In Vitro Evaluation of the Effects of Tylosin on the Composition and Metabolism of Canine Fecal Microbiota
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set Up
2.2. Chemical Analyses
2.3. Microbial Analysis
2.4. Statistical Analyses
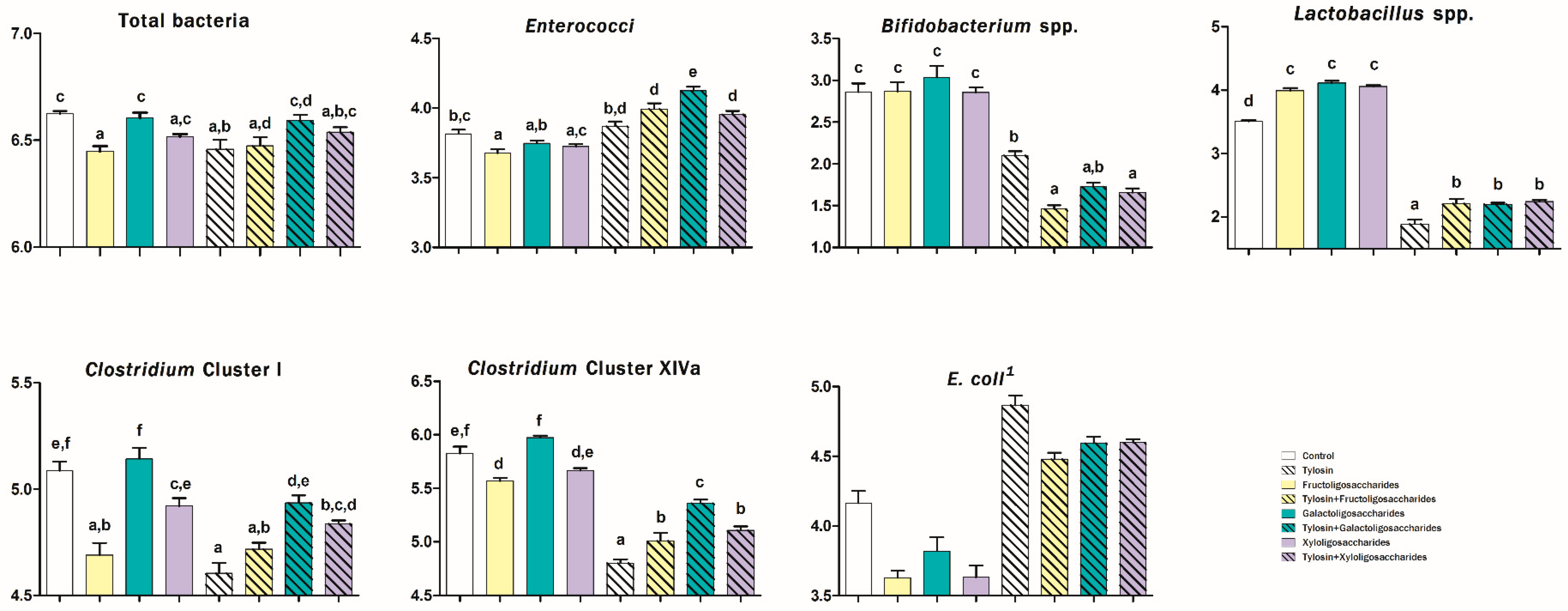
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Westermarck, E. Chronic Diarrhea in Dogs: What Do We Actually Know About It? Top. Companion Anim. Med. 2016, 31, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Westermarck, E.; Skrzypczak, T.; Harmoinen, J.; Steiner, J.M.; Ruaux, C.G.; Williams, D.A.; Eerola, E.; Sundbäck, P.; Rinkinen, M. Tylosin-Responsive Chronic Diarrhea in Dogs. J. Vet. Intern. Med. 2005, 19, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, S.; Spillmann, T.; Syrja, P.; Skrzypczak, T.; Louhelainen, M.; Westermarck, E. Effect of tylosin on dogs with suspected tylosin-responsive diarrhea: A placebo-controlled, randomized, double-blinded, prospective clinical trial. Acta Vet. Scand. 2011, 53, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpinen, S.; Spillmann, T.; Westermarck, E. Efficacy of two low-dose oral tylosin regimens in controlling the relapse of diarrhea in dogs with tylosin-responsive diarrhea: A prospective, single-blinded, two-arm parallel, clinical field trial. Acta Vet. Scand. 2014, 56, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsic, B.; Barber, J.; Čikoš, A.; Mladenovic, M.; Stankovic, N.; Novak, P. 16-membered macrolide antibiotics: A review. Int. J. Antimicrob. Agents 2018, 51, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Dong, M.; Shen, J.Z.; Wu, B.B.; Wu, C.M.; Du, X.D.; Wang, Z.; Qi, Y.T.; Li, B.Y. Tilmicosin and tylosin have anti-inflammatory properties via modulation of COX-2 and iNOS gene expression and production of cytokines in LPS-induced macrophages and monocytes. Int. J. Antimicrob. Agents 2006, 27, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Dowd, S.E.; Westermarck, E.; Steiner, J.M.; Wolcott, R.D.; Spillmann, T.; Harmoinen, J.A. The effect of the macrolide antibiotic tylosin on microbial diversity in the canine small intestine as demonstrated by massive parallel 16S rRNA gene sequencing. BMC Microbiol. 2009, 9, 210. [Google Scholar] [CrossRef] [Green Version]
- Kilpinen, S.; Rantala, M.; Spillmann, T.; Björkroth, J.; Westermarck, E. Oral tylosin administration is associated with an increase of faecal enterococci and lactic acid bacteria in dogs with tylosin-responsive diarrhoea. Vet. J. 2015, 205, 369–374. [Google Scholar] [CrossRef]
- Jackson, C.R.; Fedorka-Cray, P.J.; Davis, J.A.; Barrett, J.B.; Frye, J.G. Prevalence, species distribution and antimicrobial resistance of enterococci isolated from dogs and cats in the United States. J. Appl. Microbiol. 2009, 107, 1269–1278. [Google Scholar] [CrossRef]
- Leener, E.D.; Decostere, A.; De Graef, E.M.; Moyaert, H.; Haesebrouck, F. Presence and mechanism of antimicrobial resistance among enterococci from cats and dogs. Microb. Drug Resist. 2005, 11, 395–403. [Google Scholar] [CrossRef]
- Manchester, A.C.; Webb, C.B.; Blake, A.B.; Sarwar, F.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Long-term impact of tylosin on fecal microbiota and fecal bile acids of healthy dogs. J. Vet. Intern. Med. 2019, 33, 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- Honneffer, J.B.; Minamoto, Y.; Suchodolski, J.S. Microbiota Alterations in Acute and Chronic Gastrointestinal Inflammation of Cats and Dogs. World J. Gastroenterol. 2014, 20, 16489–16497. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Swanson, K.S.; Fahey, G.C., Jr.; Garleb, K.A. Perspective: Physiologic Importance of Short-Chain Fatty Acids from Nondigestible Carbohydrate Fermentation. Adv. Nutr. 2019, 10, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S.; et al. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, M.A.; Konnikova, L.; Gerber, J.S. Impact of Antibiotics on Necrotizing Enterocolitis and Antibiotic-Associated Diarrhea. Gastroenterol. Clin. N. Am. 2017, 46, 61–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macfarlane, S.; Macfarlane, G.T.; Cummings, J.H. Review article: Prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2006, 24, 701–714. [Google Scholar] [CrossRef]
- Preidis, G.A.; Versalovic, J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: Gastroenterology enters the metagenomics era. Gastroenterology 2009, 136, 2015–2031. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. 2010, 7, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.P.; Antoine, J.M.; Midtvedt, T.; van Hemert, S. Manipulating the gut microbiota to maintain health and treat disease. Microb. Ecol. Health Dis. 2015, 26, 25877–25886. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, A.K. Responses of feeding prebiotics on nutrient digestibility, faecal microbiota composition and short-chain fatty acid concentrations in dogs: A meta-analysis. Animal 2011, 5, 1743–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinna, C.; Biagi, G. The Utilisation of Prebiotics and Synbiotics in Dogs. Ital. J. Anim. Sci. 2014, 13, 169–178. [Google Scholar] [CrossRef]
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Biagi, G.; Cipollini, I.; Grandi, M.; Pinna, C.; Vecchiato, C.G.; Zaghini, G. A new in vitro method to evaluate digestibility of commercial diets for dogs. Ital. J. Anim. Sci. 2016, 15, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Sunvold, G.D.; Fahey, G.C., Jr.; Merchen, N.R.; Reinhart, G.A. In vitro fermentation of selected fibrous substrates by dog and cat fecal inoculum: Influence of diet composition on substrate organic matter disappearance and short-chain fatty acid production. J. Anim. Sci. 1995, 73, 1110–1122. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 17th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Stefanelli, C.; Carati, D.; Rossoni, C. Separation of N1- and N8-acetylspermidine isomers by reversed-phase column liquid chromatography after derivatization with dansyl chloride. J. Chromatogr. 1986, 375, 49–55. [Google Scholar] [CrossRef]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Malinen, E. Comparison of real-time PCR with SYBR Green I or 5’-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology 2003, 149, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Malinen, E.; Rinttila, T.; Kajander, K.; Matto, J.; Kassinen, A.; Krogius, L.; Saarela, M.; Korpela, R.; Palva, A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am. J. Gastroenterol. 2005, 100, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, C.; Finegold, S.M. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 2004, 70, 6459–6465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclercq, R.; Courvalin, P. Intrinsic and unusual resistance to macrolide, lincosamide, and streptogramin antibiotics in bacteria. Antimicrob. Agents Chemother. 1991, 35, 1273–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papizadeh, M.; Rohani, M.; Nahrevanian, H.; Javadi, A.; Pourshafie, M.R. Probiotic characters of Bifidobacterium and Lactobacillus are a result of the ongoing gene acquisition and genome minimization evolutionary trends. Microb. Pathog. 2017, 111, 118–131. [Google Scholar] [CrossRef]
- Schmitz, S.; Suchodolski, J.S. Understanding the canine intestinal microbiota and its modification by pro-, pre- and synbiotics—What is the evidence? Vet. Med. Sci. 2016, 2, 71–94. [Google Scholar] [CrossRef]
- Poole, T.L.; Genovese, K.J.; Knape, K.D.; Callaway, T.R.; Bischoff, K.M.; Nisbet, D.J. Effect of subtherapeutic concentrations of tylosin on the inhibitory stringency of a mixed anaerobe continuous-flow culture of chicken microflora against Escherichia coli O157:H7. J. Appl. Microbiol. 2003, 94, 73–79. [Google Scholar] [CrossRef]
- Kolida, S.; Tuohy, K.; Gibson, G.R. Prebiotic effects of inulin and oligofructose. Br. J. Nutr. 2002, 87, S193–S197. [Google Scholar] [CrossRef] [Green Version]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; McCartney, A.L.; Rastall, R.A. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 2005, 93, S31–S34. [Google Scholar] [CrossRef]
- Turroni, F.; van Sinderen, D.; Ventura, M. Genomics and ecological overview of the genus Bifidobacterium. Int. J. Food Microbiol. 2011, 149, 37–44. [Google Scholar] [CrossRef]
- Pinna, C.; Vecchiato, C.G.; Bolduan, C.; Grandi, M.; Stefanelli, C.; Windisch, W.; Zaghini, G.; Biagi, G. Influence of dietary protein and fructooligosaccharides on fecal fermentative end-products, fecal bacterial populations and apparent total tract digestibility in dogs. BMC Vet. Res. 2018, 14, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macfarlane, G.T.; Macfarlane, L.E. Acquisition, evolution and maintenance of the normal gut microbiota. Dig. Dis. 2009, 27, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Ladirat, S.E.; Schols, H.A.; Nauta, A.; Schoterman, M.H.; Keijser, B.J.; Montijn, R.C.; Gruppen, H.; Schuren, F.H. High-throughput analysis of the impact of antibiotics on the human intestinal microbiota composition. J. Microbiol. Methods 2013, 92, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Suchodolski, J.S. Intestinal microbiota of dogs and cats: A bigger world than we thought. Vet. Clin. North. Am. Small Anim. Pract. 2011, 41, 261–272. [Google Scholar] [CrossRef]
- Blachier, F.; Mariotti, F.; Huneau, J.F.; Tomé, D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids. 2007, 33, 547–562. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Studies on amine production in the human colon: Enumeration of amine forming bacteria and physiological effects of carbohydrate and pH. Anaerobe 1996, 2, 285–297. [Google Scholar] [CrossRef]
- Pinna, C.; Vecchiato, C.G.; Zaghini, G.; Grandi, M.; Nannoni, E.; Stefanelli, C.; Biagi, G. In vitro influence of dietary protein and fructooligosaccharides on metabolism of canine fecal microbiota. BMC Vet. Res. 2016, 12, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinna, C.; Stefanelli, C.; Biagi, G. In vitro effect of dietary protein level and nondigestible oligosaccharides on feline fecal microbiota. J. Anim. Sci. 2014, 92, 5593–5602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Propst, E.L.; Flickinger, E.A.; Bauer, L.L.; Merchen, N.R.; Fahey, G.C., Jr. A dose-response experiment evaluating the effects of oligofructose and inulin on nutrient digestibility, stool quality, and fecal protein catabolites in healthy adult dogs. J. Anim. Sci. 2003, 81, 3057–3066. [Google Scholar] [CrossRef] [PubMed]
- Dohrmann, A.B.; Walz, M.; Löwen, A.; Tebbe, C.C. Clostridium cluster I and their pathogenic members in a full-scale operating biogas plant. Appl. Microbiol. Biotechnol. 2015, 99, 3585–3598. [Google Scholar] [CrossRef]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B.; et al. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64 (Suppl. 3), S95–S100. [Google Scholar] [CrossRef] [Green Version]
- Ramlachan, N.; Anderson, R.C.; Andrews, K.; Harvey, R.B.; Nisbet, D.J. A comparative study on the effects of tylosin on select bacteria during continuous flow culture of mixed populations of gut microflora derived from a feral and a domestic pig. Foodborne Pathog. Dis. 2008, 5, 21–31. [Google Scholar] [CrossRef] [Green Version]

| CTRL | FOS | GOS | XOS | TYL | TYL + FOS | TYL + GOS | TYL + XOS | ANOVA p-Value | Pooled SEM | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TYL × PRE | PRE | TYL | ||||||||||
| pH | 6.03 | 5.74 | 5.71 | 5.54 | 6.34 | 6.14 | 5.98 | 5.96 | 0.572 | <0.001 2 | <0.001 | 0.061 |
| Ammonia | 27.9 | 23.2 | 26.8 | 25.7 | 25.2 | 24.0 | 24.6 | 23.6 | 0.302 | 0.028 3 | 0.033 | 0.987 |
| Acetic acid | 12.4 d | 11.0 b,c | 10.3 b | 11.5 c,d | 6.58 a | 6.20 a | 6.13 a | 6.53 a | 0.020 | <0.001 | <0.001 | 0.249 |
| Propionic acid | 6.35 c,d | 7.17 e | 6.69 d,e | 8.04 f | 4.52 a | 4.90 a | 5.16 a,b | 5.72 b,c | 0.037 | <0.001 | <0.001 | 0.150 |
| iso-Butyric acid | 0.37 c | 0.23 b | 0.24 b | 0.27 b | 0.06 a | 0.05 a | 0.05 a | 0.04 a | <0.001 | <0.001 | <0.001 | 0.010 |
| n-Butyric acid | 3.95 a | 4.95 d,e | 5.22 e | 4.39 b,c | 3.73 a | 4.01 a,b | 4.69 c,d | 3.96 a | 0.001 | <0.001 | <0.001 | 0.083 |
| iso-Valeric acid | 0.59 e | 0.36 c | 0.46 b | 0.42 d | 0.10 a | 0.09 a | 0.09 b | 0.10 a | <0.001 | <0.001 | <0.001 | 0.009 |
| Total VFA | 23.9 | 23.7 | 22.9 | 24.7 | 15.0 | 15.2 | 16.1 | 16.4 | 0.148 | 0.085 | <0.001 | 0.469 |
| CTRL | FOS | GOS | XOS | TYL | TYL + FOS | TYL + GOS | TYL + XOS | ANOVA p-Value | Pooled SEM | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TYL × PRE | PRE | TYL | ||||||||||
| pH | 5.45 | 5.22 | 5.28 | 5.18 | 5.93 | 5.61 | 5.70 | 5.59 | 0.101 | <0.001 2 | <0.001 | 0.019 |
| Ammonia | 36.9 | 35.1 | 36.0 | 34.6 | 36.0 | 34.8 | 35.7 | 36.1 | 0.268 | 0.136 | 0.958 | 1.255 |
| Acetic acid | 14.8 c | 14.2 c | 13.7 c | 13.9 c | 7.44 a,b | 7.12 a,b | 6.90 a | 8.37 b | 0.015 | 0.012 | <0.001 | 0.284 |
| Propionic acid | 11.3 c | 12.0 c | 11.5 c | 12.2 c | 7.80 a | 9.75 b | 8.85 a,b | 11.8 c | <0.001 | <0.001 | <0.001 | 0.270 |
| iso-Butyric acid | 0.53 e | 0.41 c | 0.46 d | 0.42 c | 0.07 b | 0.03 a | 0.03 a | 0.02 a | <0.001 | <0.001 | <0.001 | 0.009 |
| n-Butyric acid | 4.82 | 6.12 | 6.37 | 5.31 | 4.65 | 5.97 | 6.16 | 5.34 | 0.708 | <0.001 3 | 0.121 | 0.112 |
| iso-Valeric acid | 0.83 c | 0.63 b | 0.78 c | 0.64 b | 0.14 a | 0.13 a | 0.12 a | 0.13 a | <0.001 | <0.001 | <0.001 | 0.011 |
| Total VFA | 32.5 c | 33.7 c | 33.1 c | 32.8 c | 20.1 a | 23.0 a,b | 22.1 a | 25.6 b | 0.003 | <0.001 | <0.001 | 0.658 |
| CTRL | FOS | GOS | XOS | TYL | TYL + FOS | TYL + GOS | TYL + XOS | ANOVA p-Value | Pooled SEM | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TYL × PRE | PRE | TYL | ||||||||||
| 6 h | ||||||||||||
| Putrescine | 150 | 144 | 164 | 179 | 103 | 161 | 91.8 | 136 | 0.341 | 0.478 | 0.046 | 25.0 |
| Cadaverine | 48.0 | 28.8 | 21.4 | 160 | 90.4 | 95.2 | 46.4 | 145 | 0.606 | 0.003 2 | 0.178 | 30.6 |
| Spermidine | 41.0 | 19.5 | 14.2 | 15.3 | 50.4 | 58.6 | 29.0 | 75.0 | 0.170 | 0.191 | 0.001 | 12.3 |
| Spermine | 23.2 | 14.9 | 11.4 | 9.00 | 20.4 | 16.5 | 3.40 | 24.7 | 0.421 | 0.285 | 0.761 | 7.34 |
| 24 h | ||||||||||||
| Putrescine | 152 | 161 | 189 | 157 | 206 | 207 | 233 | 210 | 0.671 | <0.001 3 | <0.001 | 4.75 |
| Cadaverine | 52.2 a,b | 32.4 a | 55.6 a,b | 54.6 a,c | 117 d,e | 161 e | 102 b,c,d,f | 125 e,f | 0.009 | 0.476 | <0.001 | 11.7 |
| Spermidine | 8.02 | 17.2 | 33.4 | 11.1 | 18.2 | 30.2 | 34.0 | 24.6 | 0.665 | 0.008 4 | 0.030 | 5.82 |
| Spermine | 6.94 | 0.88 | 4.86 | 3.78 | 3.76 | 2.48 | 4.96 | 2.30 | 0.375 | 0.048 5 | 0.462 | 1.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinna, C.; Vecchiato, C.G.; Grandi, M.; Mammi, L.M.E.; Stefanelli, C.; Biagi, G. In Vitro Evaluation of the Effects of Tylosin on the Composition and Metabolism of Canine Fecal Microbiota. Animals 2020, 10, 98. https://doi.org/10.3390/ani10010098
Pinna C, Vecchiato CG, Grandi M, Mammi LME, Stefanelli C, Biagi G. In Vitro Evaluation of the Effects of Tylosin on the Composition and Metabolism of Canine Fecal Microbiota. Animals. 2020; 10(1):98. https://doi.org/10.3390/ani10010098
Chicago/Turabian StylePinna, Carlo, Carla Giuditta Vecchiato, Monica Grandi, Ludovica Maria Eugenia Mammi, Claudio Stefanelli, and Giacomo Biagi. 2020. "In Vitro Evaluation of the Effects of Tylosin on the Composition and Metabolism of Canine Fecal Microbiota" Animals 10, no. 1: 98. https://doi.org/10.3390/ani10010098
APA StylePinna, C., Vecchiato, C. G., Grandi, M., Mammi, L. M. E., Stefanelli, C., & Biagi, G. (2020). In Vitro Evaluation of the Effects of Tylosin on the Composition and Metabolism of Canine Fecal Microbiota. Animals, 10(1), 98. https://doi.org/10.3390/ani10010098






