Novel Low-Voltage Electro-Ejaculation Approach for Sperm Collection from Zoo Captive Lanyu Miniature Pigs (Sus barbatus sumatranus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, Antibodies
2.2. Animals
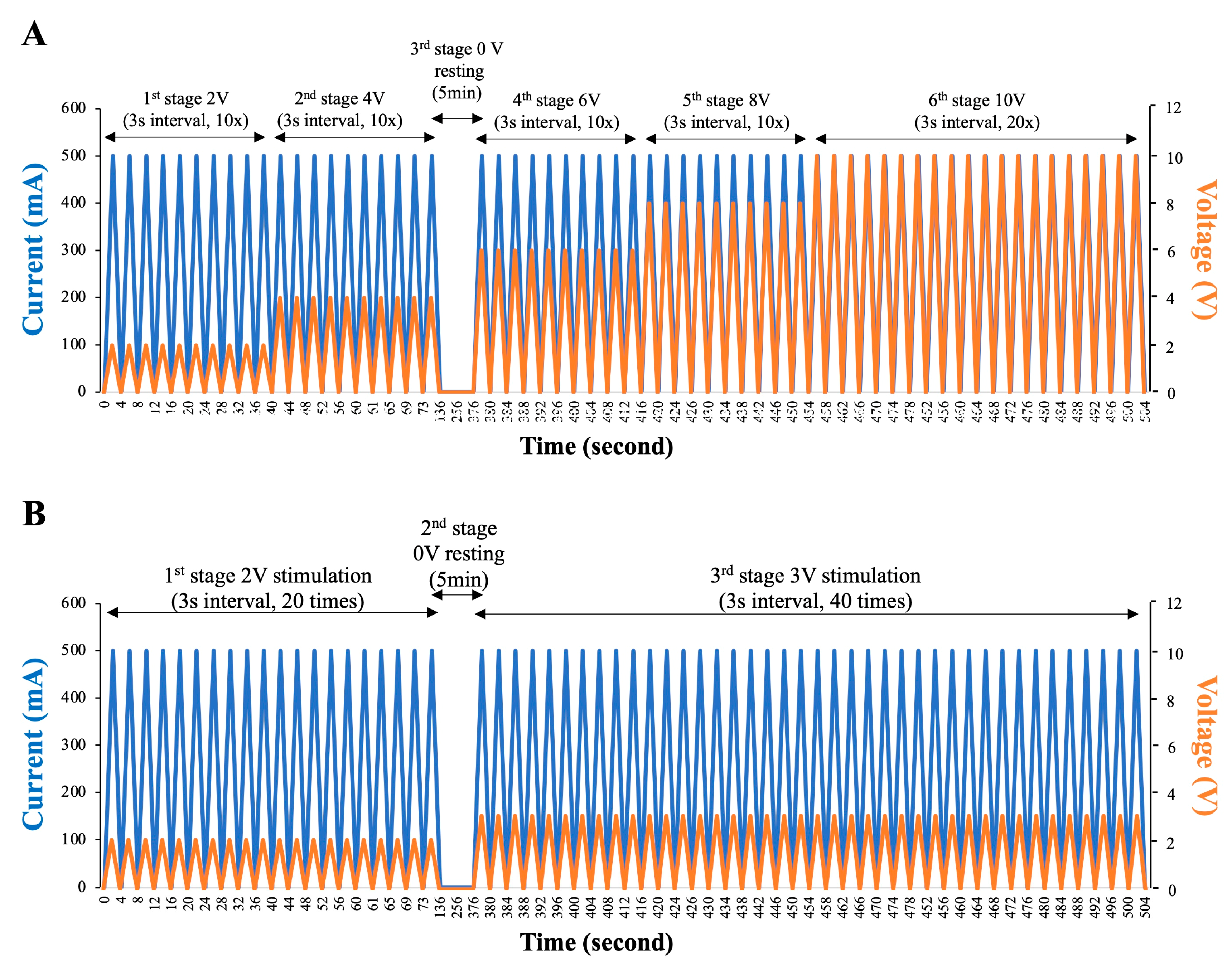
2.3. Low-Voltage Electro-Ejaculation Protocol for Semen Collecting
2.4. Semen Preparation and Sperm Analyses
2.5. Indirect Immunofluorescent (IFA) Assays for Acrosome Integrity
2.6. Sperm Membrane Potential Evaluation by Flow Cytometry
2.7. Statistical Analyses
3. Results
3.1. Physiological Parameters of Lanyu Miniature Pig during Traditional High-Voltage and Novel Low-Voltage EEJ
3.2. Sperm Viability, Morphology, and Motility Were Maintained with Low Voltage EEJ
3.3. Sperm Acrosome Integrity Was Maintained, but Mitochondria Membrane Potential Was Compromised
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asturiano, J.F.; Cabrita, E.; Horvath, A. Progress, challenges and perspectives on fish gamete cryopreservation: A mini-review. Gen. Comp. Endocrinol. 2017, 245, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gook, D.A.; Edgar, D.H. Cryopreservation of female reproductive potential. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 55, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Olaciregui, M.; Luno, V.; Malo, C.; Gonzalez, N.; Martinez, F. Current status of freeze-drying technology to preserve domestic animals sperm. Reprod. Domest. Anim. 2014, 49 (Suppl. 4), 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, K. Assisted reproductive techniques in mares. Reprod. Domest. Anim. 2018, 53 (Suppl. 2), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Luvoni, G.C. Gamete cryopreservation in the domestic cat. Theriogenology 2006, 66, 101–111. [Google Scholar] [CrossRef]
- Luvoni, G.C.; Chigioni, S.; Beccaglia, M. Embryo production in dogs: From in vitro fertilization to cloning. Reprod. Domest. Anim. 2006, 41, 286–290. [Google Scholar] [CrossRef]
- Meyers, S.; Bulkeley, E.; Foutouhi, A. Sperm mitochondrial regulation in motility and fertility in horses. Reprod. Domest. Anim. 2019, 54 (Suppl. 3), 22–28. [Google Scholar] [CrossRef]
- Parrish, J.J. Bovine in vitro fertilization: In vitro oocyte maturation and sperm capacitation with heparin. Theriogenology 2014, 81, 67–73. [Google Scholar] [CrossRef]
- Rienzi, L.F.; Iussig, B.; Dovere, L.; Fabozzi, G.; Cimadomo, D.; Ubaldi, F.M. Perspectives in Gamete and Embryo Cryopreservation. Semin. Reprod. Med. 2018, 36, 253–264. [Google Scholar] [CrossRef]
- Yu, J.F.; Lai, Y.H.; Wang, T.E.; Wei, Y.S.; Chang, Y.J.; Li, S.H.; Chin, S.C.; Joshi, R.; Chang, H.W.; Tsai, P.S. The effects of type I collagenase on the degelification of chimpanzee (Pan troglodytes) semen plug and sperm quality. BMC Vet. Res. 2018, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Vandevoort, C.A.; Neville, L.E.; Tollner, T.L.; Field, L.P. Noninvasive semen collection from an adult orangutan. Zoo Biol. 1993, 12, 257–265. [Google Scholar] [CrossRef]
- Tollner, T.L.; VandeVoort, C.A.; Overstreet, J.W.; Drobnis, E.Z. Cryopreservation of spermatozoa from cynomolgus monkeys (Macaca fascicularis). J. Reprod. Fertil. 1990, 90, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Chu, H.P.; Jiang, Y.N.; Li, S.H.; Wang, Y.; Chen, C.H.; Chen, K.J.; Lin, C.Y.; Ju, Y.T. Genetic variation and phylogenetics of Lanyu and exotic pig breeds in Taiwan analyzed by nineteen microsatellite markers. J. Anim. Sci. 2009, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.N.; Wu, C.Y.; Huang, C.Y.; Chu, H.P.; Ke, M.W.; Kung, M.S.; Li, K.Y.; Wang, C.H.; Li, S.H.; Wang, Y.; et al. Interpopulation and intrapopulation maternal lineage genetics of the Lanyu pig (Sus scrofa) by analysis of mitochondrial cytochrome b and control region sequences. J. Anim. Sci. 2008, 86, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Bollen, P.; Ellegaard, L. Developments in Breeding Göttingen Minipigs; Plenum Press: New York, NY, USA, 1996. [Google Scholar]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Cops, J.; Haesen, S.; De Moor, B.; Mullens, W.; Hansen, D. Current animal models for the study of congestion in heart failure: An overview. Heart Fail. Rev. 2019, 24, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Dolezalova, D.; Hruska-Plochan, M.; Bjarkam, C.R.; Sorensen, J.C.; Cunningham, M.; Weingarten, D.; Ciacci, J.D.; Juhas, S.; Juhasova, J.; Motlik, J.; et al. Pig models of neurodegenerative disorders: Utilization in cell replacement-based preclinical safety and efficacy studies. J. Comp. Neurol. 2014, 522, 2784–2801. [Google Scholar] [CrossRef]
- Whitlock, B.K.; Coffman, E.A.; Coetzee, J.F.; Daniel, J.A. Electroejaculation increased vocalization and plasma concentrations of cortisol and progesterone, but not substance P, in beef bulls. Theriogenology 2012, 78, 737–746. [Google Scholar] [CrossRef]
- Kruger, T.F.; Ackerman, S.B.; Simmons, K.F.; Swanson, R.J.; Brugo, S.S.; Acosta, A.A. A quick, reliable staining technique for human sperm morphology. Arch. Androl. 1987, 18, 275–277. [Google Scholar] [CrossRef]
- Broekhuijse, M.L.; Sostaric, E.; Feitsma, H.; Gadella, B.M. Application of computer-assisted semen analysis to explain variations in pig fertility. J. Anim. Sci. 2012, 90, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Nowicka-Bauer, K.; Lepczynski, A.; Ozgo, M.; Kamieniczna, M.; Fraczek, M.; Stanski, L.; Olszewska, M.; Malcher, A.; Skrzypczak, W.; Kurpisz, M.K. Sperm mitochondrial dysfunction and oxidative stress as possible reasons for isolated asthenozoospermia. J. Physiol. Pharmacol. 2018, 69. [Google Scholar] [CrossRef]
- Pabon, D.; Meseguer, M.; Sevillano, G.; Cobo, A.; Romero, J.L.; Remohi, J.; de Los Santos, M.J. A new system of sperm cryopreservation: Evaluation of survival, motility, DNA oxidation, and mitochondrial activity. Andrology 2019, 7, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Abril-Sanchez, S.; Freitas-de-Melo, A.; Giriboni, J.; Santiago-Moreno, J.; Ungerfeld, R. Sperm collection by electroejaculation in small ruminants: A review on welfare problems and alternative techniques. Anim. Reprod. Sci. 2019, 205, 1–9. [Google Scholar] [CrossRef]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef]
- Brackett, N.L.; Lynne, C.M. The method of assisted ejaculation affects the outcome of semen quality studies in men with spinal cord injury: A review. NeuroRehabilitation 2000, 15, 89–100. [Google Scholar] [CrossRef]
- Ohl, D.A.; Denil, J.; Cummins, C.; Menge, A.C.; Seager, S.W. Electroejaculation does not impair sperm motility in the beagle dog: A comparative study of electroejaculation and collection by artificial vagina. J. Urol. 1994, 152, 1034–1037. [Google Scholar] [CrossRef]
- Amaral, A.; Lourenco, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef]
- Abou-Haila, A.; Tulsiani, D.R. Mammalian sperm acrosome: Formation, contents, and function. Arch. Biochem. Biophys. 2000, 379, 173–182. [Google Scholar] [CrossRef]
- Duan, Y.G.; Wehry, U.P.; Buhren, B.A.; Schrumpf, H.; Olah, P.; Bunemann, E.; Yu, C.F.; Chen, S.J.; Muller, A.; Hirchenhain, J.; et al. CCL20-CCR6 axis directs sperm-oocyte interaction and its dysregulation correlates/associates with male infertility. Biol. Reprod. 2020. [Google Scholar] [CrossRef]
- Zuccarello, D.; Ferlin, A.; Garolla, A.; Menegazzo, M.; Perilli, L.; Ambrosini, G.; Foresta, C. How the human spermatozoa sense the oocyte: A new role of SDF1-CXCR4 signalling. Int. J. Androl. 2011, 34, e554–e565. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.R.; Meyers, S. The sperm mitochondrion: Organelle of many functions. Anim. Reprod. Sci. 2018, 194, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Tesi, M.; Sabatini, C.; Vannozzi, I.; Di Petta, G.; Panzani, D.; Camillo, F.; Rota, A. Variables affecting semen quality and its relation to fertility in the dog: A retrospective study. Theriogenology 2018, 118, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Tremoen, N.H.; Gaustad, A.H.; Andersen-Ranberg, I.; van Son, M.; Zeremichael, T.T.; Frydenlund, K.; Grindflek, E.; Vage, D.I.; Myromslien, F.D. Relationship between sperm motility characteristics and ATP concentrations, and association with fertility in two different pig breeds. Anim. Reprod. Sci. 2018, 193, 226–234. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Singh, D.; Agarwal, A.; Henkel, R. Causes and consequences of sperm mitochondrial dysfunction. Andrologia 2020, e13666. [Google Scholar] [CrossRef]



| (A) | ||||
| Zoo Captive Lanyu Miniature Pig (n = 15) | ||||
| Age of the animals (year) | 2.67 ± 0.4 (2–3) | |||
| Weight of the animals (kg) | 52.9.1 ± 12.1 (30–75) | |||
| Length of right testis (cm) | 10.5 ± 1.5 (9.1–12.5) | |||
| Length of left testis (cm) | 10.9 ± 1.1 (9.7–13.4) | |||
| Length of anus to prostate gland (cm) | 15.4 ± 1.3 (14.0–18.5) | |||
| Depth of electro-probe insertion (cm) | 19.4 ± 1.6 (14.8–22.0) | |||
| (B) | ||||
| Traditional EEJ(7 Individuals, 12 Ejaculates) | Low-Voltage EEJ(8 Individuals, 13 Ejaculates) | |||
| Core body temperature (°C) | Pre-EEJ | Post-EEJ | Pre-EEJ | Post-EEJ |
| Respiratory rate ( /min) | 38.8 ± 0.6 | 38.4 ± 0.7 | 38.6 ± 0.9 | 38.0 ± 1.1 |
| Heart rate ( /min) | 27.7 ± 4.1 | 34.0 ± 3.5 * | 28.4 ± 3.0 | 29.4 ± 2.8 |
| Systolic blood pressure (mmHg) | 103.7 ± 19.1 | 131.4 ± 12.1 * | 96.0 ± 9.1 | 101.9 ± 22.5 |
| (C) | ||||
| Traditional EEJ(7 Individuals, 12 Ejaculates) | Low-Voltage EEJ(8 Individuals, 13 Ejaculates) | |||
| Semen volume (mL) | 12.7 ± 4.1 (3.6–20.5) | 13.1 ± 7.6 (3–32.3) | ||
| Semen pH | 8.45 ± 0.1 (8.4–8.6) | 7.98 ± 0.41 (7.1–8.5) | ||
| Semen concentration (106/mL) | 2.11 ± 1.87 (0.6–5.9) | 1.57 ± 1.48 (0.4–6.8) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Yu, J.-F.; Chang, Y.-J.; Chin, S.-C.; Wang, L.-C.; Lin, H.-L.; Tsai, P.-S. Novel Low-Voltage Electro-Ejaculation Approach for Sperm Collection from Zoo Captive Lanyu Miniature Pigs (Sus barbatus sumatranus). Animals 2020, 10, 1825. https://doi.org/10.3390/ani10101825
Chen Y-H, Yu J-F, Chang Y-J, Chin S-C, Wang L-C, Lin H-L, Tsai P-S. Novel Low-Voltage Electro-Ejaculation Approach for Sperm Collection from Zoo Captive Lanyu Miniature Pigs (Sus barbatus sumatranus). Animals. 2020; 10(10):1825. https://doi.org/10.3390/ani10101825
Chicago/Turabian StyleChen, Yu-Hsin, Jane-Fang Yu, Yu-Jia Chang, Shih-Chien Chin, Lih-Chiann Wang, Hsiu-Lien Lin, and Pei-Shiue Tsai. 2020. "Novel Low-Voltage Electro-Ejaculation Approach for Sperm Collection from Zoo Captive Lanyu Miniature Pigs (Sus barbatus sumatranus)" Animals 10, no. 10: 1825. https://doi.org/10.3390/ani10101825
APA StyleChen, Y.-H., Yu, J.-F., Chang, Y.-J., Chin, S.-C., Wang, L.-C., Lin, H.-L., & Tsai, P.-S. (2020). Novel Low-Voltage Electro-Ejaculation Approach for Sperm Collection from Zoo Captive Lanyu Miniature Pigs (Sus barbatus sumatranus). Animals, 10(10), 1825. https://doi.org/10.3390/ani10101825






