Emergence of Third-Generation Cephalosporin-Resistant Morganella morganii in a Captive Breeding Dolphin in South Korea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Origin of Clinical Isolate M. morganii Strain KC-Tt-01
2.3. Determination of Phenotypic Antibiotic Resistance of M. morganii Strain KC-Tt-01
2.4. Sequencing and Analysis of the M. morganii Strain KC-Tt-01 Genome
2.5. Culture Deposition and Nucleotide Sequence Accession No.
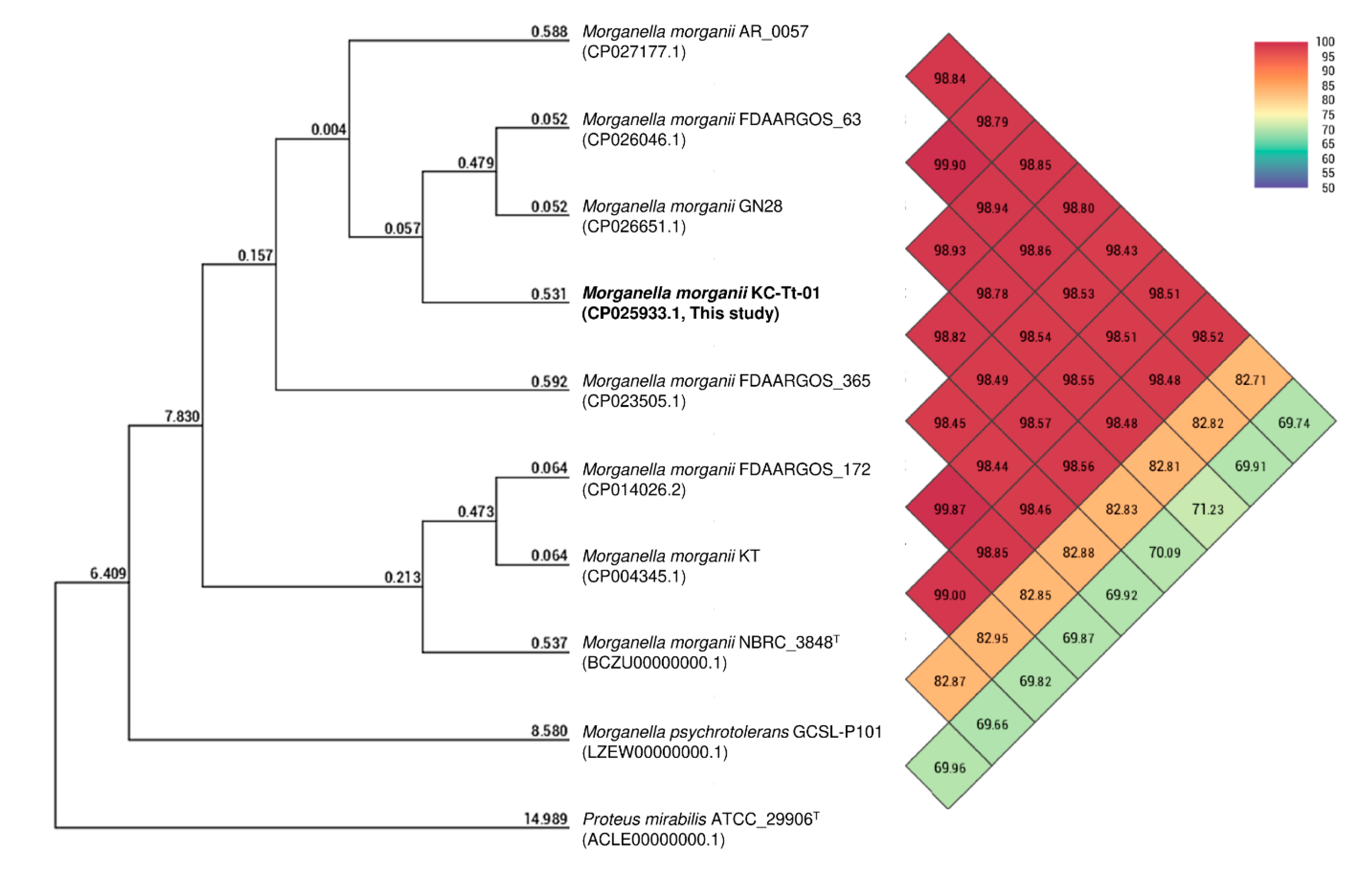
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Ethics Statement
References
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Dold, C. Cetacea (Whales, Dolphins, Porpoises). In Fowler’s Zoo and Wild Animal Medicine; Volume 8-E-Book; Elsevier Health Sciences: St. Louis, MO, USA, 2014; Volume 8, p. 422. [Google Scholar]
- Schaefer, A.M.; Goldstein, J.D.; Reif, J.S.; Fair, P.A.; Bossart, G.D. Antibiotic-resistant organisms cultured from Atlantic bottlenose dolphins (Tursiops truncatus) inhabiting estuarine waters of Charleston, SC and Indian River Lagoon, FL. Ecohealth 2009, 6, 33–41. [Google Scholar] [CrossRef]
- Prichula, J.; Pereira, R.I.; Wachholz, G.R.; Cardoso, L.A.; Tolfo, N.C.C.; Santestevan, N.A.; Medeiros, A.W.; Tavares, M.; Frazzon, J.; D’Azevedo, P.A.; et al. Resistance to antimicrobial agents among enterococci isolated from fecal samples of wild marine species in the southern coast of Brazil. Mar. Pollut. Bull. 2016, 105, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wong, S. Ocean sentinels: Marine mammals and antimicrobial resistance. In Proceedings of the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 27–30 September 2002. [Google Scholar]
- Rozanova, E.I.; Alekseev, A.Y.; Abramov, A.V.; Rassadkin, Y.N.; Shestopalov, A.M. Death of the killer whale Orsinus orca from bacterial pneumonia in 2003. Russ. J. Mar. Biol. 2007, 33, 321–323. [Google Scholar] [CrossRef]
- Gili, C.; Biancani, B.; Gulland, F.; Mazzariol, S. Meticillin-resistant Staphylococcus aureus (MRSA) associated dolphin mortality and the subsequent facility decolonisation protocol. Vet. Rec. Case Rep. 2017, e000444. [Google Scholar] [CrossRef]
- Mazzariol, S.; Corrò, M.; Tonon, E.; Centelleghe, C.; Biancani, B.; Gili, C. Death associated to methicillin resistant Staphylococcus aureus ST8 infection in two dolphins maintained under human care, Italy. Front. Immunol. 2018, 9, 2726. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, J.; Hu, Q.; Rao, X. Morganella morganii, a non-negligent opportunistic pathogen. Int. J. Infect Dis. 2016, 50, 10–17. [Google Scholar] [CrossRef]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Elfadl, A.K.; Lee, S.W.; Kim, J.H.; Lee, K.L.; Ullah, H.A.; Chung, M.J.; Ghim, S.G.; Lee, E.J.; Kim, Y.D.; Kim, S.M.; et al. Fatal fibrino-hemorrhagic bronchopneumonia associated with Morganella morganii in a bottlenose dolphin: A case report. Dis. Aquat. Organ. 2017, 127, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing: 24th Informational Supplement M100-S24; CLSI: Wayne, PA, USA, 2014. [Google Scholar]
- Holt, J.G.; Kreig, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams and Wilkins Co.: Baltimore, MD, USA, 1994. [Google Scholar]
- Stock, I.; Wiedemann, B. Identification and natural antibiotic susceptibility of Morganella morganii. Diagn. Microbiol. Infect. Dis. 1998, 30, 153–165. [Google Scholar] [CrossRef]
- Chen, Y.T.; Peng, H.L.; Shia, W.C.; Hsu, F.R.; Ken, C.F.; Tsao, Y.M.; Chen, C.H.; Liu, C.E.; Hsieh, M.F.; Chen, H.C.; et al. Whole-genome sequencing and identification of Morganella morganii KT pathogenicity-related genes. BMC Genom. 2012, 13, S4. [Google Scholar] [CrossRef] [PubMed]
- Tessier, F.; Arpin, C.; Allery, A.; Quentin, C. Molecular characterization of a TEM-21 β-lactamase in a clinical isolate of Morganella morganii. Antimicrob. Agents Chemother. 1998, 42, 2125–2127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barroso, H.; Freitas-Vieira, A.; Duarte, A. Molecular characterization of a ceftazidime-resistant Morganella morganii isolate producing a TEM-10 β-lactamase. Antimicrob. Agents Chemother. 1999, 43, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Kuga, A.; Okamoto, R.; Inoue, M. ampR gene mutations that greatly increase class C β-lactamase activity in Enterobacter cloacae. Antimicrob. Agents Chemother. 2000, 44, 561–567. [Google Scholar] [CrossRef] [PubMed]
| Test Group | Antimicrobial Agent | Disk Diffusion | MIC (μg/mL) | |
|---|---|---|---|---|
| Disk Content (μg) | Result | |||
| Penicillins | ||||
| Ampicillin ‡ | 10 | R | >256 (R) | |
| β-lactam/β-lactamase inhibitor combinations | ||||
| Amoxicillin | 10 | R | >256 (R) | |
| Amoxicillin-clavulanate ‡ | 20/10 | R | >256 (R) | |
| Ampicillin-sulbactam | 10/10 | R | ND § | |
| Piperacillin-tazobactam | 100/10 | S | ND | |
| Cephems (including cephalosporins 1st, 2nd, 3rd, and 4th) | ||||
| 1st ‡ | Cephalothin | 30 | R | ND |
| Cephazolin | 30 | R | ND | |
| 2nd ‡ | Cefoxitin | 30 | R | ND |
| Cefuroxime | 30 | R | ND | |
| 3rd | Cefotaxime | 30 | R | 64 (R) |
| Ceftazidime | 30 | R | ND | |
| 4th | Cefepime | 30 | S | ND |
| Monobactams | ||||
| Aztreonam | 30 | R | ND | |
| Carbapenems | ||||
| Imipenem ¶ | 10 | I | 16 | |
| Meropenem | 10 | S | 0.12 | |
| Aminoglycosides | ||||
| Gentamicin | 10 | S | 1 | |
| Amikacin | 30 | S | 2 | |
| Tetracyclines | ||||
| Tetracycline ‡ | 30 | S | 4 | |
| Fluoroquinolones | ||||
| Ciprofloxacin | 5 | S | 0.008 | |
| Levofloxacin | 5 | S | 0.12 | |
| Enrofloxacin | 5 | S | ND | |
| Quinolones | ||||
| Nalidixic acid | 30 | S | ND | |
| Folate pathway inhibitors | ||||
| Trimethoprim- sulfamethoxazole | 1.25/23.75 | S | ND | |
| Macrolides | ||||
| Erythromycin | 15 | R | >256 (R) | |
| Phenicols | ||||
| Chloramphenicol | 30 | S | ND | |
| Polymyxin | ||||
| Polymyxin B ‡ | 300 | R | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.Y.; Lee, K.; Cho, Y.; Lim, S.R.; Kwon, H.; Han, J.E.; Kim, J.H. Emergence of Third-Generation Cephalosporin-Resistant Morganella morganii in a Captive Breeding Dolphin in South Korea. Animals 2020, 10, 2052. https://doi.org/10.3390/ani10112052
Park SY, Lee K, Cho Y, Lim SR, Kwon H, Han JE, Kim JH. Emergence of Third-Generation Cephalosporin-Resistant Morganella morganii in a Captive Breeding Dolphin in South Korea. Animals. 2020; 10(11):2052. https://doi.org/10.3390/ani10112052
Chicago/Turabian StylePark, Seon Young, Kyunglee Lee, Yuna Cho, Se Ra Lim, Hyemin Kwon, Jee Eun Han, and Ji Hyung Kim. 2020. "Emergence of Third-Generation Cephalosporin-Resistant Morganella morganii in a Captive Breeding Dolphin in South Korea" Animals 10, no. 11: 2052. https://doi.org/10.3390/ani10112052
APA StylePark, S. Y., Lee, K., Cho, Y., Lim, S. R., Kwon, H., Han, J. E., & Kim, J. H. (2020). Emergence of Third-Generation Cephalosporin-Resistant Morganella morganii in a Captive Breeding Dolphin in South Korea. Animals, 10(11), 2052. https://doi.org/10.3390/ani10112052






