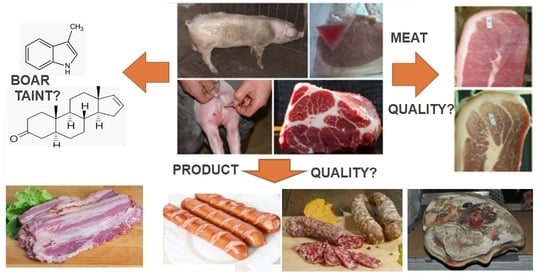
The Use of Pork from Entire Male and Immunocastrated Pigs for Meat Products—An Overview with Recommendations
Abstract
Simple Summary
Abstract
1. Introduction
2. Fat and Meat Quality
2.1. Fat Tissue Quality and Composition
2.2. Meat Color and pH Value
| pH 45 min | Ultimate pH | Colour L* | ||||
|---|---|---|---|---|---|---|
| Meta-analytical studies 1 | ||||||
| EM–SC | NS | [14,41] | EM > SC | [41] | SC > EM | [14] |
| SC > EM | [14] | NS | [41] | |||
| EM–IC | NS | [14,41] | EM > IC | [29] | IC > EM | [14,29,41] |
| IC–SC | NS | [14,41] | SC > IC | [14] | NS | [14,29,41] |
| NS | [29] | |||||
| Individual studies 2 | ||||||
| EM–SC | NS | [22,48,55] | EM > SC | [55] | EM > SC | [52] |
| SC > EM | [56] | |||||
| NS | [22,48,52,57] | NS | [22,48,56,57] | |||
| EM–IC | EM > IC | [58] | EM > IC | [58,59] | EM > IC | [60] |
| NS | [22,55,59,61,62] | IC > EM | [56,60] | IC > EM | [58] | |
| NS | [22,42,55,57,61,62] | NS | [22,42,56,57,59,61,62] | |||
| IC–SC | NS | [22,55,63,64,65,66] | SC > IC | [65] | IC > SC | [66] |
| NS | [22,49,55,56,57,63,64,66,67,68] | SC > IC | [64] | |||
| NS | [22,49,56,57,63,65,67,68,69] | |||||
2.3. Muscle Water Holding Capacity
2.4. Meat Tenderness
2.5. Recommendations in Regard to Fat and Meat Quality
3. Meat Product Quality
3.1. Dry-Cured Ham
3.2. Dry-Fermented Sausages
3.3. Cured and/or Thermally Processed Bellies and Bacon
3.4. Other Meat Products
3.5. Recommendations in Regard to Meat Products
4. Mitigating the Risk of Boar Taint in Meat and Meat Products
4.1. Dry-Cured Ham
4.2. Dry-Fermented Sausages
4.3. Fresh Meat (Primals, Subprimals, Meat Cuts, Fresh Minced Meat Products like Patties or Sausages)
4.4. Cooked Meat Products (Cooked Ham, Bacon and Emulsion-Type Sausages)
4.5. Recommendations in Regard to the Mitigation of Boar Taint in Meat and Meat Products
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bee, G.; Chevillon, P.; Bonneau, M. Entire male pig production in Europe. Anim. Prod. Sci. 2015, 55, 1347–1359. [Google Scholar] [CrossRef]
- Backus, G.; Higuera, M.; Juul, N.; Nalon, E.; de Bryne, N. Second Progress Report 2015–2017 on the European Declaration on Alternatives to Surgical Castration of Pigs. Available online: https://www.boarsontheway.com/wp-content/uploads/2018/08/Second-progress-report-2015-2017-final-1.pdf (accessed on 8 July 2020).
- De Roest, K.; Montanari, C.; Fowler, T.; Baltussen, W. Resource efficiency and economic implications of alternatives to surgical castration without anaesthesia. Animal 2009, 3, 1522–1531. [Google Scholar] [CrossRef]
- von Borell, E.; Baumgartner, J.; Giershing, M.; Jäggin, N.; Prunier, A.; Tuyttens, F.A.M.; Edwards, S.A. Animal welfare implications of surgical castration and its alternatives in pigs. Animal 2009, 3, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.; Weiler, U.; Herzog, A. Physiological aspects of androstenone and skatole formation in the boar—A review with experimental data. Meat Sci. 1994, 38, 289–305. [Google Scholar] [CrossRef]
- Lundström, K.; Matthews, K.R.; Haugen, J.-E. Pig meat quality from entire males. Animal 2009, 3, 1497–1507. [Google Scholar] [CrossRef]
- Bonneau, M.; Weiler, U. Pros and cons of alternatives to piglet castration: Welfare, boar taint and other meat quality traits. Animals 2019, 9, 884. [Google Scholar] [CrossRef] [PubMed]
- Dunshea, F.R.; Colantoni, C.; Howard, K.; McCauley, I.; Jackson, P.; Long, K.A.; Lopaticki, S.; Nugent, E.A.; Simons, J.A.; Walker, J. Vaccination of boars with a GnRH vaccine (Improvac) eliminates boar taint and increases growth performance. J. Anim. Sci. 2001, 79, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Batorek-Lukač, N.; Dubois, S.; Noblet, J.; Čandek-Potokar, M.; Labussière, E. Effect of high dietary fat content on heat production and lipid and protein deposition in growing immunocastrated male pigs. Animal 2016, 10, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Čandek-Potokar, M.; Škrlep, M.; Batorek Lukač, N. Raising entire males or immunocastrates—Outlook on meat quality. Proc. Food Sci. 2015, 5, 30–33. [Google Scholar] [CrossRef]
- Škrlep, M.; Čandek-Potokar, M. Meat quality issues in entire male and immunocastrated pigs. Adv. Anim. Biosci. 2018, 9, 31. [Google Scholar]
- Kouba, M.; Sellier, P. A review of the factors influencing the development of intermuscular adipose tissue in the growing pig. Meat Sci. 2011, 88, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 43–358. [Google Scholar] [CrossRef] [PubMed]
- Pauly, K.; Luginbühl, W.; Ampuero, S.; Bee, G. Expected effects on carcass and pork quality when surgical castration is omitted. Meat Sci. 2012, 92, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Gandemer, G. Lipids in muscles and addipose tissues, changes during processing and sensory properties of meat products. Meat Sci. 2002, 62, 309–321. [Google Scholar] [CrossRef]
- Čandek-Potokar, M.; Škrlep, M. Factors in pig production that impact the quality of dry-cured ham: A review. Animal 2012, 6, 327–338. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Whittington, F.M.; Moncrieff, C.B.; Kempster, A.J. Backfat composition in pigs: Differences between fat thickness groups and sexes. Livest. Prod. Sci. 1989, 22, 351–362. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone: A metabolic hormone in health and disease. J. Endocrinol. 2013, 217, R25–R45. [Google Scholar] [CrossRef]
- Boler, D.D.; Clark, D.L.; Baer, A.A.; Meeuwse, D.M.; King, V.L.; McKeith, F.K.; Killefer, J. Effects of increasing lysine on further processed product characteristics from immunologically castrated male pigs. J. Anim. Sci. 2011, 89, 2200–2209. [Google Scholar] [CrossRef]
- Kyle, J.M.; Bohrer, B.M.; Schroeder, A.L.; Matulis, R.J.; Boler, D.D. Effects of immunological castration (Improvest) on further processed belly characterisitics and commercial slicing yields of finishing pigs. J. Anim. Sci. 2014, 92, 4223–4233. [Google Scholar] [CrossRef]
- Škrlep, M.; Poklukar, K.; Kress, K.; Vrecl, M.; Fazarinc, G.; Batorek Lukač, N.; Weiler, U.; Stefanski, V.; Čandek-Potokar, M. Effect of immunocastration and housing conditions on pig carcass and meat quality traits. Transl. Anim. Sci. 2020, 4, 55. [Google Scholar] [CrossRef]
- Babol, J.; Squires, E.J. Quality of meat from entire male pigs. Food Res. Int. 1995, 28, 201–212. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M. Comparison of boars and castrates for bacon production 2. Composition of muscle and subcutaneous fat, and changes in side weight during curing. Anim. Prod. 1982, 35, 65–74. [Google Scholar] [CrossRef]
- Sather, A.P.; Jones, S.D.M.; Robertson, W.M.; Zawadski, S. Sex effects on fat hardness meter readings of market weight pigs. Can. J. Anim. Sci. 1995, 75, 509–515. [Google Scholar] [CrossRef]
- Wood, J.D. Fat quality in pig meat. In Fat Quality in Lean Pigs; Wood, J.D., Ed.; AFRC Meat Research Institute: Langford, Bristol, UK, 1984; pp. 9–14. [Google Scholar]
- Gispert, M.; Oliver, M.A.; Velarde, A.; Suarez, P.; Pérez, J.; i Furnols, M.F. Carcass and meat quality characteristics of immunocastrated male, surgically castrated male, entire male and female pigs. Meat Sci. 2010, 85, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.; Lacorn, M.; Danowski, K.; Pearce, M.C.; Bauer, A. Short-term endocrine and metabolic reactions before and after second immunization against GnRH in boars. Vaccine 2007, 25, 4689–4696. [Google Scholar] [CrossRef]
- Batorek, N.; Čandek-Potokar, M.; Bonneau, M.; Van Milgen, J. Meta-analysis of the effect of immunocastration on production, reproductive organs and boar taint compounds in pigs. Animal 2012, 6, 1330–1338. [Google Scholar] [CrossRef]
- Nautrup, B.P.; Van Vlaenderen, I.; Aldaz, A.; Mah, C.K. The effect of immunization against gonadotropin-releasing factor on growth performance, carcass characteristics and boar taint relevant to pig producers and the pork packing industry: A meta-analysis. Res. Vet. Sci. 2018, 119, 182–195. [Google Scholar] [CrossRef]
- Lealiifano, A.K.; Pluske, J.R.; Nicholls, R.R.; Dunshea, F.R.; Campbell, R.G.; Hennessy, D.P.; Miller, D.W.; Hansen, C.F.; Mullan, B.P. Reducing the length of time between slaughter and the secondary gonadotropin-releasing factor immunization improves growth performance and clears boar taint compounds in male finishing pigs. J. Anim. Sci. 2011, 89, 2782–2792. [Google Scholar] [CrossRef]
- Berryman, D.E.; List, E.O.; Sackmann-Sala, L.; Lubbers, E.; Munn, R.; Kopchick, J.J. Growth hormone and adipose tissue: Beyond the adipocyte. Growth Horm. IGF Res. 2011, 21, 113–123. [Google Scholar] [CrossRef]
- Hauser, N.; Mourot, J.; De Clercq, L.; Genart, C.; Remacle, C. The cellularity of developing adipose tissues in Pietrain and Meishan pigs. Reprod. Nutr. Dev. 1997, 37, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Poklukar, K.; Čandek-Potokar, M.; Fazarinc, M.V.; Lukač, N.B.; Fazarinc, G.; Kress, K.; Weiler, U.; Stefanski, V.; Škrlep, M. Effect of immunocastration on adipose tissue deposition and composition in pigs. Animal 2020. under review. [Google Scholar]
- Mackay, J.; Pearce, M.C.; Thevasagayam, S.; Doran, O. Fatty acid composition and lipogenic enzyme protein expression in subcutaneous adipose tissue of male pigs vaccinated against boar taint, barrows, and entire boars. J. Anim. Sci. 2013, 91, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Tavárez, M.A.; Puls, C.L.; Schroeder, A.L.; Dilger, A.C. Effects of immunocastration and time after second Improvest dose on adipose tissue fatty acid profile of finishing barrows. J. Anim. Sci. 2014, 92, 3736–3744. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.K.; Mellencamp, M.A.; Johnston, L.J.; Cox, R.B.; Shurson, G.C. Effectiveness of different corn dried distillers grains with solubles feeding strategies and increasing the time intervals between the second Improvest dose and slaughter of immunologically castrated pigs on belly and pork fat quality. Meat Sci. 2018, 135, 62–73. [Google Scholar] [CrossRef]
- Asmus, M.D.; Tavarez, M.A.; Tokach, M.D.; Dritz, S.S.; Schroeder, A.L.; Nelssen, J.L.; Godband, R.D.; DeRouchey, J.M. The effects of immunological castration and corn dried distillers grains with solubles withdrawal on growth performance, carcass characteristics, fatty acid analysis, and iodine value of pork fat depots. J. Anim. Sci. 2014, 92, 2116–2132. [Google Scholar] [CrossRef]
- Batorek, N.; Škrlep, M.; Prunier, A.; Louveau, I.; Noblet, J.; Bonneau, M.; Čandek-Potokar, M. Effect of feed restriction on hormones, performance, carcass traits, and meat quality in immunocastrated pigs. J. Anim. Sci. 2012, 90, 4593–4603. [Google Scholar] [CrossRef]
- Minelli, G.; Macchini, P.; Mezzetti, F.; Belmonte, A.M.; Volpelli, L.A.; Faeti, V.; Lo Fiego, D.P. Characteristics of lipids from immunocastrated medium-heavy pigs fed either restricted or ad libitum. Ital. J. Food Sci. 2019, 31, 98–109. [Google Scholar] [CrossRef]
- Trefan, L.; Doeschl-Wilson, A.; Rooke, J.A.; Terulow, C.; Bünger, L. Meta-analysis of effects of gender in combination with carcass weight and breed on pork quality. J. Anim. Sci. 2013, 91, 1480–1492. [Google Scholar] [CrossRef]
- Park, J.; Campbell, C.P.; Squires, E.J.; de Lange, C.F.M.; Mandell, I.B. Effects of pig genotype, immunological castration, and use of ractopamine on growth performance, carcass traits and pork quality of entire male pigs. Can. J. Anim. Sci. 2019, 99, 82–106. [Google Scholar] [CrossRef]
- Lawrie, R.A. Lawrie’s Meat Science, 6th ed.; Woodhead Publishing Limited: Cambridge, UK, 2002; pp. 58–118. [Google Scholar]
- Cronin, G.M.; Dunshea, F.R.; Butler, K.L.; McCaules, I.; Barnett, J.L.; Hemsworth, P.H. The effects of immune- and surgical-castration on the behaviour and consequently growth of group-housed, male finisher pigs. Appl. Anim. Behav. Sci. 2003, 81, 111–126. [Google Scholar] [CrossRef]
- Sather, A.P.; Jones, S.D.M.; Squires, E.J.; Schaefer, A.L.; Robertson, W.M.; Tong, A.K.W.; Zawadski, S. Antemortem handling effects on the behaviour, carcass yield and meat quality of market weight entire male pigs. Can. J. Anim. Sci. 1995, 75, 45–56. [Google Scholar]
- Moss, B.W.; Robb, J.D. The effect of pre-slaughter lairage on serum thyroxine and cortisol levels at slaughter, and meat quality of boars, hogs and gilts. J. Sci. Food Agric. 1978, 29, 689–696. [Google Scholar] [CrossRef]
- Fàbrega, E.; Velarde, A.; Cros, J.; Gispert, M.; Suárez, P.; Tibau, J.; Soler, J. Effect of vaccination against gonadotropin-releasing hormone, using Improvac, on growth performance, body composition, behaviour and acute phase proteins. Livest. Sci. 2010, 132, 53–59. [Google Scholar] [CrossRef]
- Holinger, M.; Früh, M.; Stoll, P.; Pedan, V.; Kreuzer, M.; Bérard, J.; Hillman, E. Long-term effects of castration, chronic intermittent social stress, provision of grass silage and their interactions on performance and meat and adipose tissue properties in growing-finishing pigs. Meat Sci. 2018, 145, 40–50. [Google Scholar] [CrossRef]
- Rocha, L.M.; Bridi, A.M.; Foury, A.; Mormède, P.; Weschenfelder, A.V.; Devillers, N.; Bertoloni, W.; Faucitano, L. Effects of ractopamine administration and castration method on the response to preslaughter stress and carcass and meat quality in pigs of two Pietrain genotypes. Anim. Sci. 2013, 91, 3965–3977. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olivan, M.; González, J.; Bassols, A.; Díaz, F.; Carreras, R.; Mainau, E.; Arroyo, L.; Peña, R.; Potes, Y.; Coto-Montes, A.; et al. Effect of sex and RYR1 gene mutation on the muscle proteomic profile and main physiological biomarkers at slaughter. Meat Sci. 2018, 141, 81–90. [Google Scholar] [CrossRef]
- Grandin, T. Assessment of stress during handling and transport. J. Anim. Sci. 1997, 75, 249–257. [Google Scholar] [CrossRef]
- Škrlep, M.; Tomažin, U.; Lukač, N.B.; Poklukar, K.; Čandek-Potokar, M. Proteomic profiles of the longissimus muscles of entire male and castrated pigs as related to meat quality. Animals 2019, 9, 74. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Baas, T.J.; Malek, M.; Dekkers, J.C.M.; Prusa, K.; Rothschild, M.F. Correlations among selected pork quality traits. J. Anim. Sci. 2002, 80, 617–627. [Google Scholar] [CrossRef]
- Jones-Hamlow, K.A.; Tavarez, M.A.; Boler, D.D.; Schroeder, A.L.; Prusa, K.J.; Dilger, A.C. Colour stability and sensory characteristics of fresh and enhanced pork loins from immunologically castrated barrows. J. Anim. Sci. 2015, 93, 794–801. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aluwé, M.; Millet, S.; Škrlep, M.; Čandek-Potokar, M.; Żakowska-Biemans, S.; Kostyra, E.; Van den Broeke, A. Évaluation de la qualité de la viande des mâles entires, cestres et immunocatrés: Evaluation of meat quality of boars, barrows and immunocatrates. In Proceedings of the 52émes Journées de la Recherche Porcine, Paris, France, 4–5 February 2020; Volume 52, pp. 61–62. [Google Scholar]
- Aluwé, M.; Langendries, K.C.M.; Beakert, K.M.; Tuyttens, F.A.M.; De Brabander, D.L.; De Smet, S.; Millet, S. Effect of surgical castration, immunocastration and chicory-diet on the meat quality and palatability of boars. Meat Sci. 2013, 94, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Boler, D.D.; Puls, C.L.; Clark, D.L.; Matzat, P.D.; Killefer, J.; McKeith, F.K.; Dilger, A.C. Effects of immunological castration (Improvest) on changes in dressing percentage and carcass characteristics in finishing pigs. J. Anim. Sci. 2014, 91, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Akit, H.; Frobose, H.; Channon, H.A.; D’Souza, D.N.; Dunshea, F.R. Effect of sex and dietary lecithin on eating quality of pork. In Proceedings of the 60th International Congress of Meat Science and Technology, Punta del Este, Uruguay, 17–22 August 2014. [Google Scholar]
- Van den Broeke, A.; Leen, F.; Aluwé, M.; Ampe, B.; Van Meensel, J.; Millet, S. The effect of GnRH vaccination on performance, carcass, and meat quality and hormonal regulation in boar, barrows and gilts. J. Anim. Sci. 2016, 94, 2811–2820. [Google Scholar] [CrossRef]
- Oliviero, C.; Ollila, A.; Andersson, M.; Heinonen, M.; Voutila, L.; Serenius, T.; Peltoniemi, O. Strategic use of anti-GnRH vaccine allowing selection of breeding without adverse effects on reproductive production performances. Theriogenology 2016, 85, 476–482. [Google Scholar] [CrossRef]
- Moore, K.L.; Mullan, B.P.; Dunshea, F.R. Boar taint, meat quality and fail rate in entire male pigs and male pigs immunized against gonadotrophin releasing factor as related to body weight and feeding regime. Meat Sci. 2017, 125, 95–101. [Google Scholar] [CrossRef]
- Needham, T.; Hoffman, L.C. Physical meat quality and chemical composition of the Longissimus thoracis of entire male and immunocastrated pigs fed varying dietary protein levels. Meat Sci. 2015, 110, 101–108. [Google Scholar] [CrossRef]
- Bahelka, I.; Tomka, J.; Hanusova, E. Immunocastrated pigs as an laternative to pork production with surgical castrates. In Proceedings of the Internationsl Symposium on Animal Science, Belgrade-Zemun, Serbia, 24–25 November 2016. [Google Scholar]
- Caldara, F.; Moi, M.; dos Santos, L.; Paz, I.; Garcia, R.; Nääs, I.; Fernandez, A. Carcass characteristics and qualitative attributes of pork from immunocastrated animals. Asian-Australas. J. Anim. Sci. 2013, 26, 1630–1636. [Google Scholar] [CrossRef]
- Martinez-Macipe, M.; Rodríguez, P.; Izquierdo, M.; Gispert, M.; Manteca, X.; Mainauc, E.; Hernández, F.I.; Claret, A.; Guerrero, L.; Dalmau, A. Comparison of meat quality parameters in surgical castrated versus vaccinated against gonadotrophin-releasing factor male and female Iberian pigs reared in free-ranging conditions. Meat Sci. 2016, 111, 116–121. [Google Scholar] [CrossRef]
- Seiquer, I.; Palma-Granados, P.; Haro, A.; Lara, L.; Lachica, M.; Fernández-Fígares, I.; Nieto, R. Meat quality traits in longissimus lumborum and gluteus medius muscles from immunocastrated and surgically castrated Iberian pigs. Meat Sci. 2019, 150, 77–84. [Google Scholar] [CrossRef]
- Li, H.; Gariépy, C.; Jin, Y.; Furnols, M.F.; Fortin, J.; Rocha, L.M.; Faucitano, L. Effects of ractopamine administration and castration method on muscle fiber characteristics and sensory quality of the longissimus muscle in two Piétrain pig genotypes. Meat Sci. 2015, 102, 27–34. [Google Scholar] [CrossRef]
- Lowe, B.K.; Gerleman, G.D.; Carr, S.N.; Rinckler, P.J.; Schroeder, A.L.; Petry, D.B.; McKeith, F.K.; Allee, G.L.; Dilger, A.C. Effects of feeding ractopamine hydrochloride (Paylean) to physical and immunological castrates (Improvest) in a commercial setting on carcass cutting yields and loin quality. J. Anim. Sci. 2014, 92, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Daza, A.; Latorre, M.A.; Olivares, A.; López-Bote, C.J. The effects of male and female immunocastration on growth performances and carcass and meat quality of pigs intended for dry-cured ham production: A preliminary study. Livest. Sci. 2016, 190, 20–26. [Google Scholar] [CrossRef]
- den Hertog-Meischke, M.J.A.; van Laack, R.J.L.M.; Smulders, F.J.M. The water holding capacity of fresh meat. Vet. Q. 1997, 19, 175–181. [Google Scholar] [CrossRef]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Lepetit, J. Collagen contribution to meat toughness: Theoretical aspects. Meat Sci. 2008, 80, 960–967. [Google Scholar] [CrossRef]
- Font i Furnols, M.; González, J.; Gispert, M.; Oliver, M.A.; Hortós, M.; Pérez, J.; Suárez, P.; Guerrero, L. Sensory characterization of meat from pigs vaccinated against gonadotropin releasing factor compared to meat from surgically castrated, entire male and female pigs. Meat Sci. 2009, 83, 438–442. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.N.; Mullan, B.P. The effect of genotype and castration method on the eating quality characteristics of pork from entire male pigs. Anim. Sci. 2003, 77, 67–72. [Google Scholar] [CrossRef]
- Channon, H.A.; D’Souza, D.N.; Dunshea, F.R. Developing a cuts-based system to improve consumer acceptability of pork: Impact of gender, ageing period, endpoint temperature and cooking method. Meat Sci. 2016, 121, 216–227. [Google Scholar] [CrossRef]
- Petersen, J.S.; Berge, P.; Henckel, P.; Soerensen, M.T. Collagen characteristics and meat texture of pigs exposed to different levels of physical activity. J. Muscle Foods 1997, 8, 47–61. [Google Scholar] [CrossRef]
- Nold, R.A.; Romans, R.J.; Costello, W.J.; Libal, G.W. Characterization of muscles from boars, barrows and gilts slaughtered at 100 and 110 kilograms: Differences in fat moisture, colour, water-holding capacity and collagen. J. Anim. Sci. 1999, 77, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Aleksić, J.Z. The Investigation into the Efficacy od Immunocastration Aimed at the Prevention of Sex Odour in Boar’s Meat. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2012. [Google Scholar]
- Kaltnekar, T.; Škrlep, M.; Batorek Lukač, N.; Tomažin, U.; Prevolnik Povše, M.; Labussière, E.; Demšar, L.; Čandek-Potokar, M. Effects of salting duration and boar taint level on quality of dry-cured hams. Acta Agric. Slov. 2016, S5, 132–137. [Google Scholar]
- Škrlep, M.; Poklukar, K.; Lukač, N.B.; Kress, K.; Čandek-Potokar, M. Myofibrillar Fragmentation in Entire Male, Immunocastrated or Surgically Castrated Pig, Proceedings of the 60th International Meat Industry Conference, Kopaonik, Serbia, 22–25 September 2019; Furnols, M.F., Lakičević, B., Eds.; IOP Publishing: Bristol, UK, 2019. [Google Scholar] [CrossRef]
- Channon, H.A.; Kerr, M.G.; Walker, P.J. Effect of Duroc content, sex and ageing period on meat eating quality attributes of pork loin. Meat Sci. 2004, 66, 881–888. [Google Scholar] [CrossRef]
- Kristensen, L.; Therkildsen, M.; Riis, B.; Sørensen, T.; Oksbjerg, N.; Purslow, P.P.; Ertbjerg, P. Dietary-induced changes of muscle growth rate in pigs: Effects on in vivo and postmortem muscle proteolysis and meat quality. J. Anim. Sci. 2002, 80, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.; Therkildsen, M.; Aaslyng, M.D.; Oksbjerg, N.; Ertbjerg, P. Compensatory growth improves meat tenderness in gilts but not in barrows. J. Anim. Sci. 2004, 82, 3617–3624. [Google Scholar] [CrossRef] [PubMed]
- Font-i-Furnols, M.; Luo, X.; Brun, A.; Lizardo, R.; Esteve-Garcia, E.; Soler, J.; Gispert, M. Computed tomography evaluation of gilt growth performance and carcass quality under feeding restriction and compensatory growth effects on sensory quality of pork. Livest. Sci. 2020, 237, 104023. [Google Scholar] [CrossRef]
- Therkildsen, M.; Riis, B.; Karlsson, A.; Kristensen, L.; Ertbjerg, P.; Purslow, P.P.; Aaslyng, M.D.; Oksbjerg, N. Compensatory growth response in pigs, muscle protein turn-over and meat texture: Effects of restriction/realimentation period. Anim. Sci. 2002, 75, 367–377. [Google Scholar] [CrossRef]
- Stolzenbach, S.; Therkildsen, M.; Oksbjerg, N.; Lazarotti, R.; Ertbjerg, P.; Lametsch, R.; Bryne, D.V. Compensatory growth response as a strategy to enhance tenderness in entire male and female pork M. longissimus thoracis. Meat Sci. 2009, 81, 163–170. [Google Scholar] [CrossRef]
- Gondret, F.; Lebret, B. Feeding intensity and dietary protein level affect adipocyte cellularity and lipogenic capacity of muscle homogenates in growing pigs, without modification of the expression of sterol regulatory binding protein. J. Anim. Sci. 2002, 80, 3184–3193. [Google Scholar] [CrossRef]
- Nicolau-Solano, S.I.; Whittington, F.M.; Wood, J.D.; Doran, O. Relationship between carcass weight, adipose tissue androstenone level and expression of hepatic 3β-hydroxysteroid dehydrogenase in entire commercial pigs. Animal 2007, 1, 1053–1059. [Google Scholar] [CrossRef]
- Škrlep, M.; Čandek-Potokar, M.; Batorek Lukač, N.; Šegula, B.; Prevolnik, M.; Pugliese, C.; Bonneau, M. Length of the interval between immunocastration and slaughter in relation to boar taint and carcass traits. Acta Agric. Slov. 2012, 100 (Suppl. 3), 247–251. [Google Scholar]
- Adzitey, F. Effect of pre-slaughter animal handling on carcass and meat quality. Int. Food Res. J. 2011, 18, 484–490. [Google Scholar]
- Warner, R.D. The eating quality of meat—IV Water-holding capacity and juiciness. In Lawrie’s Meat Science; Toldrá, F., Ed.; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 419–459. [Google Scholar] [CrossRef]
- Škrlep, M.; Batorek Lukač, N.; Šegula, B.; Kubale, V.; Fazarinc, G.; Čandek-Potokar, M. Incidenca kožnih poškodb na klavnih trupih prašičev: Primerjava merjascev, imunokastratov in kirurških kastratov. Slov. Vet. Res. 2011, 48 (Suppl. 13), 203–206. [Google Scholar]
- Russo, V.; Costa, L.N. Suitability of pig meat for salting and the production of quality processed products. Pig News Inf. 1995, 16, 17–26. [Google Scholar]
- Čandek-Potokar, M.; Škrlep, M. Dry ham (“Kraški pršut”) processing losses as affected by raw material properties and manufacturing practice. J. Food Proc. Preserv. 2011, 35, 96–111. [Google Scholar] [CrossRef]
- Chevillon, P.; Le Strat, P.; Vendeuvre, J.L.; Gault, E.; Lhommeau, T.; Bonneau, M.; Mourot, J. Acceptabilité par le consommateur de jambon sec issu de porcs mâles entiers, de females ou de mâles castrés. J. Rech. Porc. 2011, 43, 61–62. [Google Scholar]
- Chevillon, P.; Le Strat, P.; Vendeuvre, J.L.; Gault, E.; Lhommeau, T.; Bonneau, M.; Mourot, J. Acceptabilité par le consommateur du jambon sec de mâles entiers. Viand. Prod. Carn. 2015, 31, 7. [Google Scholar]
- Čandek-Potokar, M.; Škrlep, M.; Kostyra, E.; Żakowska-Biemans, S.; Poklukar, K.; Batorek-Lukač, N.; Kress, K.; Weiler, U.; Stefanski, V. Quality of dry-cured ham from entire, surgically castrated and immunocastrated males: Case study on Kraški pršut. Animals 2020, 10, 239. [Google Scholar] [CrossRef]
- Toldrá, F.; Flores, M. The role of muscle proteases and lipases in flavour development during the processing of dry-cured ham. Crit. Rev. Food Sci. 1998, 38, 331–352. [Google Scholar] [CrossRef]
- Bañón, S.; Costa, E.; Gil, M.D.; Garrido, M.D. A comparative study of boar taint in cooked and dry-cured meat. Meat Sci. 2003, 63, 381–388. [Google Scholar] [CrossRef]
- Škrlep, M.; Čandek-Potokar, M.; Lukač, N.B.; Povše, M.P.; Pugliese, C.; Labussière, E.; Flores, M. Comparison of entire and immunocastrated pigs for dry-cured ham production under two salting regimes. Meat Sci. 2016, 111, 27–37. [Google Scholar] [CrossRef]
- Arnau, J.; Hugas, M.; Monfort, J.M. El Jamón Curado: Aspectos Técnicos; Institut de Recerca i Tecnologia Agroalimentaries: Monells, Spain, 1987; p. 173. [Google Scholar]
- Wilson, B.R.; Pearson, A.M.; Shorland, F.B. Effect of total lipids and phospholipids on warmed out flavour in red and white muscle from several species as measured by thiobarbituric acid analysis. J. Agric. Food Chem. 1976, 24, 7–11. [Google Scholar] [CrossRef]
- Pinna, A.; Schivazappa, C.; Virgili, R.; Parolari, G. Effect of vaccination against gonadotropin-releasing hormone (GnRH) in heavy male pigs for Italian typical dry-cured ham production. Meat Sci. 2015, 110, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Font-i-Furnols, M.; Francás, C.; Claret, A.; Guerrero, L.; Romero, A.; Gispert, M. Sensory characterization of muscle biceps femoris of dry-cured ham from pigs of different sexes. In Proceedings of the Production and Utilization of Meat from Entire Male Pigs: EAAP Working Group, IRTA, Monells, Girona, Spain, 2–3 December 2013. [Google Scholar]
- Corral, S.; Salvador, A.; Flores, M. Effect of the use of entire male fat in the production of reduced salt fermented sausages. Meat Sci. 2016, 116, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Corral, S.; Belloch, C.; López-Díez, J.J.; Salvador, A.; Flores, M. Yeast inoculation as a strategy to improve the physico-chamical and sensory properties of reduced salt fermented sausages produced with entire male fat. Meat Sci. 2017, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Corral, S.; Belloch, C.; López-Díez, J.J.; Flores, M. Lipolysis and aroma generation as mechanisms involved in masking boar taint in sodium reduced fermented sausages inoculated with Debaryomyces hansenii yeast. J. Sci. Food Agric. 2018, 98, 2121–2130. [Google Scholar] [CrossRef]
- Garrido, M.D.; Egea, M.; Linares, M.B.; Borrisser-Pairó, F.; Rubio, B.; Viera, C.; Martínez, B. Sensory characteristics of meat and meat products from entire male pigs. Meat Sci. 2017, 129, 50–53. [Google Scholar] [CrossRef]
- Martínez, B.; Vieira, C.; Rubio, B.; Garrido, M.D.; Egea, M.; Linares, M.B.; Panella-Riera, N. Effect of androstenone level in characteristics of dry fermented sausage manufactured with meat from entire male pigs. Arch. Zootec. 2018, 67 (Suppl. 1), 213–216. [Google Scholar]
- Müller, T.; Stiebing, A.; Dederer, I. Rohschinken und Rohwürste aus Eberfleisch. Fleischwirtschaft 2012, 1, 93–98. [Google Scholar]
- Xiong, Y.L. Protein oxidation and implication for muscle food quality. In Antioxidants in Muscle Foods; Decker, E.A., Faustman, C.L., Lopez-Bote, C.J., Eds.; John Wiley and Sons: New York, NY, USA, 2000; pp. 85–111. [Google Scholar]
- Sebranek, J.G.; Bacus, J.N. Cured meat products without direct addition of nitrate and nitrite: What are the issues? Meat Sci. 2007, 77, 136–147. [Google Scholar] [CrossRef]
- Gallas, L.; Borilova, G.; Svobodova, I.; Steinhauserova, I.; Steinhouser, L. Usability of meat from immunologically castrated male pigs for the production of dry-fermented sausages. In Proceedings of the 57th International Congress of Meat Acience and Technology, Ghent, Belgium, 7–12 August 2011. [Google Scholar]
- Stiebing, A. Ohne jede Einschränkung geeignet. Fleischwirtschaft 2019, 99, 9. [Google Scholar]
- Lesser, D.; Baron, P.J.; Robb, J. Boar bacon: A consumer survey. J. Sci. Food Agric. 1977, 28, 1120–1131. [Google Scholar] [CrossRef]
- Ellis, M.; Smith, W.C.; Clark, J.B.K.; Innes, N. A comparison of boars, gilts and castrates for bacon manufacture. Anim. Prod. 1983, 37, 1–9. [Google Scholar] [CrossRef]
- Smith, W.C.; Ellis, M.; Clark, J.B.K.; Innes, N. A comparison of boars, gilts and castrates for bacon manufacture 2. Curing characteristics, bacon yield and quality. Anim. Prod. 1983, 37, 11–15. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wood, J.D.; Patterson, R.L.S. Comparison of boars and castrates for bacon production 3. Composition and eating quality of bacon. Anim. Prod. 1982, 35, 75–80. [Google Scholar] [CrossRef]
- Čandek-Potokar, M.; Prevolnik-Povše, M.; Škrlep, M.; Font-i-Furnols, M.; Batorek-Lukač, N.; Kress, K.; Stefanski, V. Acceptability of dry-cured belly (Pancetta) from entire males, immunocastrates or surgical castrates: Study with Slovenian consumers. Foods 2019, 8, 122. [Google Scholar] [CrossRef]
- Cowan, C.A.; Joseph, R.L. Production and quality of boar and castrate bacon 2. Consumer panel response to bacon and fat samples. Ir. J. Food Sci. Technol. 1981, 5, 105–116. [Google Scholar]
- Lowe, B.K.; Overholt, M.F.; Gerlemann, G.D.; Carr, S.N.; Rincker, P.J.; Schroeder, A.L.; Petry, D.B.; McKeith, F.K.; Allee, G.B.; Dilger, A.C. Ham and belly processing characteristics of immunological castrated barrows (Improvest) fed ractopamine hydrochloride (Paylean). Meat Sci. 2016, 112, 103–109. [Google Scholar] [CrossRef]
- Boler, D.D.; Killefer, J.; Meeuwse, D.M.; King, V.L.; McKeith, F.K.; Dilger, A.C. Effect of slaughter time post-second injection on carcass cutting yields and bacon characteristics of immunologically castrated male pigs. J. Anim. Sci. 2012, 90, 334–344. [Google Scholar] [CrossRef]
- Tavárez, M.A.; Bohrer, B.M.; Asmus, M.D.; Schroeder, A.L.; Boler, F.K.; McKeith, F.K.; Dilger, A.C. Effects of immunological castration and distiller’s grains with soluble on carcass cutability and commercial bacon slicing yields of barrows slaughtered at different time points. J. Anim. Sci. 2014, 92, 3149–3160. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Choi, J.-H.; Choi, Y.-S.; Han, D.-J.; Kim, H.-Y.; Lee, M.-A.; Lee, D.-H.; Kim, C.-J. The effects of immunocastration on meat quality and sensory properties of pork bellies. Korean J. Food Sci. Anim. Resour. 2011, 31, 372–380. [Google Scholar] [CrossRef]
- Person, R.C.; McKenna, D.R.; Griffin, D.B.; McKeith, F.K.; Scanga, J.A.; Belk, K.E.; Smith, G.C.; Savell, J.W. Benchmarking value in the pork supply chain: Processing characteristics and consumer evaluation of pork bellies of thickness when manufactured into bacon. Meat Sci. 2005, 70, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, S.D.; Miller, M.F.; Haydon, K.D.; Lovegren, N.V.; Lyon, C.E.; Reagan, J.O. Acceptability of bacon as influences by the feeding of elevated levels of monounsaturated fats to growing-finishing swine. J. Food Sci. 1990, 55, 621–624. [Google Scholar] [CrossRef]
- Font-i-Furnols, M.; Brun, A.; Marcos, B.; Albano, M.; Tejeda, J.F.; Gispert, M. Morphological, composition and mechanical characteristics of bellies from immunocastrated and entire male pigs. In Proceedings of the 66th International Congress of Meat Science and Technology, Orlando, FL, USA, 2–7 August 2020. [Google Scholar]
- Herrick, R.T.; Tavárez, M.A.; Hersh, B.N.; Mellencamp, M.A.; Boler, D.D.; Dilger, A.C. Effect of immunological castration management strategy on lipid oxidation and sensory characteristics of bacon stored under simulated food service conditions. J. Anim. Sci. 2016, 94, 3084–3092. [Google Scholar] [CrossRef] [PubMed]
- Little, K.J.; Kyle, J.M.; Bohrer, B.M.; Schroeder, A.L.; Fedler, C.A.; Prusa, K.J.; Boler, D.D. A comparison of slice characteristics and sensory characteristics of bacon from immunologically castrated barrows with bacon from physically castrated barrows, boars, and gilts. J. Anim. Sci. 2014, 92, 5769–5777. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Upmann, M.; Hölscher, M.; Nolte, T.; Zapp, J.; Lautenschläger, R.; Stiebing, A. Ebermast: Entwicklung eines Konzepts für die Produktion, Schlachtung und Vermarktung ökologisch ezeugter Eber entlang der gesamten Wertschöpfungskette. Teilbericht der Hochschule Ostwestfalen-Lippe. 2016. Available online: https://www.orgprints.org/32010/ (accessed on 8 July 2020).
- Zapeda, C.M.G.; Kastner, C.L.; Kropf, D.H.; Hunt, M.C.; Kenny, P.B.; Schwenke, J.R.; Schleusener, D.S. Utilization of surimi-like products from pork with sex odour in restructured, precooked pork roast. J. Food Sci. 1993, 58, 53–58. [Google Scholar] [CrossRef]
- Hallensvedt, E.; Kjos, N.P.; Rehnberg, A.C.; Øverland, M.; Thomassen, M. Fish oil in feeds for entire male and female pigs: Changes in muscle fatty acid composition and stability of sensory quality. Meat Sci. 2010, 85, 182–190. [Google Scholar] [CrossRef]
- Meier-Dinkel, L.; Gertheiss, J.; Schnäckel, W.; Mörlein, D. Consumers’ perception and acceptance of boiled and fermented sausages from strongly boar tainted meat. Meat Sci. 2016, 118, 34–42. [Google Scholar] [CrossRef]
- Jones-Hamlow, K.A.; Tavarez, M.A.; Schroeder, A.L.; Dilger, A.C. Lipid oxidation, sensory characteristics, and colour of fresh pork sausages from immunologically castrated pigs stored frozen for up to 12 weeks. Food Sci. Nutr. 2016, 4, 355–363. [Google Scholar] [CrossRef]
- Brewer, M.S.; Stites, C.R.; McKeith, F.K.; Bechtel, P.J.; Novakofski, J.E.; Bruggen, K.A. Belly thickness effects on the proximate composition, processing, and sensory characteristics of bacon. J. Muscle Foods 1995, 6, 238–296. [Google Scholar] [CrossRef]
- Knipe, C.L.; Beld, J. Bacon production. In Encyclopedia of Meat Science, 2nd ed.; Jensen, W., Devine, C., Dikeman, M., Eds.; Elsevier Science: London, UK, 2014; pp. 53–57. [Google Scholar] [CrossRef]
- Ockerman, H.W.; Basu, L.; Crespo, L.; Cespedes Sanchez, F.J. Comparison of European and American System of Production and Consumption of Dry-Cured Hams. Fact Sheet, Pork Info. Gateway. 2002. Available online: http://porkgateway.org/wp-content/uploads/2015/07/comparison-of-european-american-systems-of-production-and-consumption-of-dry-cured-hams1.pdf (accessed on 8 July 2020).
- Wauters, J.; Vercruysse, V.; Aluwé, M.; Verplanken, K.; Vanhaecke, L. Boar taint compound levels in back fat versus meat products: Do they correlate? Food Chem. 2016, 206, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tørngren, M.A.; Claudi-Magnussen, C.; Støier, S.; Kristensen, L. Boar taint reduction in smoked, cooked ham. In Proceedings of the 57th International Congress of Meat Science and Technology, Ghent, Belgium, 7–12 August 2011. [Google Scholar]
- Wauters, J.; Verplanken, K.; Vercruysse, V.; Ampe, B.; Aluwé, M.; Vanhaecke, L. Sensory evaluation of boar meat products by trained experts. Food Chem. 2017, 237, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Diestre, A.; Oliver, M.A.; Gispert, M.; Arpa, I.; Arnau, J. Consumer responses to fresh meat and meat products from barrows and boars with different levels of boar taint. Anim. Sci. 1990, 50, 519–530. [Google Scholar] [CrossRef]
- Lundström, K.; Zamaratskaia, G.; Matthews, K.R.; Haugen, J.E.; Squires, E.J. Pigmeat quality implications of surgical castration and its alternatives. PIGCAS Deliv. 2008, D3, 45–87. [Google Scholar]
- Stolzenbach, S.; Lindahl, G.; Lundström, K.; Chen, G.; Byrne, D.V. Perceptual masking of boar taint in Swedish fermented sausages. Meat Sci. 2009, 81, 580–588. [Google Scholar] [CrossRef]
- Marro, P.; Bauer, A.; Stefanski, V.; Weiler, U. Effect of processing on the concentrations of boar taint compounds skatole and androstenone in different types of sausage. J. Food Proc. Preserv. 2018, 42, e13580. [Google Scholar] [CrossRef]
- Verplanken, K.; Wauters, J.; Vercruysse, V.; Aluwé, M.; Vanhaecke, L. Sensory evaluation of boar-taint-containing minced meat, dry-cured ham and dry fermented sausage by a trained expert panel and consumers. Food Chem. 2017, 233, 247–255. [Google Scholar] [CrossRef]
- Font-i-Furnols, M. Consumer studies on sensory acceptability of boar taint: A review. Meat Sci. 2012, 92, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Čandek-Potokar, M.; Škrlep, M.; Lukač Batorek, N.; Labussière, E. Reduction of boar taint compounds in Kraški pršut through dry-curing process. Proc. Food Sci. 2015, 5, 34–37. [Google Scholar] [CrossRef]
- Parolari, G.; Virgili, R.; Schivazappa, C. Relationship between cathepsin B activity and compositional parameters in dry-cured hams of normal and defective texture. Meat Sci. 1994, 38, 117–122. [Google Scholar] [CrossRef]
- Virgili, R.; Parolari, G.; Schivazappa, C. Sensory and texture quality of dry-cured ham as affected by endogenous cathepsin B activity and muscle composition. J. Food Sci. 1995, 60, 1183–1186. [Google Scholar] [CrossRef]
- Martín, L.; Cordoba, J.J.; Timón, T.; Ventanas, J. Effects of salt and temperature on proteolysis during ripening of Iberian ham. Meat Sci. 1998, 49, 145–153. [Google Scholar] [CrossRef]
- Breslin, P.A.S.; Beauchamp, G.K. Salt enhances flavour by suppressing bitterness. Nature 1997, 387, 563. [Google Scholar] [CrossRef] [PubMed]
- Desmoulin, B.; Bonneau, M.; Frouin, A.; Bidard, J.P. Consumer testing of pork and processed meat from boars. Livest. Prod. Sci. 1982, 9, 707–715. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Støier, S.; Koch, A.G. Fermentation is not a strategy to mask boar taint. Adv. Anim. Biosci. 2018, 9, 46. [Google Scholar]
- Toldrá, F.; Flores, M. Sausages, types of: Dry and semidry. In Encyclopedia of Meat Science, 2nd ed.; Jensen, W., Devine, C., Dikeman, M., Eds.; Elsevier Science: London, UK, 2014; pp. 248–255. [Google Scholar] [CrossRef]
- Haugen, J.-E.; Brunius, C.; Zamaratskaia, G. Review of analytical methods to measure boar taint compounds in porcine adipose tissue: The need for harmonised methods. Meat. Sci. 2012, 90, 9–19. [Google Scholar] [CrossRef]
- Peñaranda, I.; Garrido, M.D.; Moumeh, B.; Linares, M.B. Use of masking strategies to avoid the boar taint perception in chorizo: Consumers’ acceptability. Meat Sci. 2020, 169, 108223. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Koch, A.G. The use of smoke as a strategy for masking boar taint in sausages and bacon. Food Res. Int. 2018, 108, 378–395. [Google Scholar] [CrossRef]
- Denhard, M.; Claus, R.; Herbert, E.; Hillenbrand, M. Skatol und Androstenonkonzentrationen in Fleischerzeugnissen aus Eberschlachtkörpern. In Die Ebermast; Heft 449, Rheie A: Schriftenreihe des Bundesministeriums für Ernährung, Landwirtschaft und Forsten; Landwirtschaftsverlag GmbH: Münster, Germany, 1995; pp. 55–71. [Google Scholar]
- Engesser, D.; Braun, P.G. Alternatives for boar taint reduction by processing boar meat. Fleischwirtschaft 2017, 97, 60–70. [Google Scholar]
- Mottram, D.S. The effect of cooking conditions on the formation of volatile heterocyclic compounds in pork. J. Sci. Food. Agric. 1985, 36, 377–382. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Peñaranda, I.; Garrido, M.D.; Egea, M.; Díaz, P.; Álvarez, D.; Oliver, M.A.; Linares, M.B. Sensory perception of meat from entire male pigs processed by different heating methods. Meat Sci. 2017, 134, 98–102. [Google Scholar] [CrossRef]
- Amoore, J.E.; Buttery, R.G. Partition coefficients and comparative olfactometry. Chem. Sens. 1978, 3, 57–71. [Google Scholar] [CrossRef]
- Windholz, M.; Budavari, S.; Blumetti, R.F.; Otterbein, E.S. The Merck Index: An Encyclopedia of Chemicals, Drugs and Biologicals, 10th ed.; Merck & Co.: Rahway, NJ, USA, 1983. [Google Scholar]
- Aaslyng, M.D.; Støier, S.; Koch, A.G. Cooking meat for long time at low temperatures does not decrease boar taint. Adv. Anim. Biosci. 2018, 9, 47. [Google Scholar]
- Borrisser-Pairó, F.; Panella-Riera, N.; Gil, M.; Kallas, Z.; Linares, M.B.; Egea, M.; Garrido, M.D.; Oliver, M.A. Consumers’ sensitivity to androstenone and the evaluation of different cooking methods to mask boar taint. Meat Sci. 2017, 123, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Egea, M.; Díaz, P.; Álvarez, D.; Garrido, M.D.; Linares, M.B. Effect of cooking methods (vacuum vs frying) on boar taint perception. Arch. Latinoam. Prod. Anim. 2014, 22, 391–393. [Google Scholar]
- Anastasijević, V.; Tadić, I.; Stanković, M.; Đorđević, M.; Josipović, S. Influence of curing on the organoleptic quality of neck and bacon from entire and castrated male pigs; b) Meat from entire male pigs (abstract). In Proceedings of the 36th Annual Meeting of the EAAP, Kallithea, Greece, 30 September–3 October 1985. [Google Scholar]
- Ampuero Kragten, S.; Raemy, M.; Bee, G. About boar taint compounds determination in adipose tissue or in liquid fats. In Proceedings of the Production and Utilization of Meat from Entire Male Pigs: EAAP Working Group, IRTA, Monells, Girona, Spain, 2–3 December 2013. [Google Scholar]
- Egelandsdal, B.; Løvlund, E.; Choinsky, J.; Koller, A.; Mielnik, M. Shifting sensorial thresholds of pre-cooked entire male using the marinating technology. In Proceedings of the 50th International Congress of Meat Science and Technology, Helsinki, Finland, 8–13 August 2004. [Google Scholar]
- Lunde, K.; Egelendsdal, B.; Choinski, J.; Mielnik, M.; Flåtten, A.; Kubberød, E. Marinating as a technology to shift sensory theresholds in ready-to eat entire male pork meat. Meat Sci. 2008, 80, 1264–1272. [Google Scholar] [CrossRef]
- Egea, M.; Linares, M.B.; Gil, M.; López, M.B.; Garrido, M.D. Reduction of androstenone perception in pan-fried boar meat by different masking strategies. J. Sci. Food Agric. 2018, 98, 2251–2257. [Google Scholar] [CrossRef]
- Danish Technological Institute. Applications for Meat from Sorted Entire Male Pigs. Available online: https://www.dti.dk/specialists/applications-for-meat-from-sorted-entire-male-pigs/39302 (accessed on 8 July 2020).
- Hemeryck, L.Y.; Wauters, J.; Dewulf, L.; Decloedt, A.; Aluwé, M.; de Smet, S.; Fraeye, I.; Vanhaecke, L. Valorisation of tainted boar meat patties, frankfurter sausages and cooked ham by means of targeted dilution, cooking and smoking. Food Chem. 2020, 330, 126897. [Google Scholar] [CrossRef]
- Tørngren, M.A.; Kristensen, L.; Claudi-Magnussen, C. How to use “tainted” boar meat for processing whole meat cuts. In Proceedings of the 58th International Congress of Meat Science and Technology, Montreal, QC, Canada, 12–17 August 2012. [Google Scholar]
- Coker, M.D.; West, R.L.; Brendemuhl, J.H.; Johnson, D.D.; Stelzleni, A.M. Effects of live weight and processing on the sensory traits, androstenedione concentration and 5-alpha-androst-16-en-3-one (androstenone) concentration in boar meat. Meat Sci. 2009, 82, 399–404. [Google Scholar] [CrossRef]
- EFSA. Welfare Aspects of the Castration of Piglets. Scientific Report of the Scientific Panel for Animal Health and Welfare on Request from the Comission Related to Welfare Aspects of the Castration of Piglets. European Food Safety Authority AHAW/04-087. EFSA J. 2004, 91, 1–18. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/aw_prac_farm_pigs_cast-alt_sci_efsa_opinion_welfare-aspects.pdf (accessed on 22 July 2020).
- Martínez, B.; Rubio, B.; Viera, C.; Linares, M.B.; Panella-Riera, N.; Garrido, M.D. Evaluation of different strategies to mask boar taint in cooked sausage. Meat Sci. 2016, 116, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, M.; Desmoulin, B.; Frouin, A.; Bidard, J.-P. Conséquences des processus technologiques de transformation des viands de porc mâle sur la teneur en androsténone des graisses. Ann. Technol. Agric. 1980, 29, 69–73. [Google Scholar]
- Mörlein, J.; Meier-Dinkel, L.; Gertheiss, J.; Schnäckel, W.; Mörlein, D. Sustainable use of tainted boar meat: Blending is a strategy for processed products. Meat Sci. 2019, 152, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Walstra, P. Fattening of young boars: Quantification of negative and positive aspects. Livest. Prod. Sci. 1974, 1, 187–196. [Google Scholar] [CrossRef]





| Drip Loss | Cooking Loss | Shear Force | ||||
|---|---|---|---|---|---|---|
| Meta-analytical studies 1 | ||||||
| EM–SC | NS | [14,41] | EM > SC | [14] | ||
| EM–IC | NS | [14,29,41] | EM > IC | [14,29] | ||
| IC–SC | NS | [14,29,41] | IC > SC | [14] | ||
| NS | [29] | |||||
| Individual studies 2 | ||||||
| EM–SC | EM > SC | [52,56] | EM > SC | [22,52,56,75] | EM > SC | [22,52,75] |
| NS | [48,56,57,67] | NS | [48,57] | NS | [48,56] | |
| EM–IC | EM > IC | [57] | EM > IC | [62] | EM > IC | [22,58] |
| NS | [22,42,55,56,59,61,62] | IC > EM | [56,58] | NS | [42,56,59,61,62] | |
| NS | [22,42,57,59,61] | |||||
| IC–SC | IC > SC | [49,56,66,68] | IC > SC | [22,55] | IC > SC | [49,65,68] |
| SC > IC | [57,64] | NS | [57,64,66] | NS | [22,56,63,64,67] | |
| NS | [22,55,63,67] | |||||
| Content | EM | IC |
|---|---|---|
| Fat tissue quality and composition |
| |
| Meat colour, pH and WHC |
|
|
| Meat tendernes |
|
|
| Content | EM | IC |
|---|---|---|
| Dry-cured ham |
|
|
| Dry fermented sausages |
|
|
| Cured and/or thermally processed bellies and bacon |
| |
| Other meat products |
|
|
| Products. | Boar Taint Level | Masking Capacity without Strategy 1 | Strategies |
|---|---|---|---|
| Dry-cured ham | Low-Medium | + |
|
| High | + |
| |
| Dry-fermented sausages | Low-Medium | +++ | |
| High | + |
| |
| Fresh meat | Low-Medium | + | |
| High | + |
| |
| Cooked meat products | Low-Medium | ++ |
|
| High | + |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škrlep, M.; Tomašević, I.; Mörlein, D.; Novaković, S.; Egea, M.; Garrido, M.D.; Linares, M.B.; Peñaranda, I.; Aluwé, M.; Font-i-Furnols, M. The Use of Pork from Entire Male and Immunocastrated Pigs for Meat Products—An Overview with Recommendations. Animals 2020, 10, 1754. https://doi.org/10.3390/ani10101754
Škrlep M, Tomašević I, Mörlein D, Novaković S, Egea M, Garrido MD, Linares MB, Peñaranda I, Aluwé M, Font-i-Furnols M. The Use of Pork from Entire Male and Immunocastrated Pigs for Meat Products—An Overview with Recommendations. Animals. 2020; 10(10):1754. https://doi.org/10.3390/ani10101754
Chicago/Turabian StyleŠkrlep, Martin, Igor Tomašević, Daniel Mörlein, Saša Novaković, Macarena Egea, María Dolores Garrido, María Belén Linares, Irene Peñaranda, Marijke Aluwé, and Maria Font-i-Furnols. 2020. "The Use of Pork from Entire Male and Immunocastrated Pigs for Meat Products—An Overview with Recommendations" Animals 10, no. 10: 1754. https://doi.org/10.3390/ani10101754
APA StyleŠkrlep, M., Tomašević, I., Mörlein, D., Novaković, S., Egea, M., Garrido, M. D., Linares, M. B., Peñaranda, I., Aluwé, M., & Font-i-Furnols, M. (2020). The Use of Pork from Entire Male and Immunocastrated Pigs for Meat Products—An Overview with Recommendations. Animals, 10(10), 1754. https://doi.org/10.3390/ani10101754