Comparison of the Effectiveness of Two Different Vaccination Regimes for Avian Influenza H9N2 in Broiler Chicken
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Birds and Vaccines
2.3. Experiment Design
2.3.1. Laboratory Groups
2.3.2. Field-monitored Groups
2.4. Hemagglutination Inhibition (HI) Test
2.5. Challenge Virus
2.6. qRT-PCR for Virus Shedding
2.7. Statistical Analysis
3. Results
3.1. Different AIV-H9N2 Virus Hemagglutinin Segment Amino Acid Identity Degrees
3.2. Immune Response to Other Vaccines at 28th Day of Life
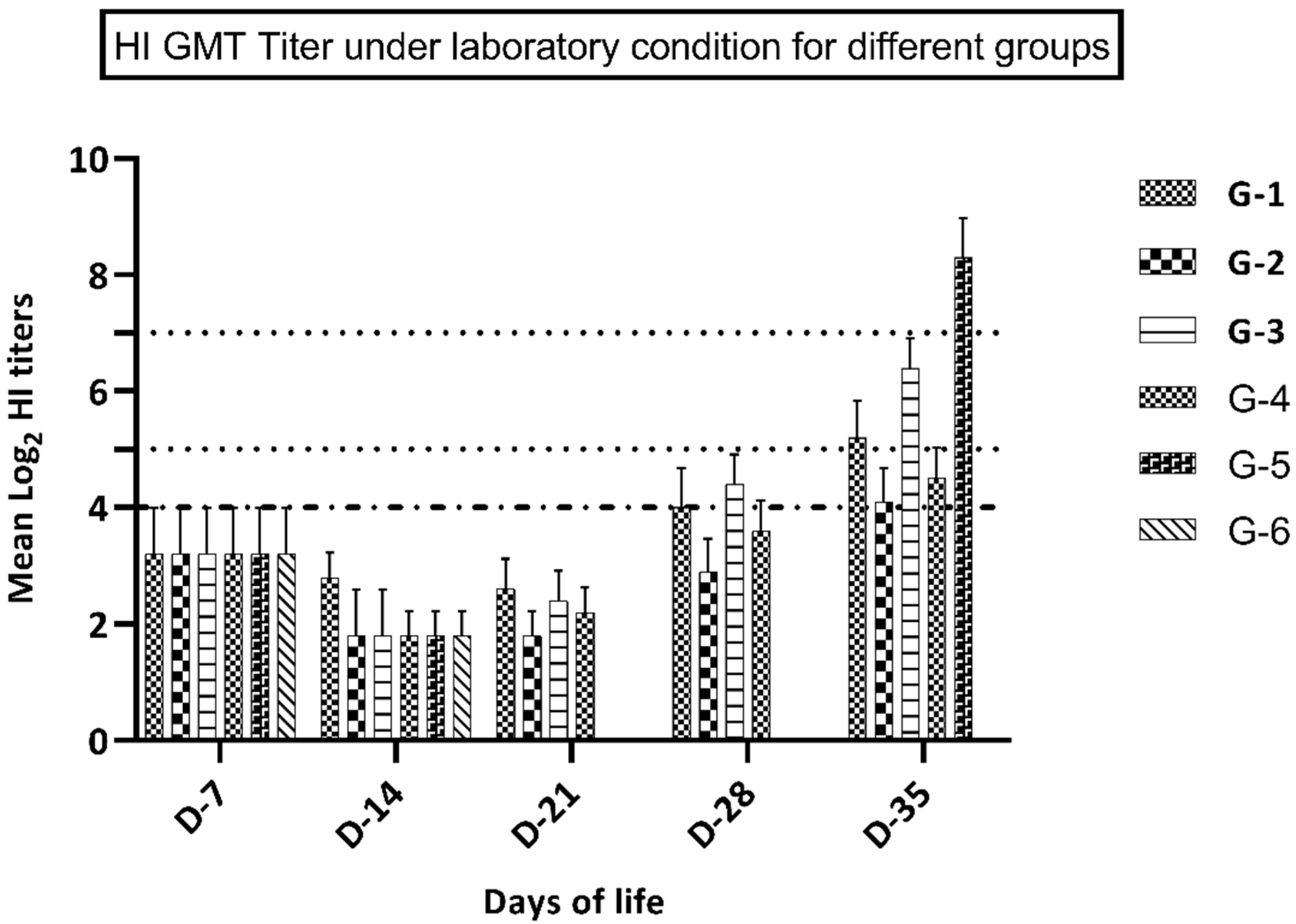
3.3. Immune Response in Groups Kept under Laboratory Conditions
3.4. Protection Following Challenge with a Wild Type H9N2 at the 28th Day of Life
3.4.1. Seroconversion 7-DPC with Recent Middle Eastern H9N2
3.4.2. Virus Shedding Following Challenge with H9N2 at 28 Days of Life
3.5. Immune Response in Groups Kept under Field Condition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dia, M.S.; Hafez, M.S.A.E.; Ashry, M.A.; Elfeil, W.K. Occurrence of avian influenza h5n1 among chicken, duck farms and human in Egypt. Am. J. Anim. Vet. Sci. 2019, 14, 26–32. [Google Scholar] [CrossRef]
- Eid, H.M.; Algammal, A.M.; Elfeil, W.K.; Youssef, F.M.; Harb, S.M.; Abd-Allah, E.M. Prevalence, molecular typing, and antimicrobial resistance of bacterial pathogens isolated from ducks. Vet. World 2019, 12, 677–683. [Google Scholar] [CrossRef]
- Sedeik, M.E.; Awad, A.M.; Rashed, H.; Elfeil, W.K. Variations in Pathogenicity and Molecular Characterization of Infectious Bursal Disease Virus (IBDV) in Egypt. Am. J. Anim. Vet. Sci. 2018, 13, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Eid, H.I.; Algammal, A.M.; Nasef, S.A.; Elfeil, W.K.; Mansour, G.H. Genetic variation among avian pathogenic E. coli strains isolated from broiler chickens. Asian J. Anim. Vet. Adv. 2016, 11, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, M.A.; Elfeil, W.K.; El Boraey, D.; Hammam, H.; Nossair, M.A. Evaluation of some vaccination programs in protection of experimentally challenged broiler chicken against newcastle disease virus. Am. J. Anim. Vet. Sci. 2019, 14, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Sultan, H.A.; Talaat, S.; Elfeil, W.K.; Selim, K.; Kutkat, M.A.; Amer, S.A.; Choi, K.S. Protective efficacy of the Newcastle disease virus genotype VII–matched vaccine in commercial layers. Poult. Sci. 2020, 99, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Elfeil, W.K.; Ezzat, M.E.; Fathi, A.; Alkilany, M.-A.A.; Abouelmaatti, R.R. Prevalence and Genotypic Analysis and Antibiotic Resistance of Salmonella Species Isolated from Imported and Freshly Slaughtered Chicken. Am. J. Anim. Vet. Sci. 2020, 15, 134–144. [Google Scholar] [CrossRef]
- Fawzy, M.; Ali, R.; Elfeil, W.; Saleh, A.; Eltarabilli, M. Efficacy of inactivated velogenic Newcastle disease virus genotype VII vaccine in broiler chickens. Vet. Res. Forum 2020, 11, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Sultan, H.A.; Ali, A.; El Feil, W.K.; Bazid, A.H.I.; Zain El-Abideen, M.A.; Kilany, W.H. Protective Efficacy of Different Live Attenuated Infectious Bronchitis Virus Vaccination Regimes against Challenge with IBV Variant-2 Circulating in the Middle East. Front. Vet. Sci. 2019, 6, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rady, M.; Ezz-El-Din, N.; Mohamed, K.F.; Nasef, S.; Samir, A.; Elfeil, W.K. Correlation between ESβL Salmonella Serovars Isolated from Broilers and their Virulence Genes. J. Hell. Vet. Med Soc. 2020, 71, 2163–2170. [Google Scholar] [CrossRef]
- Capua, I. Avian Influenza and Newcastle Disease: A Field and Laboratory Manual; Springer: Milan, Italy, 2009. [Google Scholar]
- Swayne, D.E.; Glisson, J.R. Diseases of Poultry; Wiley-Blackwell: Ames, Iowa, 2013. [Google Scholar]
- Abouelmaatti, R.R.; Algammal, A.M.; Li, X.; Ma, J.; Abdelnaby, E.A.; Elfeil, W.M.K. Cloning and analysis of Nile tilapia Toll-like receptors type-3 mRNA. Cent. Eur. J. Immunol. 2013, 38, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Elfeil, W.K.; Abouelmaatti, R.R.; Sun, C.; Han, W.; Li, X.; Ma, J.; Lei, L.; Liu, S.; Yang, Y.; Wang, Y.; et al. Identification, cloning, expression of a novel functional anasplatyrhynchos mRNA TLR4. J. Anim. Vet. Adv. 2012, 11, 1727–1733. [Google Scholar] [CrossRef]
- Elfeil, W.M.K.; Algammal, A.M.; Abouelmaatti, R.R.; Gerdouh, A.; Abdel-Daim, M.M. Molecular characterization and analysis of TLR-1 in rabbit tissues. Cent. Eur. J. Immunol. 2016, 41, 236–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abouelmaatti, R.R.; Algammal, A.M.; Elfeil, W.M.K.; Elshaffy, N.M.; Li, X.; Ma, J.; Fawzy, M.; Wahdan, A.; El-Tarabili, R.; Shabana, I.I. Genetic characterization, cloning, and expression of Toll-like Receptor 1 mRNA Oreochromis niloticus. Vet. Arh. 2020, 90, 193–204. [Google Scholar] [CrossRef]
- Capua, I.; Alexander, D.J. Avian influenza: Recent developments. Avian Pathol. 2004, 33, 393–404. [Google Scholar] [CrossRef]
- Spackman, E. Avian Influenza Virus; Humana Press: Totowa, NJ, USA, 2008; Volume 436. [Google Scholar]
- El-Zoghby, E.F.; Arafa, A.S.; Hassan, M.K.; Aly, M.M.; Selim, A.; Kilany, W.H.; Selim, U.; Nasef, S.; Aggor, M.G.; Abdelwhab, E.M.; et al. Isolation of H9N2 avian influenza virus from bobwhite quail (Colinus virginianus) in Egypt. Arch. Virol. 2012, 157, 1167–1172. [Google Scholar] [CrossRef]
- Monne, I.; Hussein, H.A.; Fusaro, A.; Valastro, V.; Hamoud, M.M.; Khalefa, R.A.; Dardir, S.N.; Radwan, M.I.; Capua, I.; Cattoli, G. H9N2 influenza A virus circulates in H5N1 endemically infected poultry population in Egypt. Influenza Other Respir. Viruses 2013, 7, 240–243. [Google Scholar] [CrossRef] [Green Version]
- El Sayed, M.; Alyousef, Y.; Al Sayed, A.; Elfeil, W. Evaluation of the antibody response of two local Saudi lines and commercial chickens vaccinated against newcastle diseases virus and infectious bursal disease virus. Sci. J. King Faisal Univ. 2019, 20, 105–113. [Google Scholar]
- Gharaibeh, S. Pathogenicity of an Avian Influenza Virus Serotype H9N2 in Chickens. Avian Dis. 2008, 52, 106–110. [Google Scholar] [CrossRef]
- Hsu, S.M.; Chen, T.H.H.; Wang, C.H. Efficacy of Avian Influenza Vaccine in Poultry: A Meta-analysis. Avian Dis. 2010, 54, 1197–1209. [Google Scholar] [CrossRef]
- Ahad, A.; Thornton, R.N.; Rabbani, M.; Yaqub, T.; Younus, M.; Muhammad, K.; Mahmood, A.; Shabbir, M.Z.; Kashem, M.A.; Islam, M.Z. Risk factors for H7 and H9 infection in commercial poultry farm workers in provinces within Pakistan. Prev. Vet. Med. 2014, 117, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Qiang, F.; Youxiang, D. The effects of H9N2 influenza A on the immune system of broiler chickens in the Shandong Province. Transbound. Emerg. Dis. 2011, 58, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Farzin, H.; Toroghi, R.; Haghparast, A. Up-regulation of pro-inflammatory cytokines and chemokine production in avian influenza H9N2 virus-infected human lung epithelial cell line (A549). Immunol. Investig. 2016, 45, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Arafat, N.; Eladl, A.H.; Marghani, B.H.; Saif, M.A.; El-shafei, R.A. Enhanced infection of avian influenza virus H9N2 with infectious laryngeotracheitis vaccination in chickens. Vet. Microbiol. 2018, 219, 8–16. [Google Scholar] [CrossRef]
- Bonfante, F.; Cattoli, G.; Leardini, S.; Salomoni, A.; Mazzetto, E.; Davidson, I.; Haddas, R.; Terregino, C. Synergy or interference of a H9N2 avian influenza virus with a velogenic Newcastle disease virus in chickens is dose dependent. Avian Pathol. 2017, 46, 488–496. [Google Scholar] [CrossRef]
- Nagy, A.; Mettenleiter, T.C.; Abdelwhab, E.M. A brief summary of the epidemiology and genetic relatedness of avian influenza H9N2 virus in birds and mammals in the Middle East and North Africa. Epidemiol. Infect. 2017, 145, 3320–3333. [Google Scholar] [CrossRef] [Green Version]
- Sultan, A.H.; Abd El-Razik, G.A.; Allam, S.T.; El-Deeb, A.H. Inactivated oil emulsion H9N2 vaccine in broiler chickens: Pathogenesis and Clinicopathological studies. Am. J. Res. Commun. 2015, 3, 38–53. [Google Scholar]
- Elfeil, W.; Abouelmaatti, R.; Diab, M.; Mandour, M.; Rady, M. Experimental Infection of Chickens by Avian Influenza H9N2 Virus: Monitoring of Tissue Tropism and Pathogenicity. J. Egypt. Vet. Med. Assoc 2018, 78, 369–383. [Google Scholar]
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals-Avian Influenza. In OIE Terrestrial Manual 2015; OIE: Rome, Italy, 2015. [Google Scholar]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statics; Mcgraw Hill Book Comp. Inc.: London, UK, 1960. [Google Scholar]
- Kilany, W.; Bazid, A.-H.; Ali, A.; El-Deeb, A.; Zain El Abideen, M.; Sayed, M.; El-Kady, M. Comparative Effectiveness of Two Oil Adjuvant–Inactivated Avian Influenza H9N2 Vaccines. Avian Dis. 2016, 60, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Khalil, A.; Hussein, H.; Tolba, S.; El-Sanousi, A. Preparation and Evaluation of H9n2 Vaccine Adjuvated with Montanide ISA71 VG. Glob. Vet. 2015, 15, 670–674. [Google Scholar] [CrossRef]
- Elfeil, W.K.; Abouelmaatti, R.R.; Ibrahim, H.H.; Ali, A.; Kilany, W.H.; Rady, A.; El-Sayed, M. Comparison of the effectiveness of two local and imported inactivated combined H9N2-ND vaccines in broiler flocks in Egypt. In Proceedings of the IX International Veterinary Conference: One World, One Health, Sharm El Sheikh City, Egypt, 9–13 March 2009. [Google Scholar]
- El-Deeb, A.H.; Kilany, W.; Hanafie, A.; Echeverry, F.; El-Kady, M. Comparative Field Evaluation of Protective Efficacy of Commercially Available H9N2 Vaccines in Breeders in Egypt. In Proceedings of the 19th World Veterinary Poultry Association Congress, Cape Town, South Africa, 7–11 September 2015. [Google Scholar]
- Elfeil, W.; Hanfie, A.; Rady, M.; Kilany, W.; Ibrahim, H.; Elkady, M.; Elsayed, M. Evaluation of Different H9N2 vaccination regime in commercial Broiler breeders flocks. In Proceedings of the XX World Veterinary Poultry Association Congress, Edinburgh International Conference Centre (EICC), Edinburgh, UK, 2 May 2017. [Google Scholar]
- Kilany, W.; Ali, A.; Bazid, A.-H.; El-Deeb, A.; Zain El Abideen, M.; Sayed, M.; El-Kady, M. A Dose-Response Study of Inactivated Low Pathogenic Avian Influenza H9N2 Virus in Specific-Pathogen-Free and Commercial Broiler Chickens. Avian Dis. 2016, 60, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Elfeil, W.; Yousef, H.; Fawzy, M.; Ali, A.; Kilany, W.; Ibrahim, H.; Elsayed, M. Protective efficacy of MEFLUVAC H9 in turkey poults in both lab and Field condition. In Proceedings of the XXIst World Veterinary Poultry Association Congress, Angkok International Trade and Exhibition Centre, Bangkok, Thailand, 16–20 September 2019. [Google Scholar]
- Sun, Y.; Pu, J.; Fan, L.; Sun, H.; Wang, J.; Zhang, Y.; Liu, L.; Liu, J. Evaluation of the protective efficacy of a commercial vaccine against different antigenic groups of H9N2 influenza viruses in chickens. Vet. Microbiol. 2012, 156, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Shahar, E.; Haddas, R.; Goldenberg, D.; Lublin, A.; Bloch, I.; Bachner Hinenzon, N.; Pitcovski, J. Newcastle disease virus: Is an updated attenuated vaccine needed? Avian Pathol. 2018, 47, 467–478. [Google Scholar] [CrossRef]
- Elhady, M.A.; Ali, A.; Kilany, W.H.; Elfeil, W.K.; Ibrahim, H.; Nabil, A.; Samir, A.; El Sayed, M. Field Efficacy of an Attenuated Infectious Bronchitis Variant 2 Virus Vaccine in Commercial Broiler Chickens. Vet. Sci. 2018, 5, 49. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

| Group No. | Bird No. | Vaccine Regime | Challenge at 28 Day of Age | Assessment of Protection | |||
|---|---|---|---|---|---|---|---|
| Vaccine Type | Age/Days | Dose/mL | |||||
| Experiment 1 lab experiment | G-1 | 20 | A | 1 | 0.3 | +++ | Follow up of immune response of vaccinated birds at weeks post vaccination to H9, H5, ND, IBD, and IB vaccines using HI and ELISA tests. Viral shedding detected by real-time PCR. |
| G-2 | 20 | B | 1 | 0.3 | +++ | ||
| G-3 | 20 | A | 7 | 0.3 | +++ | ||
| G-4 | 20 | B | 7 | 0.3 | +++ | ||
| G-5 | 20 | - | ------ | ---- | +++ | ||
| G-6 | 20 | - | ------ | --- | ----- | ||
| Experiment 2 field group | G-7 | 27K | A | 1 | 0.3 | - | Follow up of immune response of vaccinated birds on weekly basis. Measure of performance parameters and field exposure. Natural exposure monitoring by RT-PCR. |
| G-8 | 27K | B | 1 | 0.3 | - | ||
| G-9 | 27K | A | 6 | 0.3 | - | ||
| G-10 | 27K | B | 6 | 0.3 | - | ||
| Items | Alg. 2017 | Egypt 2018 | Iraq 2017 | KSA 2018 | Leb. 2017 | Libya 2015 | Mor. 2018 | Pak. 2018 | Tun. 2015 | UEA 2017 | Vaccine A | Vaccine B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alg./2017 | 94.59 | 94.78 | 96.39 | 94.59 | 96.39 | 99.28 | 91.88 | 91.76 | 98.10 | 93.68 | 92.24 | |
| Egypt/2018 | 94.59 | 94.79 | 93.68 | 94.64 | 95.54 | 94.64 | 92.54 | 94.58 | 96.46 | 98.86 | 91.79 | |
| Iraq/2017 | 94.78 | 94.79 | 94.34 | 93.32 | 93.93 | 93.21 | 94.46 | 93.11 | 93.04 | 94.43 | 90.71 | |
| KSA/2018 | 96.39 | 93.68 | 91.34 | 94.22 | 95.49 | 96.57 | 90.43 | 92.31 | 96.39 | 94.78 | 91.88 | |
| Leb./2017 | 94.59 | 94.64 | 92.32 | 94.22 | 96.79 | 94.82 | 90.89 | 93.41 | 94.64 | 94.75 | 92.86 | |
| Libya/2015 | 96.39 | 95.54 | 93.93 | 95.49 | 96.79 | 96.25 | 92.86 | 95.33 | 96.07 | 94.64 | 93.93 | |
| Mor./2018 | 99.28 | 94.64 | 93.21 | 96.57 | 94.82 | 96.25 | 91.96 | 92.31 | 99.82 | 94.75 | 92.14 | |
| Pak./2018 | 91.88 | 90.54 | 94.46 | 90.43 | 90.89 | 92.86 | 91.96 | 89.84 | 91.79 | 94.18 | 89.11 | |
| Tun./2015 | 91.76 | 92.58 | 90.11 | 92.31 | 93.41 | 95.33 | 92.31 | 89.84 | 92.31 | 95.48 | 89.56 | |
| UEA/2017 | 98.10 | 94.46 | 93.04 | 96.39 | 94.64 | 96.07 | 99.82 | 91.79 | 92.31 | 96.57 | 91.96 | |
| Vaccine A | 94.68 | 98.86 | 94.43 | 94.78 | 93.75 | 94.64 | 94.75 | 94.18 | 95.48 | 96.57 | 90.61 | |
| Vaccine B | 92.24 | 91.79 | 90.71 | 91.88 | 92.86 | 93.93 | 92.14 | 89.11 | 89.56 | 91.96 | 91.61 |
| Group No. | Bird No. | Vaccine Regime | ELISA Mean Titers | HI Titer Log2 | |||
|---|---|---|---|---|---|---|---|
| Vaccine Type | Age/days | Dose/mL | IBD | AI-H5 | ND | ||
| 28 Days | 28 Days | 28 Days | |||||
| G-1 | 20 | A | 1 | 0.3 | 17,553 ± 1105 | 3.7 ± 0.51 | 4.8 ± 0.72 |
| G-2 | 20 | B | 1 | 0.3 | 17,703 ± 1120 | 3.8 ± 0.61 | 4.9 ± 0.61 |
| G-3 | 20 | A | 7 | 0.3 | 17,612 ± 1090 | 3.6 ± 0.42 | 4.7 ± 0.66 |
| G-4 | 20 | B | 7 | 0.3 | 16,217 ± 1220 | 3.6 ± 0.52 | 4.8 ± 0.62 |
| G-5 | 20 | - | - | - | 17,533 ± 1140 | 3.7 ± 0.65 | 4.9 ± 0.56 |
| G-6 | 20 | - | - | - | 17,533 ± 1170 | 3.7 ± 0.72 | 4.8 ± 0.81 |
| Days of Life | Antigen Type | Experimental Groups | |||||
|---|---|---|---|---|---|---|---|
| G-1 | G-2 | G-3 | G-4 | G-5 | G-6 | ||
| D-7 | Antigen A | 3.2 ± 0.79 | 3.2 ± 0.79 | 3.2 ± 0.79 | 3.2 ± 0.79 | 3.2 ± 0.79 | 3.2 ± 0.79 |
| Antigen B | 2.6 ± 0.69 | 2.6 ± 0.69 | 2.6 ± 0.69 | 2.6 ± 0.69 | 2.6 ± 0.69 | 2.6 ± 0.69 | |
| D-14 | Antigen A | 2.8 ± 0.54 | 1.8 ± 0.42 | 1.8 ± 0.49 | 1.8 ± 0.56 | 1.8 ± 0.48 | 1.8 ± 0.32 |
| Antigen B | 2.1 ± 0.44 | 2.2 ± 0.54 | 1.6 ± 0.74 | 1.6 ± 0.64 | 1.6 ± 0.74 | 1.6 ± 0.64 | |
| D-21 | Antigen A | 2.6 ± 0.55 | 1.8 ± 0.67 | 2.4 ± 0.52 | 2.2 ± 0.42 | nd | nd |
| Antigen B | 2.2 ± 0.54 | 2.3 ± 0.64 | 2.1 ± 0.54 | 2.1 ± 0.54 | nd | nd | |
| D-28 | Antigen A | 4.1 ± 0.71 | 2.9 ± 0.59 | 4.4 ± 0.57 | 3.6 ± 0.46 | nd | nd |
| Antigen B | 3.1 ± 0.44 | 3.1 ± 0.54 | 3.4 ± 0.64 | 3.2 ± 0.64 | nd | nd | |
| D-35 | Antigen A | 5.4 ± 0.61 | 4.0 ± 0.57 | 6.4 ± 0.52 | 4.5 ± 0.67 | 8.3 ± 0.65 | nd |
| Antigen B | 4.2 ± 0.54 | 4.1 ± 0.48 | 5.1 ± 0.45 | 4.9 ± 0.64 | 7.4 ± 0.82 | nd | |
| Group No. | Bird No. | Vaccine Regime | GMT Log2 HI Titer (n = 10) | |||
|---|---|---|---|---|---|---|
| Vaccine Type | Age/days | Dose/mL | 7 Days Post-Challenge | |||
| Local Antigen | Imported Antigen | |||||
| 1 | 10 | A | 1 | 0.3 | 4.1 ± 1.2 * | 3.2 ± 1.21 * |
| 2 | 10 | B | 1 | 0.3 | 7.0 ± 2.57 * | 5.9 ± 2.57 * |
| 3 | 10 | A | 7 | 0.3 | 5.1 ± 0.92 * | 4.8 ± 0.89 * |
| 4 | 10 | B | 7 | 0.3 | 6.5 ± 1.67 * | 5.5 ± 1.87 * |
| 5 | 10 | - | - | - | 8.3 ± 0.65 * | 7.4 ± 0.82 * |
| 6 | 10 | - | - | - | - | - |
| Group No. | Vaccinal Regime | Assessment of Protection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine Type | Vaccine Age | 3-DPC | 7-DPC | |||||||
| Tracheal Swabs | Cloacal Swabs | Tracheal Swabs | Cloacal Swabs | |||||||
| No./EID50 | % | No./EID50 | % | No./EID50 | % | No./EID50 | % | |||
| G-1 | A | 1 | 4/10 (2.1 ± 0.9) b | 40% | 3/10 (1.5 ± 0.3) c | 30% | 3/10 (1.8 ± 0.7) b | 30% | 3/10 (1.8 ± 0.8) b | 30% |
| G-2 | B | 1 | 6/10 (2.8 ± 0.8) c | 60% | 4/10 (2.1 ± 0.4) c | 40% | 3/10 (2.3 ± 1.1) c | 30% | 4/10 (2.5 ± 0.8) c | 40% |
| G-3 | A | 7 | 2/10 (1.6 ± 0.6) a | 20% | 2/10 (1.1 ± 0.3) a | 20% | 1/10 (1.3 ± 0.0) a | 10% | 2/10 (1.8 ± 0.5) a | 20% |
| G-4 | B | 7 | 4/10 (2.4 ± 0.7) c | 40% | 3/10 (1.9 ± 0.3) c | 30% | 2/10 (1.9 ± 0.7) c | 20% | 3/10 (2.1 ± 0.6) c | 30% |
| G-5 | - | - | 10/10 (3.1 ± 0.9) d | 100% | 6/10 (2.1 ± 0.3) c | 60% | 7/10 (2.3 ± 1.9) c | 70% | 10/10 (3.1 ± 1.8) d | 100% |
| G-6 | - | - | nd | - | nd | - | nd | - | nd | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talat, S.; Abouelmaatti, R.R.; Almeer, R.; Abdel-Daim, M.M.; Elfeil, W.K. Comparison of the Effectiveness of Two Different Vaccination Regimes for Avian Influenza H9N2 in Broiler Chicken. Animals 2020, 10, 1875. https://doi.org/10.3390/ani10101875
Talat S, Abouelmaatti RR, Almeer R, Abdel-Daim MM, Elfeil WK. Comparison of the Effectiveness of Two Different Vaccination Regimes for Avian Influenza H9N2 in Broiler Chicken. Animals. 2020; 10(10):1875. https://doi.org/10.3390/ani10101875
Chicago/Turabian StyleTalat, Shaimaa, Reham R. Abouelmaatti, Rafa Almeer, Mohamed M. Abdel-Daim, and Wael K. Elfeil. 2020. "Comparison of the Effectiveness of Two Different Vaccination Regimes for Avian Influenza H9N2 in Broiler Chicken" Animals 10, no. 10: 1875. https://doi.org/10.3390/ani10101875
APA StyleTalat, S., Abouelmaatti, R. R., Almeer, R., Abdel-Daim, M. M., & Elfeil, W. K. (2020). Comparison of the Effectiveness of Two Different Vaccination Regimes for Avian Influenza H9N2 in Broiler Chicken. Animals, 10(10), 1875. https://doi.org/10.3390/ani10101875






