. Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecol. Lett. 2008, 11, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Edenius, L.; Ericsson, G.; Näslund, P. Selectivity by moose vs. the spatial distribution of aspen: A natural experiment. Ecography 2002, 25, 289–294. [Google Scholar] [CrossRef]
- Milligan, H.T.; Koricheva, J. Effects of tree species richness and composition on moose winter browsing damage and foraging selectivity: An experimental study. J. Anim. Ecol. 2013, 82, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.C.; Xiao, Q.Z.; Lu, Y. Selection and utilization ratio of winter diet, and seasonal changes in feeding and bedding habitat selection by moose in northeastern China. Dev. Anim. Vet. Sci. 1993, 26, 172–180. [Google Scholar]
- Jiang, G.; Ma, J.; Zhang, M.; Stott, P. Multiple spatial-scale resource selection function models in relation to human disturbance for moose in northeastern China. Ecol. Res. 2009, 24, 423–440. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, G.; Wang, Q. China Red Data Book of Endangered Animals: Pisces; Science Press: Beijing, China, 1998. [Google Scholar]
- Dou, H.; Jiang, G.; Stott, P.; Piao, R. Climate change impacts population dynamics and distribution shift of moose (Alces alces) in Heilongjiang Province of China. Ecol. Res. 2013, 28, 625–632. [Google Scholar] [CrossRef]
- Wam, H.K.; Hjeljord, O. Moose summer and winter diets along a large scale gradient of forage availability in southern Norway. Eur. J. Wildl. Res. 2010, 56, 745–755. [Google Scholar] [CrossRef]
- Regelin, W.L.; Schwartz, C.C.; Franzmann, A.W. Seasonal Energy Metabolism of Adult Moose. J. Wildl. Manag. 1985, 49, 388–393. [Google Scholar] [CrossRef]
- Allen, A.M.; Månsson, J.; Sand, H.; Malmsten, J.; Ericsson, G.; Singh, N.J. Scaling up movements: From individual space use to population patterns. Ecosphere 2016, 7, e01524. [Google Scholar] [CrossRef]
- Maklakov, A.A.; Simpson, S.J.; Zajitschek, F.; Hall, M.D.; Dessmann, J.; Clissold, F.; Raubenheimer, D.; Bonduriansky, R.; Brooks, R.C. Sex-Specific Fitness Effects of Nutrient Intake on Reproduction and Lifespan. Curr. Biol. 2008, 18, 1062–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morehouse, N.I.; Nakazawa, T.; Booher, C.M.; Jeyasingh, P.D.; Hall, M.D. Sex in a material world: Why the study of sexual reproduction and sex-specific traits should become more nutritionally-explicit. Oikos 2010, 119, 766–778. [Google Scholar] [CrossRef]
- Senior, A.M.; Charleston, M.A.; Lihoreau, M.; Buhl, J.; Raubenheimer, D.; Simpson, S.J. Evolving Nutritional Strategies in the Presence of Competition: A Geometric Agent-Based Model. PLoS Comput. Biol. 2015, 11, e1004111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illius, A.W.; Gordon, I.J. Modelling the nutritional ecology of ungulate herbivores: Evolution of body size and competitive interactions. Oecologia 1992, 89, 428–434. [Google Scholar] [CrossRef]
- Soest, P.J.V. Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 1994; ISBN 978-0-8014-2772-5. [Google Scholar]
- Barboza, P.S.; Bowyer, R.T. Sexual Segregation in Dimorphic Deer: A New Gastrocentric Hypothesis. J. Mammal. 2000, 81, 473–489. [Google Scholar] [CrossRef] [Green Version]
- Raubenheimer, D. The Right-Angled Mixture Triangle. Bull. Ecol. Soc. Am. 2011, 92, 294–297. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Machovsky-Capuska, G.E.; Chapman, C.A.; Rothman, J.M. Geometry of nutrition in field studies: An illustration using wild primates. Oecologia 2015, 177, 223–234. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, Z.; Raubenheimer, D.; Elser, J.J.; Wei, W.; Wei, F. Obligate herbivory in an ancestrally carnivorous lineage: The giant panda and bamboo from the perspective of nutritional geometry. Funct. Ecol. 2015, 29, 26–34. [Google Scholar] [CrossRef]
- Parker, K.L.; Barboza, P.S.; Gillingham, M.P. Nutrition integrates environmental responses of ungulates. Funct. Ecol. 2009, 23, 57–69. [Google Scholar] [CrossRef]
- Simpson, S.J.; Le Couteur, D.G.; Raubenheimer, D. Putting the Balance Back in Diet. Cell 2015, 161, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Bao, H.; Dou, H.; Ma, Y.; Liu, H.; Jiang, G. Moose winter diet components from feces and field feeding signs: Consistency and variability related to forage availability and nutritional requirements. Ecol. Res. 2017, 32, 685–692. [Google Scholar] [CrossRef]
- Efford, M.G. Estimation of population density by spatially explicit capture–recapture analysis of data from area searches. Ecology 2011, 92, 2202–2207. [Google Scholar] [CrossRef] [PubMed]
- Neumann, W.; Ericsson, G.; Dettki, H.; Radeloff, V.C. Behavioural response to infrastructure of wildlife adapted to natural disturbances. Landsc. Urban Plan. 2013, 114, 9–27. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Liu, H.; Jiang, G. Risks involved in fecal DNA-based genotyping of microsatellite loci in the Amur tiger Panthera tigris altaica: A pilot study. J. For. Res. 2018, 29, 525–531. [Google Scholar] [CrossRef]
- Shrestha, R.; Wegge, P.; Koirala, R.A. Summer diets of wild and domestic ungulates in Nepal Himalaya. J. Zool. 2005, 266, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Aryal, A.; Brunton, D.; Ji, W.; Yadav, H.K.; Adhikari, B.; Raubenheimer, D. Diet and Habitat use of Hispid Hare Caprolagus hispidus in Shuklaphanta Wildlife Reserve, Nepal. Mammal Study 2012, 37, 147–154. [Google Scholar] [CrossRef]
- Shrestha, R.; Wegge, P. Determining the Composition of Herbivore Diets in the Trans-Himalayan Rangelands: A Comparison of Field Methods. Rangel. Ecol. Manag. 2006, 59, 512–518. [Google Scholar] [CrossRef]
- Holechek, J.L.; Gross, B. Training Needed for Quantifying Simulated Diets from Fragmented Range Plants. J. Range Manag. 1982, 35, 644–647. [Google Scholar] [CrossRef] [Green Version]
- Sparks, D.R.; Malechek, J.C. Estimating percentage dry weight in diets using a microscopic technique. J. Range Manag. 1968, 21, 264–265. [Google Scholar] [CrossRef] [Green Version]
- Panthi, S.; Aryal, A.; Raubenheimer, D.; Lord, J.; Adhikari, B. Summer Diet and Distribution of the Red Panda (Ailurus fulgens fulgens) in Dhorpatan Hunting Reserve, Nepal. Zool. Stud. 2012, 51, 701–709. [Google Scholar]
- Rothman, J.; Chapman, C.; Soest, P. Methods in Primate Nutritional Ecology: A User’s Guide. Int. J. Primatol. 2011, 33, 542–566. [Google Scholar] [CrossRef]
- Maynard, L.; Loosli, J. Animal Nutrition; McGraw-Hill: New York, NY, USA, 1969. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Science Ltd.: Oxford, UK, 2004. [Google Scholar]
- Schrempp, T.V.; Rachlow, J.L.; Johnson, T.R.; Shipley, L.A.; Long, R.A.; Aycrigg, J.L.; Hurley, M.A. Linking forest management to moose population trends: The role of the nutritional landscape. PLoS ONE 2019, 14, e0219128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testa, J.W.; Adams, G.P. Body Condition and Adjustments to Reproductive Effort in Female Moose (Alces alces). J. Mammal. 1998, 79, 1345–1354. [Google Scholar] [CrossRef] [Green Version]
- Allen, A.M.; Dorey, A.; Malmsten, J.; Edenius, L.; Ericsson, G.; Singh, N.J. Habitat-performance relationships of a large mammal on a predator-free island dominated by humans. Ecol. Evol. 2017, 7, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.P.; Dahlgren, J. Browsing Damage on Pine (Pinus sylvestris and P. contorta) by a migrating moose (Alces alces) Population in Winter: Relation to Habitat Composition and Road Barriers. Scand. J. For. Res. 2002, 17, 427–435. [Google Scholar] [CrossRef]
- Ericsson, G.; Edenius, L.; Sundström, D. Factors affecting browsing by moose (Alces alces L.) on European aspen (Populus tremula L.) in a managed boreal landscape. Écoscience 2001, 8, 344–349. [Google Scholar] [CrossRef]
- Dussault, C.; Courtois, R.; Ouellet, J.-P.; Girard, I. Space use of moose in relation to food availability. Can. J. Zool. 2005, 83, 1431–1437. [Google Scholar] [CrossRef]
- Månsson, J.; Andrén, H.; Pehrson, Å.; Bergström, R. Moose browsing and forage availability: A scale-dependent relationship? Can. J. Zool. 2007, 85, 372–380. [Google Scholar] [CrossRef]
- Vivas, H.J.; Saether, B.-E. Interactions Between a Generalist Herbivore, the Moose Alces alces, and its Food Resources: An Experimental Study of Winter Foraging Behaviour in Relation to Browse Availability. J. Anim. Ecol. 1987, 56, 509–520. [Google Scholar] [CrossRef]
- Westoby, M. An Analysis of Diet Selection by Large Generalist Herbivores. Am. Nat. 1974, 108, 290–304. [Google Scholar] [CrossRef]
- Felton, A.M.; Felton, A.; Raubenheimer, D.; Simpson, S.J.; Foley, W.J.; Wood, J.T.; Wallis, I.R.; Lindenmayer, D.B. Protein content of diets dictates the daily energy intake of a free-ranging primate. Behav. Ecol. 2009, 20, 685–690. [Google Scholar] [CrossRef] [Green Version]
- Mayntz, D.; Nielsen, V.H.; Sørensen, A.; Toft, S.; Raubenheimer, D.; Hejlesen, C.; Simpson, S.J. Balancing of protein and lipid intake by a mammalian carnivore, the mink. Mustela Vison. Anim. Behav. 2009, 77, 349–355. [Google Scholar] [CrossRef]
- Felton, A.M.; Felton, A.; Raubenheimer, D.; Simpson, S.J.; Krizsan, S.J.; Hedwall, P.-O.; Stolter, C. The Nutritional Balancing Act of a Large Herbivore: An Experiment with Captive Moose (Alces alces L). PLoS ONE 2016, 11, e0150870. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, C.C.; Regelin, W.L.; Franzmann, A.W. Protein Digestion in Moose. J. Wildl. Manag. 1987, 51, 352–357. [Google Scholar] [CrossRef]
- Franzmann, A.W.; Schwartz, C.C. Ecology and Management of the North American Moose; Smithsonian Institution Press: Washington, DC, USA, 1998. [Google Scholar]
- Wam, H.K.; Felton, A.M.; Stolter, C.; Nybakken, L.; Hjeljord, O. Moose selecting for specific nutritional composition of birch places limits on food acceptability. Ecol. Evol. 2018, 8, 1117–1130. [Google Scholar] [CrossRef]
- Kandume, J.N. Sexual Segregation in Foraging of Greater Kudu (Tragelaphus strepsiceros) in a Heterogeneous Savanna, in Chobe National Park, Botswana. Master’s Thesis, Hedmark University College, Hedmark, Norway, 2012. [Google Scholar]
- Solon-Biet, S.M.; McMahon, A.C.; Ballard, J.W.O.; Ruohonen, K.; Wu, L.E.; Cogger, V.C.; Warren, A.; Huang, X.; Pichaud, N.; Melvin, R.G.; et al. The Ratio of Macronutrients, Not Caloric Intake, Dictates Cardiometabolic Health, Aging, and Longevity in Ad Libitum-Fed Mice. Cell Metab. 2014, 19, 418–430. [Google Scholar] [CrossRef] [Green Version]



| Site | Population Density (moose/km2) | Area (km2) | Sampled Moose Individuals |
|---|---|---|---|
| Hanma | 0.305 ± 0.064 | 216.3 | 38 |
| Zhanhe | 0.150 ± 0.051 | 156.44 | 17 |
| Nanwenghe | 0.111 ± 0.056 | 256.15 | 21 |
| Shuanghe | 0.056 ± 0.018 | 193.1 | 18 |
| Mohe | 0.052 ± 0.005 | 269.91 | 12 |
| Meitian | 0.028 ± 0.013 | 207.78 | 14 |
| Site | Sex | Margalef (S) | Shannon-Wiener (H’) | Species Evenness (J’) | Species Niche Breadth (B) |
|---|---|---|---|---|---|
| Mohe | F | 13 | 1.8 | 0.70 | 4.7 |
| M | 12 | 1.76 | 0.71 | 4.63 | |
| Nanwenghe | F | 13 | 2.11 | 0.82 | 6.98 |
| M | 13 | 2.15 | 0.84 | 7.28 | |
| Zhanhe | F | 13 | 2.33 | 0.91 | 8.33 |
| M | 13 | 2.2 | 0.86 | 6.68 | |
| Shuanghe | F | 13 | 2.26 | 0.88 | 8.21 |
| M | 13 | 2.29 | 0.89 | 7.99 | |
| Hanma | F | 12 | 2.14 | 0.86 | 7.11 |
| M | 11 | 1.94 | 0.81 | 5.41 | |
| Meitian | F | 11 | 2.09 | 0.87 | 7.38 |
| M | 10 | 1.91 | 0.83 | 5.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Bao, H.; Bencini, R.; Raubenheimer, D.; Dou, H.; Liu, H.; Wang, S.; Jiang, G. Macro-Nutritional Adaptive Strategies of Moose (Alces alces) Related to Population Density. Animals 2020, 10, 73. https://doi.org/10.3390/ani10010073
Ma Y, Bao H, Bencini R, Raubenheimer D, Dou H, Liu H, Wang S, Jiang G. Macro-Nutritional Adaptive Strategies of Moose (Alces alces) Related to Population Density. Animals. 2020; 10(1):73. https://doi.org/10.3390/ani10010073
Chicago/Turabian StyleMa, Yingjie, Heng Bao, Roberta Bencini, David Raubenheimer, Hongliang Dou, Hui Liu, Sirui Wang, and Guangshun Jiang. 2020. "Macro-Nutritional Adaptive Strategies of Moose (Alces alces) Related to Population Density" Animals 10, no. 1: 73. https://doi.org/10.3390/ani10010073





