Swim Bladder Disorders in Koi Carp (Cyprinus carpio)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Bacteriological Examination
2.3. Histology
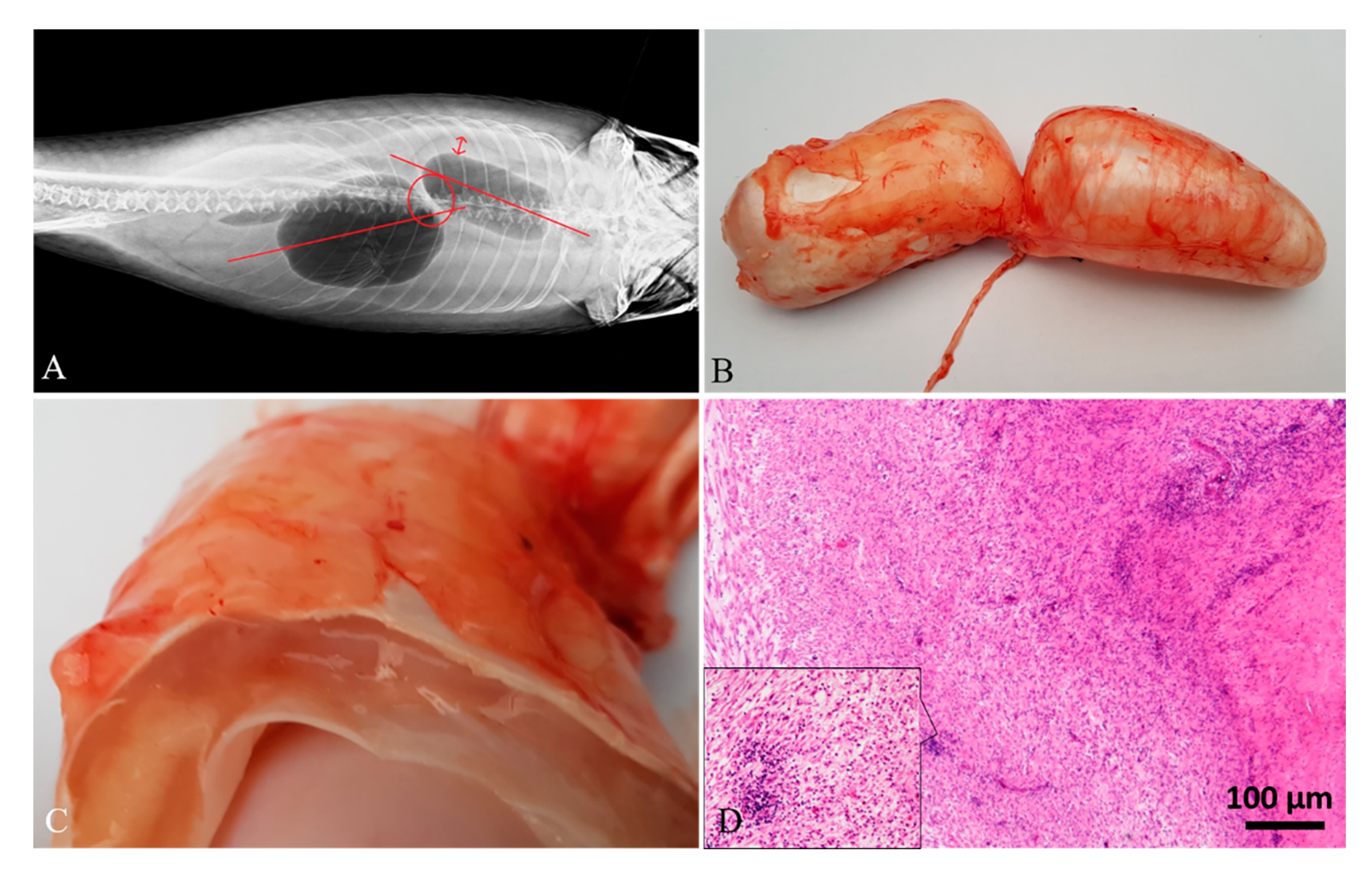
3. Results
3.1. Diagnostic Imaging and Gross Findings
3.2. Histology
3.3. Bacteriology
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balon, E.K. The oldest domesticated fishes, and the consequences of an epigenetic dichotomy in fish culture. J. Ichthyol. Aquat. Biol. 2006, 11, 47–86. [Google Scholar]
- Saint-Erne, N. Diagnostic Techniques and Treatments for Internal Disorders of Koi (Cyprinus carpio). Veter. Clin. N. Am. Exot. Anim. Pr. 2010, 13, 333–347. [Google Scholar] [CrossRef] [PubMed]
- De Kock, S.; Gomelsky, B. Japanese Ornamental Koi Carp: Origin, Variation and Genetics. In Biology and Ecology of Carp; Informa UK Limited: Boca Raton, FL, USA, 2015; pp. 27–53. [Google Scholar]
- Sanders, J. Aquatic Ambulatory Practice. Veter. Clin. N. Am. Exot. Anim. Pr. 2018, 21, 609–622. [Google Scholar] [CrossRef]
- Dey, V.K. The global trade in ornamental fish. Info Fish Internat. 2016, 4, 52–55. [Google Scholar]
- McDermott, C.; Palmeiro, B. Updates on Selected Emerging Infectious Diseases of Ornamental Fish. Veter. Clin. N. Am. Exot. Anim. Pr. 2020, 23, 413–428. [Google Scholar] [CrossRef]
- Sirri, R.; Ciulli, S.; Barbé, T.; Volpe, E.; Lazzari, M.; Franceschini, V.; Errani, F.; Sarli, G.; Mandrioli, L. Detection of Cyprinid Herpesvirus 1 DNA in cutaneous squamous cell carcinoma of koi carp (Cyprinus carpio). Veter. Dermatol. 2017, 29, 60–e24. [Google Scholar] [CrossRef]
- Knüsel, F.O.; Knüsel, R.; Doherr, M.G.; Schmidt-Posthaus, H. Frequency and histologic characterization of coelomatic neoplasms in koi Cyprinus carpio koi. Dis. Aquat. Org. 2016, 119, 219–229. [Google Scholar] [CrossRef]
- Wildgoose, W.H. Buoyancy disorders of ornamental fish: A review of cases seen in veterinary practice. Fish Vet. J. 2007, 9, 22–37. [Google Scholar]
- Pees, M.; Pees, K.; Kiefer, I. The use of computed tomography for assessment of the swim bladder in koi carp (cyprinus carpio). Veter. Radiol. Ultrasound 2010, 51, 294–298. [Google Scholar] [CrossRef]
- Chang, H.K.; Chen, Y.-A.; Tsai, Y.-L.; Wu, Y.-C.; Shien, J.-H.; Chang, W.-F.; Hsuan, S.-L.; Lin, C.-C. Outbreak of swim bladder inflammation caused by Sphaerospora dykovae in Koi (Cyprinus carpio koi) in Taiwan. Pak. Vet. J. 2016, 36, 499–502. [Google Scholar]
- Dyková, I.; Lom, J. Sphaerospora renicola n.sp., a myxosporean from carp kidney, and its pathogenicity. Parasitol. Res. 1982, 68, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Holzer, A.S.; Hartigan, A.; Patra, S.; Pecková, H.; Eszterbauer, E. Molecular fingerprinting of the myxozoan community in common carp suffering Swim Bladder Inflammation (SBI) identifies multiple etiological agents. Parasites Vectors 2014, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Caccamo, D.; Di Cello, F.; Fani, R.; Gugliandolo, C.; Maugeri, T.L. Polyphasic approach to the characterisation of marine luminous bacteria. Res. Microbiol. 1999, 150, 221–230. [Google Scholar] [CrossRef]
- BLAST. National Center for Biotechnology Information: Bethesda, MD, USA, 2011. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 12 October 2011).
- Markey, B.; Leonard, F.; Archambault, M.; Cullinane, A.; Maguire, D. Clinical Veterinary Microbiology, 2nd ed.; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Newton, A.L. Swim Bladder Disorders. In Fish Diseases and Medicine; Informa UK Ltd.: London, UK, 2019; pp. 230–243. [Google Scholar]
- Vergneau-Grosset, C.; Nadeau, M.-E.; Groff, J.M. Fish Oncology. Veter. Clin. N. Am. Exot. Anim. Pr. 2017, 20, 21–56. [Google Scholar] [CrossRef]
- Holland, M.F.; Frank, P.M. What Is Your Diagnosis? J. Am. Veter. Med. Assoc. 2006, 229, 1247–1248. [Google Scholar] [CrossRef]
- Kocylowski, B.; Antychowicz, J.; Zelazny, J. Studies on the etiology and pathogenesis of carp swimbladder inflammation. Riv. It. Piscic. Ittiopat. 1970, 5, 59. [Google Scholar]
- Galuppi, R.; Fioravanti, M.L.; Delgado, M.L.; Quaglio, F.; Caffara, M.; Tampieri, M.P. Segnalazione di due casi di micosi della vescica natatoria in Sparus aurata e Carassius auratus. Two cases of mycosis of the swimbladder in Sparus aurata and Carassius auratus. Boll. Soc. It. Patol. Ittica. 2001, 32, 26. [Google Scholar]
- Hoole, D.; Bucke, D.; Burgess, P.; Welby, I. Diseases of Carp and Other Cyprinid Fishes; Hoole, D., Bucke, D., Burgess, P., Wellby, I., Eds.; Fishing New Books Blackwell Ltd.: Oxford, UK, 2001; pp. 134–135. [Google Scholar]
- Daly, K.; Kelly, J.; Moran, A.W.; Bristow, R.; Young, I.S.; Cossins, A.R.; Bravo, D.; Shirazi-Beechey, S.P. Host selectively contributes to shaping intestinal microbiota of carnivorous and omnivorous fish. J. Gen. Appl. Microbiol. 2019, 65, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Monette, S.; Dallaire, A.D.; Mingelbier, M.; Groman, D.; Uhland, C.; Richard, J.-P.; Paillard, G.; Johannson, L.M.; Chivers, D.P.; Ferguson, H.W.; et al. Massive Mortality of Common Carp (Cyprinus carpio carpio) in the St. Lawrence River in 2001: Diagnostic Investigation and Experimental Induction of Lymphocytic Encephalitis. Veter. Pathol. 2006, 43, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Ciulli, S.; Volpe, E.; Sirri, R.; Passalacqua, P.; Bianchi, F.C.; Serratore, P.; Mandrioli, L. Outbreak of mortality in Russian (Acipenser gueldenstaedtii) and Siberian (Acipenser baerii) sturgeons associated with sturgeon nucleo-cytoplasmatic large DNA virus. Veter. Microbiol. 2016, 191, 27–34. [Google Scholar] [CrossRef]
- Volpe, E.; Mandrioli, L.; Errani, F.; Serratore, P.; Zavatta, E.; Rigillo, A.; Ciulli, S. Evidence of fish and human pathogens associated with doctor fish (Garra rufa, Heckel, 1843) used for cosmetic treatment. J. Fish Dis. 2019, 42, 1637–1644. [Google Scholar] [CrossRef]
- Ciulli, S.; Volpe, E.; Sirri, R.; Tura, G.; Errani, F.; Zamperin, G.; Toffan, A.; Silvi, M.; Renzi, A.; Abbadi, M.; et al. Multifactorial Causes of Chronic Mortality in Juvenile Sturgeon (Huso huso). Animals 2020, 10, 1866. [Google Scholar] [CrossRef] [PubMed]
- Paździor, E.; Pękala-Safińska, A.; Wasyl, D. Phenotypic diversity and potential virulence factors of the Shewanella putrefaciens group isolated from freshwater fish. J. Veter. Res. 2019, 63, 321–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocchi, M.; De Zan, G.; Di Giusto, T.; Deotto, S.; Pretto, T.; Manfrin, A.; Brunetta, R.; Toffan, A. Systemic polymicrobic infection involving Shewanella putrefaciens group in koi. J. Fish Dis. 2018, 41, 1929–1931. [Google Scholar] [CrossRef] [PubMed]
- Jung-Schroers, V.; Jung, A.; Ryll, M.; Bauer, J.; Teitge, F.; Steinhagen, D. Methods for identification and differentiation of different Shewanella spp. isolates for diagnostic use. J. Fish Dis. 2017, 41, 689–714. [Google Scholar] [CrossRef]
- Pękala, A.; Kozińska, A.; Paździor, E.; Głowacka, H. Phenotypical and genotypical characterization ofShewanella putrefaciens strains isolated from diseased freshwater fish. J. Fish Dis. 2014, 38, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhu, M.; Xu, J. First report of Shewanella sp. and Listonella sp. infection in freshwater cultured loach, Misgurnus anguillicaudatus. Aquac. Res. 2012, 45, 602–608. [Google Scholar] [CrossRef]




| Case # | Clinical Investigations | Outcome | Laboratory Analyses | Histological Findings |
|---|---|---|---|---|
| 1 | Ultrasonographic investigation, echo-guided needle aspiration | Euthanized | Bacteriology, histology | Submucosal and muscularis mucosae inflammation, lamina propria edema, mucosal hyperplasia with squamous metaplasia |
| 2 | Ultrasonographic investigation, echo-guided needle aspiration | Euthanized | Bacteriology, histology | Submucosal, muscularis mucosae and lamina propria inflammation, mucosal hyperplasia with mucous metaplasia and intraepithelial parasitic stages |
| 3 | Ultrasonographic investigation | Euthanized | Histology | Submucosal inflammation |
| 4 | Ultrasonographic investigation, echo-guided needle aspiration | Euthanized | Bacteriology, histology | Submucosal inflammation, muscularis mucosae fibrosis, mucosal hyperplasia with intraepithelial parasitic stages. |
| 5 | X-ray | Euthanized | Histology | Muscularis mucosae fibrosis, mucosal hyperplasia and inflammation (papillary type) |
| 6 | X-ray, ultrasonographic investigation | Euthanized | Bacteriology, histology | Submucosal and muscularis mucosae inflammation, muscularis mucosae fibrosis |
| 7 | Ultrasonographic investigation, echo-guided needle aspiration | Recovered | Bacteriology | NA |
| 8 | Ultrasonographic investigation, echo-guided needle aspiration | Recovered | Bacteriology | NA |
| 9 | Ultrasonographic investigation, echo-guided needle aspiration | Recovered | Bacteriology | NA |
| Case # | Isolated Bacteria | Method of Identification |
|---|---|---|
| 1 | S. xiamenensis | 16 rDNA sequencing |
| 2 | Aeromonas hydrophila/cavia group | API 20 NE |
| 4 | Aeromonas hydrophila/cavia group | API 20 NE |
| 6 | Negative | NA |
| 7 | S. xiamenensis | 16 rDNA sequencing |
| 8 | S. xiamenensis | 16 rDNA sequencing |
| 9 | S. xiamenensis | 16 rDNA sequencing |
| Case # | Isolated Bacteria | Strain Name | Accession Number | Antimicrobial Susceptibility | |||||
|---|---|---|---|---|---|---|---|---|---|
| Amikacin | Ciprofloxacin | Sulfamethoxazole/ Trimethoprim | Tetracycline | Ampicillin/ Sulbactam | Chloramphenicol | ||||
| 1 | S. xiamenensis | 402.2 | MW131343 | S | S | S | S | S | S |
| 7 | S. xiamenensis | 402.3 | MW131344 | S | S | S | S | S | S |
| 8 | S. xiamenensis | 402.1 | MW131345 | S | R | S | R | S | S |
| 9 | S. xiamenensis | 402.4 | MW131346 | S | S | S | S | S | S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirri, R.; Mandrioli, L.; Zamparo, S.; Errani, F.; Volpe, E.; Tura, G.; Barbé, T.; Ciulli, S. Swim Bladder Disorders in Koi Carp (Cyprinus carpio). Animals 2020, 10, 1974. https://doi.org/10.3390/ani10111974
Sirri R, Mandrioli L, Zamparo S, Errani F, Volpe E, Tura G, Barbé T, Ciulli S. Swim Bladder Disorders in Koi Carp (Cyprinus carpio). Animals. 2020; 10(11):1974. https://doi.org/10.3390/ani10111974
Chicago/Turabian StyleSirri, Rubina, Luciana Mandrioli, Samuele Zamparo, Francesca Errani, Enrico Volpe, Giorgia Tura, Tim Barbé, and Sara Ciulli. 2020. "Swim Bladder Disorders in Koi Carp (Cyprinus carpio)" Animals 10, no. 11: 1974. https://doi.org/10.3390/ani10111974





