Oral Transmucosal or Intramuscular Administration of Dexmedetomidine–Methadone Combination in Dogs: Sedative and Physiological Effects
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Statistical Analysis
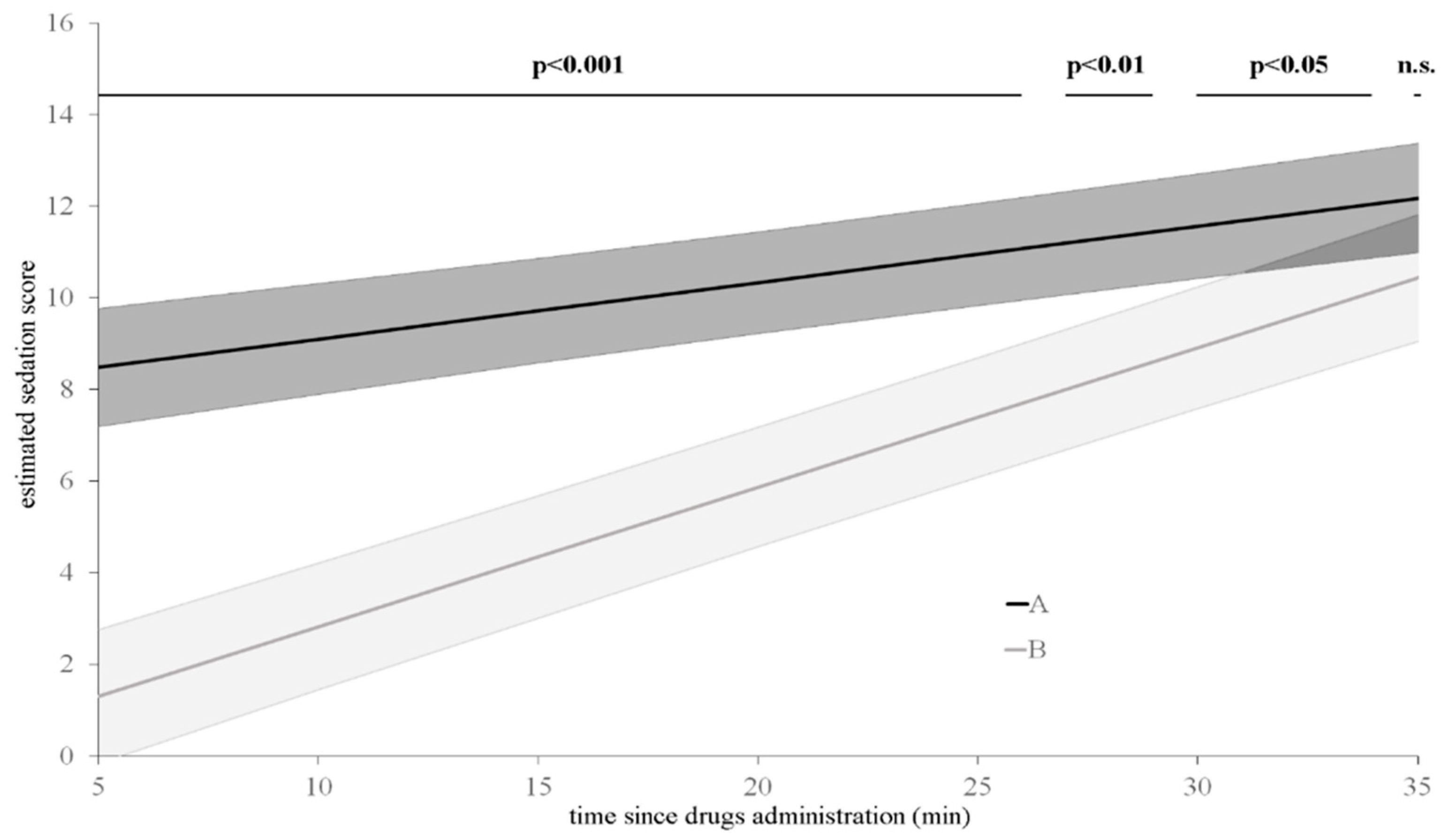
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brioschi, F.A.; Di Cesare, F.; Gioeni, D.; Rabbogliatti, V.; Ferrari, F.; D’Urso, E.S.; Amari, M.; Ravasio, G. Oral Transmucosal Cannabidiol Oil Formulation as Part of a Multimodal Analgesic Regimen: Effects on Pain Relief and Quality of Life Improvement in Dogs Affected by Spontaneous Osteoarthritis. Animals 2020, 10, 1505. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.E.; Bennett, S.L. Oral transmucosal administration of dexmedetomidine for sedation in 4 dogs. Can. Vet. J. 2015, 56, 1144. [Google Scholar] [PubMed]
- Porters, N.; Bosmans, T.; Debille, M.; De Rooster, H.; Duchateau, L.; Polis, I. Sedative and antinociceptive effects of dexmedetomidine and buprenorphine after oral transmucosal or intramuscular administration in cats. Vet. Anaesth. Analg. 2014, 41, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.C.; Freeman, L.J.; Barletta, M.; Weil, A.B.; Payton, M.E.; Johnson, B.M.; Inoue, T. Efficacy of oral transmucosal and intravenous administration of buprenorphine before surgery for postoperative analgesia in dogs undergoing ovariohysterectomy. J. Am. Vet. Med. Assoc. 2011, 238, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Porters, N.; De Rooster, H.; Bosmans, T.; Baert, K.; Cherlet, M.; Croubels, S.; De Backer, P.; Polis, I. Pharmacokinetics of oral transmucosal and intramuscular dexmedetomidine combined with buprenorphine in cats. J. Vet. Pharmacol. Ther. 2015, 38, 203–208. [Google Scholar] [CrossRef]
- Ferreira, T.H.; Rezende, M.L.; Mama, K.R.; Hudachek, S.F.; Aguiar, A.J.A. Plasma concentrations and behavioral, antinociceptive, and physiologic effects of methadone after intravenous and oral transmucosal administration in cats. Am. J. Vet. Res. 2011, 72, 764–771. [Google Scholar] [CrossRef]
- Robertson, S.A.; Lascelles, B.D.X.; Taylor, P.M.; Sear, J.W. PK-PD modeling of buprenorphine in cats: Intravenous and oral transmucosal administration 1. J. Vet. Pharmacol. Ther. 2005, 28, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hopfensperger, M.J.; Messenger, K.M.; Papich, M.G.; Sherman, B.L. The use of oral transmucosal detomidine hydrochloride gel to facilitate handling in dogs. J. Vet. Behav. 2013, 8, 114–123. [Google Scholar] [CrossRef]
- Slingsby, L.S.; Taylor, P.M.; Monroe, T. Thermal antinociception after dexmedetomidine administration in cats: A comparison between intramuscular and oral transmucosal administration. J. Feline Med. Surg. 2009, 11, 829–834. [Google Scholar] [CrossRef]
- Ansah, O.B.; Raekallio, M.; Vainio, O. Comparing oral and intramuscular administration of medetomidine in cats. Vet. Anaesth. Analg. 1998, 25, 41–46. [Google Scholar] [CrossRef]
- Pypendop, B.H.; Ilkiw, J.E.; Shilo-Benjamini, Y. Bioavailability of morphine, methadone, hydromorphone, and oxymorphone following buccal administration in cats. J. Vet. Pharmacol. Ther. 2014, 37, 295–300. [Google Scholar] [CrossRef]
- Catbagan, D.L.; Quimby, J.M.; Mama, K.R.; Rychel, J.K.; Mich, P.M. Comparison of the efficacy and adverse effects of sustained-release buprenorphine hydrochloride following subcutaneous administration and buprenorphine hydrochloride following oral transmucosal administration in cats undergoing ovariohysterectomy. Am. J. Vet. Res. 2011, 72, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Wells, S.M.; Glerum, L.E.; Papich, M.G. Pharmacokinetics of butorphanol in cats after intramuscular and buccal transmucosal administration. Am. J. Vet. Res. 2008, 69, 1548–1554. [Google Scholar] [CrossRef]
- Dent, B.T.; Aarnes, T.K.; Wavreille, V.A.; Lakritz, J.; Lerche, P.; KuKanich, B.; Riccó Pereira, C.H.; Bednarski, R.M. Pharmacokinetics and pharmacodynamic effects of oral transmucosal and intravenous administration of dexmedetomidine in dogs. Am. J. Vet. Res. 2019, 80, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Messenger, K.M.; Hopfensperger, M.; Knych, H.K.; Papich, M.G. Pharmacokinetics of detomidine following intravenous or oral-transmucosal administration and sedative effects of the oral-transmucosal treatment in dogs. Am. J. Vet. Res. 2016, 77, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Abbo, L.A.; Ko, J.C.H.; Maxwell, L.K.; Galinsky, R.E.; Moody, D.E.; Johnson, B.M.; Fang, W.B. Pharmacokinetics of buprenorphine following intravenous and oral transmucosal administration in dogs. Vet. Ther. 2008, 9, 83. [Google Scholar]
- Streisand, J.B.; Zhang, J.; Niu, S.; McJames, S.; Natte, R.; Pace, N.L. Buccal absorption of fentanyl is pH-dependent in dogs. Anesthesiol. J. Am. Soc. Anesthesiol. 1995, 82, 759–764. [Google Scholar] [CrossRef]
- Di Cesare, F.; Gioeni, D.; Ravasio, G.; Pellegrini, A.; Lucatello, L.; Bisutti, V.; Villa, R.; Cagnardi, P. Clinical pharmacokinetics of a dexmedetomidine–methadone combination in dogs undergoing routine anaesthesia after buccal or intramuscular administration. J. Vet. Pharmacol. Ther. 2019, 42, 392–400. [Google Scholar] [CrossRef]
- Murrell, J.C.; Hellebrekers, L.J. Medetomidine and dexmedetomidine: A review of cardiovascular effects and antinociceptive properties in the dog. Vet. Anaesth. Analg. 2005, 32, 117–127. [Google Scholar] [CrossRef]
- Gorman, A.L.; Elliott, K.J.; Inturrisi, C.E. The d-and l-isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci. Lett. 1997, 223, 5–8. [Google Scholar] [CrossRef]
- Codd, E.E.; Shank, R.P.; Schupsky, J.J.; Raffa, R.B. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: Structural determinants and role in antinociception. J. Pharmacol. Exp. Ther. 1995, 274, 1263–1270. [Google Scholar]
- Nishimura, L.T.; Auckburally, A.; Santilli, J.; Vieira, B.H.B.; Garcia, D.O.; Honsho, C.S.; de Mattos-Junior, E. Effects of dexmedetomidine combined with commonly administered opioids on clinical variables in dogs. Am. J. Vet. Res. 2018, 79, 267–275. [Google Scholar] [CrossRef]
- Monteiro, E.R.; Figueroa, C.D.N.; Choma, J.C.; Campagnol, D.; Bettini, C.M. Effects of methadone, alone or in combination with acepromazine or xylazine, on sedation and physiologic values in dogs. Vet. Anaesth. Analg. 2008, 35, 519–527. [Google Scholar] [CrossRef]
- Puighibet, Z.; Costa-Farré, C.; Santos, L.; Canfrán, S.; de Segura, I.A.G. The sedative effects of intramuscular low-dose medetomidine in combination with butorphanol or methadone in dogs. Vet. Anaesth. Analg. 2015, 42, 590–596. [Google Scholar] [CrossRef]
- Cardoso, C.G.; Marques, D.R.C.; da Silva, T.H.M.; de Mattos-Junior, E. Cardiorespiratory, sedative and antinociceptive effects of dexmedetomidine alone or in combination with methadone, morphine or tramadol in dogs. Vet. Anaesth. Analg. 2014, 41, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Maddern, K.; Adams, V.J.; Hill, N.A.T.; Leece, E.A. Alfaxalone induction dose following administration of medetomidine and butorphanol in the dog. Vet. Anaesth. Analg. 2010, 37, 7–13. [Google Scholar] [CrossRef]
- Gurney, M.; Cripps, P.; Mosing, M. Subcutaneous pre-anaesthetic medication with acepromazine–buprenorphine is effective as and less painful than the intramuscular route. J. Small Anim. Pract. 2009, 50, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, E.; Martinelli, E.; Brioschi, F.A.; Gioeni, D.; Corneliani, R.T.; Carotenuto, A.M. Intramuscular alfaxalone and methadone with or without ketamine in healthy cats: Effects on sedation and echocardiographic measurements. Vet. Anaesth. Analg. 2020, 47, 621–630. [Google Scholar] [CrossRef]
- Monteiro, E.R.; Nunes-Junior, J.S.; Bressan, T.F. Randomized clinical trial of the effects of a combination of acepromazine with morphine and midazolam on sedation, cardiovascular variables and the propofol dose requirements for induction of anesthesia in dogs. Vet. J. 2014, 200, 157–161. [Google Scholar] [CrossRef]
- Micieli, F.; Santangelo, B.; Reynaud, F.; Mirra, A.; Napoleone, G.; Della Valle, G.; Portier, K.G.; Vesce, G. Sedative and cardiovascular effects of intranasal or intramuscular dexmedetomidine in healthy dogs. Vet. Anaesth. Analg. 2017, 44, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Marjani, M.; Akbarinejad, V.; Bagheri, M. Comparison of intranasal and intramuscular ketamine-midazolam combination in cats. Vet. Anaesth. Analg. 2015, 42, 178–181. [Google Scholar] [CrossRef]
- Kuusela, E.; Raekallio, M.; Väisänen, M.; Mykkänen, K.; Ropponen, H.; Vainio, O. Comparison of medetomidine and dexmedetomidine as premedicants in dogs undergoing propofol-isoflurane anesthesia. Am. J. Vet. Res. 2001, 62, 1073–1080. [Google Scholar] [CrossRef]
- Granholm, M.; McKusick, B.C.; Westerholm, F.C.; Aspegrén, J.C. Evaluation of the clinical efficacy and safety of intramuscular and intravenous doses of dexmedetomidine and medetomidine in dogs and their reversal with atipamezole. Vet. Rec. 2007, 160, 891–897. [Google Scholar] [CrossRef]
- Maze, M.; Tranquilli, W. Alpha-2 adrenoceptor agonistsDefining the role in clinical anesthesia. Anesthesiol. J. Am. Soc. Anesthesiol. 1991, 74, 581–605. [Google Scholar]
- Bragg, R.F.; Bennett, J.S.; Cummings, A.; Quimby, J.M. Evaluation of the effects of hospital visit stress on physiologic variables in dogs. J. Am. Vet. Med. Assoc. 2015, 246, 212–215. [Google Scholar] [CrossRef]
- Davis, C.A.; Seddighi, R.; Cox, S.K.; Sun, X.; Egger, C.M.; Doherty, T.J. Effect of fentanyl on the induction dose and minimum infusion rate of propofol preventing movement in dogs. Vet. Anaesth. Analg. 2017, 44, 727–737. [Google Scholar] [CrossRef]
- Canfrán, S.; Bustamante, R.; González, P.; Cediel, R.; Re, M.; de Segura, I.A.G. Comparison of sedation scores and propofol induction doses in dogs after intramuscular administration of dexmedetomidine alone or in combination with methadone, midazolam, or methadone plus midazolam. Vet. J. 2016, 210, 56–60. [Google Scholar] [CrossRef]
- Karaaslan, D.; Peker, T.T.; Alaca, A.; Ozmen, S.; Kirdemir, P.; Yorgancigil, H.; Baydar, M.L. Comparison of buccal and intramuscular dexmedetomidine premedication for arthroscopic knee surgery. J. Clin. Anesth. 2006, 18, 589–593. [Google Scholar] [CrossRef]
| Criteria | Description | Score |
|---|---|---|
| Spontaneous posture | Standing | 0 |
| Sternally recumbent | 1 | |
| Laterally recumbent | 2 | |
| Eye position | Forward (normal position) | 0 |
| Rotated ventrally | 2 | |
| Response to sound (handclap) | Body movement | 0 |
| Head movement | 1 | |
| Ear twitch | 2 | |
| No reaction | 3 | |
| Resistance to lateral recumbency | Full (stands) | 0 |
| Moderate restraint required | 1 | |
| Mild restraint required | 2 | |
| No resistance | 3 | |
| Overall appearance | No sedation apparent | 0 |
| Mild sedation | 1 | |
| Moderate sedation | 2 | |
| Well sedated | 3 | |
| Total possible sedation score | 13 |
| Variable | Group | Time Points | |||
|---|---|---|---|---|---|
| T0 | T10 | T20 | T30 | ||
| Sedation | OTM | - | 1 (4) † | 5 (5) † | 9 (5) † |
| IM | - | 9 (2) † | 11 (3) † | 12 (2) † | |
| HR | OTM | 108 ± 8 | 79 ± 18 ∗† | 67 ± 18 ∗†# | 59 ± 12 ∗†#$ |
| IM | 111 ± 9 | 43 ± 6 ∗† | 38 ± 3 ∗†# | 38 ± 3 ∗†# | |
| SAP | OTM | 137 ± 15 | 136 ± 13 † | 142 ± 5 † | 144 ± 6 ∗# |
| IM | 136 ± 15 | 149 ± 7 ∗†‡ | 148 ± 6 ∗†‡ | 139 ± 7 | |
| MAP | OTM | 110 ± 15 | 111 ± 16 † | 114 ± 5 † | 117 ± 6 ∗# |
| IM | 111 ± 16 | 122 ± 7 ∗†‡ | 120 ± 6 ∗†‡ | 112 ± 7 | |
| DAP | OTM | 96 ± 15 | 97 ± 17 † | 100 ± 5 † | 104 ± 6 ∗ |
| IM | 99 ± 16 | 108 ± 7 †‡ | 107 ± 7 †‡ | 99 ± 7 | |
| fR | OTM | 63 ± 35 | 45 ± 30 ∗† | 34 ± 16 ∗†# | 30 ± 17 ∗†#$ |
| IM | 70 ± 27 | 11 ± 3 ∗† | 11 ± 3 ∗† | 10 ± 3 ∗† | |
| BT | OTM | 38.8 ± 0.4 | 38.8 ± 0.5 | 38.8 ± 0.5 | 38.7 ± 0.4 |
| IM | 39 ± 0.2 | 38.9 ± 0.2 | 38.8 ± 0.2 | 38.7 ± 0.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gioeni, D.; Brioschi, F.A.; Di Cesare, F.; Rabbogliatti, V.; Amari, M.; Zanzani, S.; Cagnardi, P.; Ravasio, G. Oral Transmucosal or Intramuscular Administration of Dexmedetomidine–Methadone Combination in Dogs: Sedative and Physiological Effects. Animals 2020, 10, 2057. https://doi.org/10.3390/ani10112057
Gioeni D, Brioschi FA, Di Cesare F, Rabbogliatti V, Amari M, Zanzani S, Cagnardi P, Ravasio G. Oral Transmucosal or Intramuscular Administration of Dexmedetomidine–Methadone Combination in Dogs: Sedative and Physiological Effects. Animals. 2020; 10(11):2057. https://doi.org/10.3390/ani10112057
Chicago/Turabian StyleGioeni, Daniela, Federica Alessandra Brioschi, Federica Di Cesare, Vanessa Rabbogliatti, Martina Amari, Sergio Zanzani, Petra Cagnardi, and Giuliano Ravasio. 2020. "Oral Transmucosal or Intramuscular Administration of Dexmedetomidine–Methadone Combination in Dogs: Sedative and Physiological Effects" Animals 10, no. 11: 2057. https://doi.org/10.3390/ani10112057
APA StyleGioeni, D., Brioschi, F. A., Di Cesare, F., Rabbogliatti, V., Amari, M., Zanzani, S., Cagnardi, P., & Ravasio, G. (2020). Oral Transmucosal or Intramuscular Administration of Dexmedetomidine–Methadone Combination in Dogs: Sedative and Physiological Effects. Animals, 10(11), 2057. https://doi.org/10.3390/ani10112057





