A Comparative Neuro-Histological Assessment of Gluteal Skin Thickness and Cutaneous Nociceptor Distribution in Horses and Humans
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Horse Skin Acquisition
2.3. Human Skin Acquisition
2.4. Histology Processing and Staining
2.5. Photographic Images
2.6. Analysis of H&E Sections
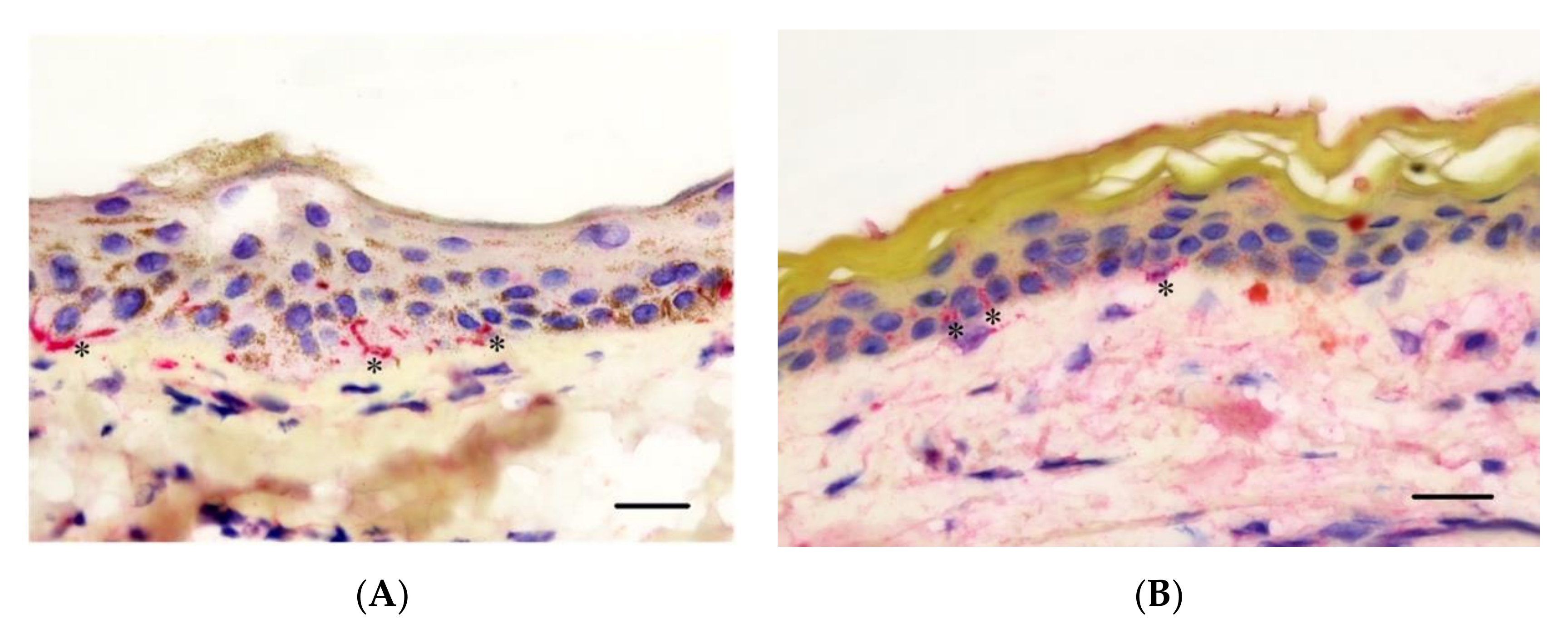
2.7. Immunohistochemistry Protocol
2.8. Analysis of IHC Sections
2.9. Statistical Analysis
3. Results
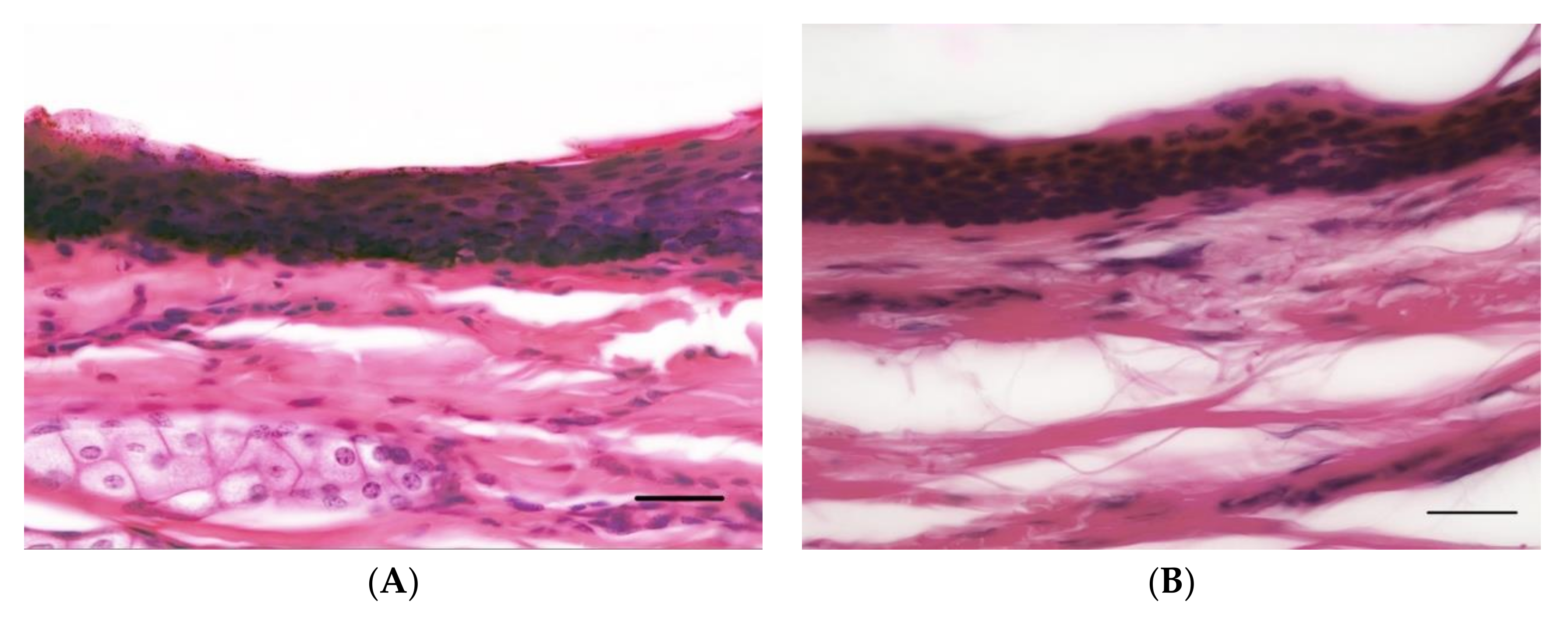
3.1. Overview
3.2. Epidermal Thickness
3.3. Dermal Thickness
3.4. Epidermal Nerve Count
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, B.; McGreevy, P.D. Ethical equitation: Applying a cost-benefit approach. J. Vet. Behav. 2010, 5, 196–202. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Oddie, C. Holding the whip hand-A note on the distribution of jockeys’ whip hand preferences in Australian Thoroughbred racing. J. Vet. Behav. 2011, 6, 287–289. [Google Scholar] [CrossRef]
- Evans, D.; McGreevy, P.D. An investigation of racing performance and whip use by jockeys in Thoroughbred races. PLoS ONE 2011, 6, e15622. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Corken, R.A.; Salvin, H.; Black, C.M. Whip use by jockeys in a sample of Australian Thoroughbred races-An observational study. PLoS ONE 2012, 7, e33398. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.; McManus, P. Changing human-animal relationships in sport: An analysis of the UK and Australian horse racing whips debates. Animals 2016, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Ban on Whip Use Would Be Positive for Racing, Suggests John Francome. Available online: http://horsetalk.co.nz/2015/11/12/ban-whip-use-positive-racing-francome/#axzz40UAtJE5b (accessed on 23 September 2020).
- McLean, A.N.; McGreevy, P.D. Ethical equitation: Capping the price horses pay for human glory. J. Vet. Behav. 2010, 5, 203–209. [Google Scholar] [CrossRef]
- Jones, B.; Goodfellow, J.; Yeates, J.; McGreevy, P.D. A Critical analysis of the British horseracing authority’s review of the use of the whip in horseracing. Animals 2015, 5, 138–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGreevy, P.D.; Hawson, L.A.; Salvin, H.; McLean, A. A note on the force of whip impacts delivered by jockeys using forehand and backhand strikes. J. Vet. Behav. 2013, 8, 395–399. [Google Scholar] [CrossRef]
- Bergmann, I. Sustainability, thoroughbred racing and the need for change. Pferdeheilkunde Equine Med. 2015, 31, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Heleski, C.R.; Anthony, R. Science alone is not always enough: The importance of ethical assessment for a more comprehensive view of equine welfare. J. Vet. Behav. 2012, 7, 169–178. [Google Scholar] [CrossRef]
- British Horseracing Authority. Responsible Regulation: A Review of the Use of the Whip in Horseracing. 2011. Available online: http://www.britishhorseracing.com/wp-content/uploads/2014/03/WhipReview.pdf (accessed on 24 September 2020).
- McGreevy, P.D.; McManus, P. Why Horse-Racing in Australia Needs a Social Licence to Operate. The Conversation, 3 November 2017. Available online: https://theconversation.com/why-horse-racing-in-australia-needs-a-social-licence-to-operate-79492 (accessed on 10 November 2020).
- McGreevy, P.D. Equine Behavior-A Guide for Veterinarians and Equine Scientists; W.B Saunders: London, UK, 2012. [Google Scholar]
- Porter, N. Thick-skinned. In Webster’s Revised Unabridged Dictionary of the English Language; C. & G. Merriam Co.: Springfield, MA, USA, 1913. [Google Scholar]
- Cuvier, G.; Vallentin, R.; Latreille, P.A.; MacMurtrie, H. The Animal Kingdom, Arranged According to Its Organization: Serving as a Foundation for the Natural History of Animals, and an Introduction to Contemporary Anatomy; G. Henderson: London, UK, 1834. [Google Scholar]
- Wakuri, H.; Mutoh, K.; Ichikawa, H.; Liu, B. Microscopic anatomy of the equine skin with special reference to the dermis. Okajimas Folia Anat. Jpn. 1995, 72, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkering, M.E. Variation of Skin Thickness over the Equine Body and the Correlation between Skin Fold Measurement and Actual Skin Thickness. Doctoral Thesis, Utrecht University, Utrecht, The Netherlands, 2009. [Google Scholar]
- Southwood, W.F.W. The thickness of the skin. Plast Reconstr Surg 1995, 15, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Artz, C.P.; Moncrief, J.A.; Pruitt, B.A. Burns: A team Approach; W. B. Saunders Company: Philadelphia, PA, USA, 1979; pp. 24–44. [Google Scholar]
- Lee, Y.; Hwang, K. Skin thickness of Korean adults. Surg. Radiol. Anat. 2002, 24, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Schummer, A.; Wilkens, H.; Vollmerhaus, B.; Habermehl, K.H. Skin and cutaneous organs of the horse. In The Circulatory System, the Skin, and the Cutaneous Organs of Domestic Mammals; Schummer, A., Wilkens, H., Vollmerhaus, B., Habermehl, K.H., Eds.; Springer: New York, NY, USA, 1981; p. 537. [Google Scholar]
- Ebenezer, G.J.; Hauer, P.; Gibbons, C.; McArthur, J.C.; Polydefkis, M. Assessment of epidermal nerve fibers. J. Neuropathol. Exp. Neurol. 2007, 66, 1059–1073. [Google Scholar] [CrossRef]
- Rosenberger, D.C.; Binzen, U.; Treede, R.-D.; Greffrath, W. The capsaicin receptor TRPV1 is the first line defense protecting from acute non damaging heat: A translational approach. J. Transl. Med. 2020, 18, 28. [Google Scholar] [CrossRef]
- Mense, S. Anatomy of nociceptors. In The Senses: A Comprehensive Reference; Bushnell, M.C., Smith, D.V., Beauchamp, G.K., Firestei, S.J., Eds.; Academic Press: New York, NY, USA, 2008; pp. 11–41. [Google Scholar]
- Talukdar, A.H.; Calhoun, M.L.; Stinson, A.W. Microscopic anatomy of the skin of the horse. Am. J. Vet. Res. 1972, 33, 2365–2390. [Google Scholar]
- Jørgensen, E.; Lazzarini, G.; Pirone, A.; Jacobsen, S.; Miragliotta, V. Normal microscopic anatomy of equine body and limb skin: A morphological and immunohistochemical study. Ann. Anat. 2018, 218, 205–212. [Google Scholar] [CrossRef]
- Sato, K.; Sugibayashi, K.; Morimoto, Y. Species differences in percutaneous absorption of nicorandil. J. Pharm. Sci. 1991, 80, 104–107. [Google Scholar] [CrossRef]
- Smith, E.S.J.; Lewin, G.R. Nociceptors: A phylogenetic view. J. Comp. Physiol. A 2009, 195, 1089–1106. [Google Scholar] [CrossRef] [Green Version]
- National Research Council (US) Committee on Recognition and Alleviation of Pain in Laboratory Animals. Pain in Research Animals: General Principles and Considerations; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Dyck, P.J.; Mellinger, J.F.; Reagan, T.J.; Horowitz, S.J.; McDonald, J.W.; Litchy, W.J.; Daube, J.R.; Fealey, R.D.; Go, V.L.; Kao, P.C.; et al. Not ‘indifference to pain’ but varieties of hereditary sensory and autonomic neuropathy. Brain 1983, 106, 373–390. [Google Scholar] [CrossRef]
- Nolano, M.; Crisci, C.; Santoro, L.; Barbieri, F.; Casale, R.; Kennedy, W.; Wendelschafer-Crabb, G.; Provitera, V.; Di Lorenzo, N.; Caruso, G. Absent innervation of skin and sweat glands in congenital insensitivity to pain with anhidrosis. Clin. Neurophysiol. 2000, 111, 1596–1601. [Google Scholar] [CrossRef]
- Lauria, G.; Cornblath, D.R.; Johansson, O.; McArthur, J.C.; Mellgren, S.I.; Nolano, M.; Rosenberg, N.; Sommer, C. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur. J. Neurol. 2005, 12, 747–758. [Google Scholar] [CrossRef] [PubMed]
- RC Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 10 November 2020).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models usinglme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lewin, G.R.; Moshourab, R. Mechanosensation and pain. J. Neurobiol. 2004, 61, 30–44. [Google Scholar] [CrossRef]
- Taylor, P.; Crosignani, N.; Lopes, C.; Rosa, A.C.; Luna, S.P.L.; Filho, J.N.P. Mechanical nociceptive thresholds using four probe configurations in horses. Vet. Anaesth. Analg. 2016, 43, 99–108. [Google Scholar] [CrossRef]
- Sluka, K.A.; Rasmussen, L.A. Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain 2010, 148, 188–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Green, P.G.; Levine, J.D. Stress enhances muscle nociceptor activity in the rat. Neuroscience 2011, 185, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinal, O.A.; Levinel, J.D.; Greenl, P.G. Enhanced cytokine-induced mechanical hyperalgesia in skeletal muscle produced by a novel mechanism in rats exposed to unpredictable sound stress. Eur. J. Pain 2011, 15, 796–800. [Google Scholar] [CrossRef] [Green Version]
- Rivat, C.; Becker, C.; Blugeot, A.; Zeau, B.; Mauborgne, A.; Pohl, M.; Benoliel, J.-J. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain 2010, 150, 358–368. [Google Scholar] [CrossRef]
- International Federation of Horseracing Authorities. International Agreement on Breeding, Racing and Wagering. Available online: http://www.horseracingintfed.com/resources/2018Agreement.pdf (accessed on 14 April 2018).
- Bessou, P.; Perl, E.R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J. Neurophysiol. 1969, 32, 1025–1043. [Google Scholar] [CrossRef] [Green Version]
- Racing Australia. Australian Rules of Racing. 2020. Available online: https://www.racingaustralia.horse/FreeServices/Australian_Rules_Of_Racing.aspx (accessed on 20 September 2020).
- Hargis, A.M.; Myers, S. The Integument. In Pathologic Basis of Veterinary Disease, 6th ed.; Zachary, J.F., Ed.; Elsevier: St. Louis, MO, USA, 2018; p. 1022. [Google Scholar]
- LaRose, C.; Richard-Yris, M.-A.; Hausberger, M.; Rogers, L.J. Laterality of horses associated with emotionality in novel situations. Laterality 2006, 11, 355–367. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.N.; McGreevy, P.D. Horse-training techniques that may defy the principles of learning theory. J. Vet. Behav. 2010, 5, 187–195. [Google Scholar] [CrossRef]
- Hall, C.; Goodwin, D.; Heleski, C.; Randle, H.; Waran, N. Is there evidence of learned helplessness in horses? J. Appl. Anim. Welf Sci. 2008, 11, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; McManus, P.; Stansall, D.; Wilson, B.J.; McGreevy, P.D. Is whip use important to thoroughbred racing integrity? What stewards’ reports reveal about fairness to punters, jockeys and horses. Animals 2020, 10, 1985. [Google Scholar] [CrossRef] [PubMed]
- Barington, K.; Jensen, H.E. The impact of force on the timing of bruises evaluated in a porcine model. J. Forensic Leg. Med. 2016, 40, 61–66. [Google Scholar] [CrossRef]






| Quartile | Horse Age Range | Number of Horse Sections | Human Age Range | Number of Human Sections |
|---|---|---|---|---|
| 1 | 3–5 | 24 | 52–61 | 12 |
| 2 | 8–10 | 42 | 67–77 | 18 |
| 3 | 11–15 | 30 | 83–87 | 18 |
| 4 | 16–25 | 30 | 92–94 | 12 |
| Coefficient | Std. Error | d.f. | t Value | p Value | |
|---|---|---|---|---|---|
| (Intercept) | 3.499 | 0.127 | 28.898 | 27.478 | <2 × 10-16 |
| SpeciesHuman | −0.185 | 0.218 | 25.565 | −0.849 | 0.404 |
| SexMale | 0.132 | 0.083 | 24.748 | 1.577 | 0.128 |
| Section | 0.005 | 0.016 | 148.977 | 0.346 | 0.730 |
| SpeciesHuman:SexMale | −0.228 | 0.145 | 25.346 | −1.572 | 0.128 |
| SpeciesHorse:Age_quantile | −0.049 | 0.039 | 24.748 | −1.238 | 0.227 |
| SpeciesHuman:Age_quantile | 0.004 | 0.056 | 24.790 | 0.076 | 0.940 |
| SpeciesHorse:SideRight | 0.071 | 0.031 | 148.977 | 2.291 | 0.023 |
| SpeciesHuman:SideRight | 0.032 | 0.047 | 152.012 | 0.694 | 0.489 |
| Estimate | Std. Error | d.f. | t Value | p Value | |
|---|---|---|---|---|---|
| (Intercept) | 4358.564 | 343.092 | 26.694 | 12.704 | 0.000 |
| SpeciesHuman | −1759.220 | 597.193 | 25.335 | −2.946 | 0.007 |
| SexMale | −193.894 | 229.847 | 24.993 | −0.844 | 0.407 |
| Section | −10.844 | 27.747 | 149.095 | −0.391 | 0.696 |
| SpeciesHuman:SexMale | 91.974 | 397.560 | 25.280 | 0.231 | 0.819 |
| SpeciesHorse:Age_quantile | −5.897 | 108.129 | 24.993 | −0.055 | 0.957 |
| SpeciesHuman:Age_quantile | 124.633 | 154.417 | 25.013 | 0.807 | 0.427 |
| SpeciesHorse:SideRight | −428.025 | 54.606 | 149.095 | −7.838 | 0.000 |
| SpeciesHuman:SideRight | −113.599 | 83.199 | 150.535 | −1.365 | 0.174 |
| Estimate | Std. Error | d.f. | t Value | p Value | |
|---|---|---|---|---|---|
| (Intercept) | 2.727 | 0.468 | 24.627 | 5.829 | 0.000 |
| SpeciesHuman | 0.041 | 0.806 | 24.215 | 0.051 | 0.960 |
| SexMale | 0.238 | 0.312 | 23.887 | 0.764 | 0.453 |
| Section2 | 0.081 | 0.076 | 142.990 | 1.072 | 0.286 |
| Section3 | 0.080 | 0.076 | 142.990 | 1.057 | 0.292 |
| SpeciesHuman:SexMale | −0.254 | 0.534 | 24.160 | −0.476 | 0.638 |
| SpeciesHorse:Age_quantile | 0.049 | 0.150 | 23.887 | 0.329 | 0.745 |
| SpeciesHuman:Age_quantile | 0.063 | 0.206 | 23.907 | 0.303 | 0.765 |
| SpeciesHorse:SideRight | 0.150 | 0.075 | 142.990 | 1.993 | 0.048 |
| SpeciesHuman:SideRight | −0.148 | 0.112 | 144.384 | −1.331 | 0.185 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, L.; Stewart, M.; Johnson, I.; Appleyard, R.; Wilson, B.; James, O.; Johnson, C.; McGreevy, P. A Comparative Neuro-Histological Assessment of Gluteal Skin Thickness and Cutaneous Nociceptor Distribution in Horses and Humans. Animals 2020, 10, 2094. https://doi.org/10.3390/ani10112094
Tong L, Stewart M, Johnson I, Appleyard R, Wilson B, James O, Johnson C, McGreevy P. A Comparative Neuro-Histological Assessment of Gluteal Skin Thickness and Cutaneous Nociceptor Distribution in Horses and Humans. Animals. 2020; 10(11):2094. https://doi.org/10.3390/ani10112094
Chicago/Turabian StyleTong, Lydia, Melinda Stewart, Ian Johnson, Richard Appleyard, Bethany Wilson, Olivia James, Craig Johnson, and Paul McGreevy. 2020. "A Comparative Neuro-Histological Assessment of Gluteal Skin Thickness and Cutaneous Nociceptor Distribution in Horses and Humans" Animals 10, no. 11: 2094. https://doi.org/10.3390/ani10112094
APA StyleTong, L., Stewart, M., Johnson, I., Appleyard, R., Wilson, B., James, O., Johnson, C., & McGreevy, P. (2020). A Comparative Neuro-Histological Assessment of Gluteal Skin Thickness and Cutaneous Nociceptor Distribution in Horses and Humans. Animals, 10(11), 2094. https://doi.org/10.3390/ani10112094






