The Effect of Stress on Reproduction and Reproductive Technologies in Beef Cattle—A Review
Abstract
:Simple Summary
Abstract
1. Introduction
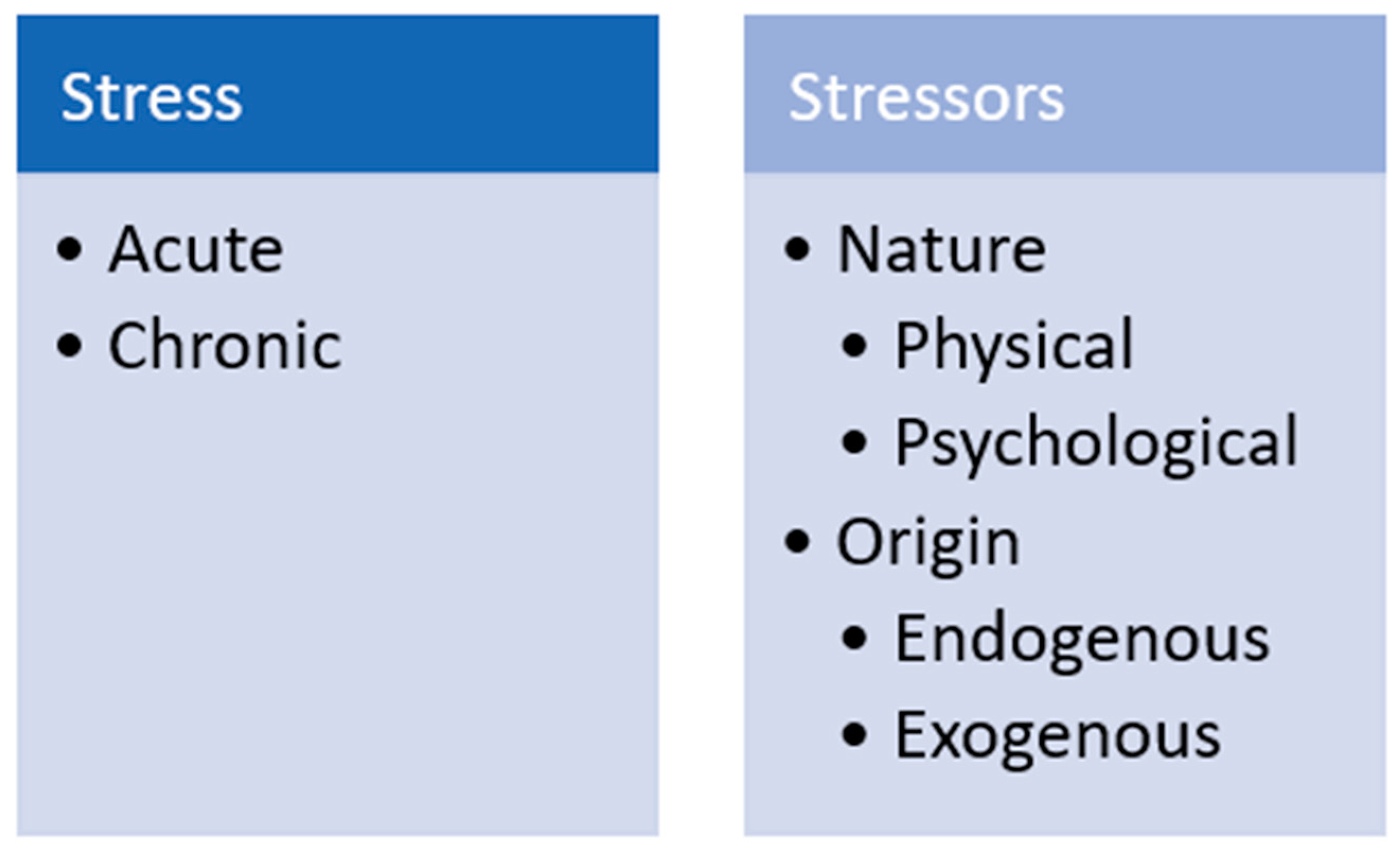
2. Definitions, Physiology and Quantification of Stress
2.1. Stress Basics
2.2. Physiology of Stress
2.3. Quantification of Stress
2.3.1. Behavioral Indicators
2.3.2. Animal Based Indicators
2.3.3. Biomarkers
3. Main Stressors in the Beef Cow
3.1. Management Stress
3.1.1. Handling Stress
3.1.2. Social Stress in the Beef Cow. Temperament and Hierarchy
3.1.3. Weaning Stress
3.2. Nutritional Stress: Under Nutrition and Imbalanced Nutrition
3.3. Thermal Stress
4. Beef Cattle Stress and Reproductive Biotechnologies
| Measured Parameter | Type of Animals | Temperament | Reference | |
|---|---|---|---|---|
| Calm | Excitable | |||
| CR at FTAI | Heifers | 60.30% | 51.90% | Kasimanickam et al. 2014 [20] |
| CR at FTAI | Heifers | 62.70% | 53.40% | Kasimanickam et al. 2018 [99] |
| CR at FTAI | Cows | 47.30% | 41.00% | Cooke et al. 2017 [101] |
| Cortisol concentration | Cows | 16.0 ± 2.1 | 12.5 ± 1.0 | Macedo et al. [171] |
| Embryo Viability | Cows | 19% less | Macedo et al. [171] | |
| P after ET | Cows | 60.20% | 52.40% | Kasimanickam et al. 2019 [169] |
| P after ET | Cows | 62.70% | 49.20% | Kasimanickam et al. 2018 [170] |
| P after ET using NSAIDs | Cows | 59.30% | 56.80% | Kasimanickam et al. 2018 [170] |
| P after ET without NSAIDs | Cows | 59.40% | 46.30% | Kasimanickam et al. 2018 [170] |
5. Stress and Reproductive Efficiency of the Beef Bull
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Givens, M.D. A clinical, evidence-based approach to infectious causes of infertility in beef cattle. Theriogenology 2006, 66, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Mee, J.F.; Geraghty, T.; O’Neill, R.; More, S.J. Bioexclusion of diseases from dairy and beef farms: Risks of introducing infectious agents and risk reduction strategies. Vet. J. 2012, 194, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Grooms, D.L. Reproductive consequences of infection with bovine viral diarrhea virus. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 5–19. [Google Scholar] [CrossRef]
- Oguejiofor, C.F.; Thomas, C.; Cheng, Z.; Wathes, D.C. Mechanisms linking bovine viral diarrhea virus (BVDV) infection with infertility in cattle. Anim. Health. Res. Rev. 2019, 20, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, B.W.; Cofield, L.G.; Walz, P.H.; Givens, M.D. Prevention of abortion in cattle following vaccination against bovine herpesvirus 1: A meta-analysis. Prev. Vet. Med. 2017, 138, 1–8. [Google Scholar] [CrossRef]
- Michi, A.N.; Favetto, P.H.; Kastelic, J.; Cobo, E.R. A review of sexually transmitted bovine trichomoniasis and campylobacteriosis affecting cattle reproductive health. Theriogenology 2016, 85, 781–791. [Google Scholar] [CrossRef]
- Collantes-Fernández, E.; Moreno-Gonzalo, J.; Sánchez-Sánchez, R.; García-Bocanegra, I.; Horcajo, P.; Ortega-Mora, L.M. Prevalence of bovine trichomonosis and associated risk factors in bulls from Spanish beef herds. Theriogenology 2019, 128, 116–121. [Google Scholar] [CrossRef]
- D’Occhio, M.J.; Baruselli, P.S.; Campanile, G. Influence of nutrition, body condition, and metabolic status on reproduction in female beef cattle: A review. Theriogenology 2019, 125, 277–284. [Google Scholar] [CrossRef]
- Butler, M.L.; Bormann, J.M.; Weaber, R.L.; Grieger, D.M.; Rolf, M.M. Selection for bull fertility: A review. Transl. Anim. Sci. 2020, 4, 423–441. [Google Scholar] [CrossRef] [Green Version]
- Barth, A.D. Review: The use of bull breeding soundness evaluation to identify subfertile and infertile bulls. Animal 2018, 12, S158–S164. [Google Scholar] [CrossRef]
- Johnston, D.J.; Barwick, S.A.; Fordyce, G.; Holroyd, R.G.; Williams, P.J.; Corbet, N.J.; Grant, T. Genetics of early and lifetime annual reproductive performance in cows of two tropical beef genotypes in northern Australia. Anim. Prod. Sci. 2014, 54, 1. [Google Scholar] [CrossRef]
- Rioja-Lang, F.C.; Connor, M.; Bacon, H.J.; Lawrence, A.B.; Dwyer, C.M. Prioritization of Farm Animal Welfare Issues Using Expert Consensus. Front. Vet. Sci. 2020, 6, 495. [Google Scholar] [CrossRef] [PubMed]
- Dobson, H.; Tebble, J.E.; Smith, R.F.; Ward, W.R. Is stress really all that important? Theriogenology 2001, 55, 65–73. [Google Scholar] [CrossRef]
- Bova, T.L.; Chiavaccini, L.; Cline, G.F.; Hart, C.G.; Matheny, K.; Muth, A.M.; Voelz, B.E.; Kesler, D.; Memili, E. Environmental stressors influencing hormones and systems physiology in cattle. Reprod. Biol. Endocrinol. 2014, 12, 58. [Google Scholar] [CrossRef] [Green Version]
- Von Borell, E.; Dobson, H.; Prunier, A. Stress, behaviour and reproductive performance in female cattle and pigs. Horm. Behav. 2007, 52, 130–138. [Google Scholar] [CrossRef]
- Broom, D.M. The scientific assessment of animal welfare. Appl. Anim. Behav. Sci. 1988, 20, 5–19. [Google Scholar] [CrossRef]
- Lynch, E.M. Characterisation of Physiological and Immune-Related Biomarkers of Weaning Stress in Beef Cattle. Ph.D. Thesis, National University of Ireland, Maynooth, Ireland, 2010. [Google Scholar]
- Chen, Y.; Arsenault, R.; Napper, S.; Griebel, P. Models and Methods to Investigate Acute Stress Responses in Cattle. Animals 2015, 5, 1268–1295. [Google Scholar] [CrossRef]
- Cooke, R.F.; Bohnert, D.W.; Cappellozza, B.I.; Mueller, C.J.; Delcurto, T. Effects of temperament and acclimation to handling on reproductive performance of Bos taurus beef females. J. Anim. Sci. 2012, 90, 3547–3555. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Asay, M.; Schroeder, S.; Kasimanickam, V.; Gay, J.; Kastelic, J.; Hall, J.; Whittier, W. Calm Temperament Improves Reproductive Performance of Beef Cows. Reprod. Dom. Anim. 2014, 49, 1063–1067. [Google Scholar] [CrossRef]
- Rivier, C.; Rivest, S. Effect of Stress on the Activity of the Hypothalamic-Pituitary-Gonadal Axis: Peripheral and Central Mechanisms. Biol. Reprod. 1991, 45, 523–532. [Google Scholar] [CrossRef]
- Tilbrook, A. Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Rev. Reprod. 2000, 5, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. A Syndrome produced by Diverse Nocuous Agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef]
- Collier, R.J.; Renquist, B.J.; Xiao, Y. A 100-Year Review: Stress physiology including heat stress. J. Dairy Sci. 2017, 100, 10367–10380. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare, 1st ed.; CABI Pub: Wallingford, CT, USA, 2000; pp. 309–377. ISBN 978-0-85199-359-1. [Google Scholar]
- Brown, E.J.; Vosloo, A. The involvement of the hypothalamopituitary-adrenocortical axis in stress physiology and its significance in the assessment of animal welfare in cattle. Onderstepoort J. Vet. Res. 2017, 84. [Google Scholar] [CrossRef]
- Collier, R.J.; Gebremedhin, K.G. Thermal Biology of Domestic Animals. Annu. Rev. Anim. Biosci. 2015, 3, 513–532. [Google Scholar] [CrossRef]
- Hughes, H.D.; Carroll, J.A.; Sanchez, N.C.B.; Richeson, J.T. Natural variations in the stress and acute phase responses of cattle. Innate Immun. 2014, 20, 888–896. [Google Scholar] [CrossRef] [Green Version]
- Olson, C.A.; Carstens, G.E.; Herring, A.D.; Hale, D.S.; Kayser, W.C.; Miller, R.K. Effects of temperament at feedlot arrival and breed type on growth efficiency, feeding behavior, and carcass value in finishing heifers. J. Anim. Sci. 2019, 97, 1828–1839. [Google Scholar] [CrossRef]
- Dahl, G.E.; Tao, S.; Laporta, J. Heat Stress Impacts Immune Status in Cows Across the Life Cycle. Front. Vet. Sci. 2020, 7, 116. [Google Scholar] [CrossRef] [Green Version]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef]
- Fike, K.; Spire, M.F. Transportation of Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2006, 22, 305–320. [Google Scholar] [CrossRef]
- Wigham, E.E.; Butterworth, A.; Wotton, S. Assessing cattle welfare at slaughter—Why is it important and what challenges are faced? Meat Sci. 2018, 145, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enríquez, D.; Hötzel, M.J.; Ungerfeld, R. Minimising the stress of weaning of beef calves: A review. Acta Vet. Scand. 2011, 53, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, B.K.; Richards, C.J.; Step, D.L.; Krehbiel, C.R. Beef Species Symposium: Best management practices for newly weaned calves for improved health and well-being. J. Anim. Sci. 2017, 95, 2170–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orihuela, A.; Galina, C.S. Effects of Separation of Cows and Calves on Reproductive Performance and Animal Welfare in Tropical Beef Cattle. Animals 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aich, P.; Jalal, S.; Czuba, C.; Schatte, G.; Herzog, K.; Olson, D.J.H.; Ross, A.R.S.; Potter, A.A.; Babiuk, L.A.; Griebel, P. Comparative Approaches to the Investigation of Responses to Stress and Viral Infection in Cattle. Omics J. Integr. Biol. 2007, 11, 413–434. [Google Scholar] [CrossRef]
- Wong, D.L.; Tank, A.W. Stress-induced catecholaminergic function: Transcriptional and post-transcriptional control. Stress 2007, 10, 121–130. [Google Scholar] [CrossRef]
- Lay, D.C.; Friend, T.H.; Randel, R.D.; Jenkins, O.C.; Neuendorff, D.A.; Kapp, G.M.; Bushong, D.M. Adrenocorticotropic hormone dose response and some physiological effects of transportation on pregnant Brahman cattle. J. Anim. Sci. 1996, 74, 1806. [Google Scholar] [CrossRef]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the hypothalamic–pituitary–adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Kumar, B. Stress and its impact on farm animals. Front. Biosci. 2012, E4, 1759–1767. [Google Scholar] [CrossRef]
- Minton, J.E. Function of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in models of acute stress in domestic farm animals. J. Anim. Sci. 1994, 72, 1891–1898. [Google Scholar] [CrossRef]
- Burdick, N.C.; Randel, R.D.; Carroll, J.A.; Welsh, T.H. Interactions between Temperament, Stress, and Immune Function in Cattle. Int. J. Zool. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dobson, H.; Ribadu, A.Y.; Noble, K.M.; Tebble, J.E.; Ward, W.R. Ultrasonography and hormone profiles of adrenocorticotrophic hormone (ACTH)-induced persistent ovarian follicles (cysts) in cattle. J. Reprod. Fertil. 2000, 120, 405–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squires, J.E. Effect on animal behavior, health and welfare. In Applied Animal Endocrinology, 1st ed.; CAB International: Oxfordshire, UK, 2003; pp. 215–217. ISBN 0-85199-594-2. [Google Scholar]
- Hein, K.G.; Allrich, R.D. Influence of exogenous adrenocorticotropic hormone on estrous behavior in cattle. J. Anim. Sci. 1992, 70, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Sapolsky, R.M. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; Cidlowski, J.A. One Hormone, Two Actions: Anti- and Pro-Inflammatory Effects of Glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef]
- Cooke, R.F.; Bohnert, D.W. Technical note: Bovine acute-phase response after corticotrophin-release hormone challenge. J. Anim. Sci. 2011, 89, 252–257. [Google Scholar] [CrossRef]
- Hodgson, P.D.; Aich, P.; Stookey, J.; Popowych, Y.; Potter, A.; Babiuk, L.; Griebel, P.J. Stress significantly increases mortality following a secondary bacterial respiratory infection. Vet. Res. 2012, 43, 21. [Google Scholar] [CrossRef] [Green Version]
- Lomborg, S.R.; Nielsen, L.R.; Heegaard, P.M.H.; Jacobsen, S. Acute phase proteins in cattle after exposure to complex stress. Vet. Res. Commun. 2008, 32, 575–582. [Google Scholar] [CrossRef]
- Kelley, R. Zebu-Cross Cattle in Northern Australia: An Ecological Experiment, 1st ed.; C.S.L.R. Aust. Bull.: Melbourne, Australia, 1943; Volume 172, pp. 7–27. [Google Scholar]
- Brownlee, A. Studies in the behaviour of domestic cattle in Britain. Bull. Anim. Behav. 1950, 8, 11–20. [Google Scholar]
- Fraser, A.F. The state of fight or flight in the bull. Anim. Behav. 1957, 5, 48–49. [Google Scholar] [CrossRef]
- Schloeth, R. Quelques moyens d’intercommunication des taureaux de Camargue. Terre Et Vie 1956, 2, 83–93. [Google Scholar]
- Tulloh, N.M. Behaviour of cattle in yards. II. A study of temperament. Anim. Behav. 1961, 9, 25–30. [Google Scholar] [CrossRef]
- Heamshaw’, H.; Barlow, R.; Want, G. Development of a temperament or handling difficulty score for cattle. Proc. Assoc. Advmt. Anim. Breed. Genet. 1979, 1, 164–166. [Google Scholar]
- Fordyce, G.; Dodt, R.; Wythes, J. Cattle temperaments in extensive beef herds in northern Queensland. Factors affecting temperament. Aust. J. Exp. Agric. 1988, 28, 683. [Google Scholar] [CrossRef]
- Grandin, T. Behavioral agitation during handling of cattle is persistent over time. Appl. Anim. Behav. Sci. 1993, 36, 1–9. [Google Scholar] [CrossRef]
- Curley, K.O.; Paschal, J.C.; Welsh, T.H.; Randel, R.D. Technical note: Exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 2006, 84, 3100–3103. [Google Scholar] [CrossRef]
- Bruno, K.; Vanzant, E.; Vanzant, K.; Altman, A.; Kudupoje, M.; McLeod, K. Relationship between quantitative measures of temperament and other observed behaviors in growing cattle. Appl. Anim. Behav. Sci. 2018, 199, 59–66. [Google Scholar] [CrossRef]
- Stookey, J.; Nickel, T.; Hanson, J.; Vandenbosch, S. A movement-measuring-device for objectively measuring temperament in beef cattle and for use in determining factors that influence handling. J. Anim. Sci. 1994, 72, 207. [Google Scholar]
- Le Neindre, P.; Trillat, G.; Sapa, J.; Ménissier, F.; Bonnet, J.N.; Chupin, J.M. Individual differences in docility in Limousin cattle. J. Anim. Sci. 1995, 73, 2249–2253. [Google Scholar] [CrossRef] [Green Version]
- Schwartzkopf-Genswein, K.S.; Stookey, J.M.; Welford, R. Behavior of cattle during hot-iron and freeze branding and the effects on subsequent handling ease. J. Anim. Sci. 1997, 75, 2064. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.P.; Navajas, E.A.; Hyslop, J.J.; Ross, D.W.; Richardson, R.I.; Prieto, N.; Bell, M.; Jack, M.C.; Roehe, R. Associations between response to handling and growth and meat quality in frequently handled Bos taurus beef cattle. J. Anim. Sci. 2011, 89, 4239–4248. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, A.C.; Paranhos da Costa, M.J.R. Validity and feasibility of qualitative behavior assessment for the evaluation of Nellore cattle temperament. Livest. Sci. 2013, 157, 254–262. [Google Scholar] [CrossRef]
- Yu, H.; Morota, G.; Celestino, E.F.; Dahlen, C.R.; Wagner, S.A.; Riley, D.G.; Hulsman Hanna, L.L. Deciphering Cattle Temperament Measures Derived From a Four-Platform Standing Scale Using Genetic Factor Analytic Modeling. Front. Genet. 2020, 11, 599. [Google Scholar] [CrossRef]
- Galán, E.; Llonch, P.; Villagrá, A.; Levit, H.; Pinto, S.; Del Prado, A. A systematic review of non-productivity-related animal-based indicators of heat stress resilience in dairy cattle. PLoS ONE 2018, 13, e0206520. [Google Scholar] [CrossRef]
- Platz, S.; Ahrens, F.; Bahrs, E.; Nüske, S.; Erhard, M.H. Association between floor type and behaviour, skin lesions, and claw dimensions in group-housed fattening bulls. Prev. Vet. Med. 2007, 80, 209–221. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. Hiv Aids 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Allen, B.J.; Rogers, S.D.; Ghilardi, J.R.; Menning, P.M.; Kuskowski, M.A.; Basbaum, A.I.; Simone, D.A.; Mantyh, P.W. Noxious Cutaneous Thermal Stimuli Induce a Graded Release of Endogenous Substance P in the Spinal Cord: Imaging Peptide Action In Vivo. J. Neurosci. 1997, 17, 5921–5927. [Google Scholar] [CrossRef] [Green Version]
- DeVane, C.L. Substance P: A New Era, a New Role. Pharmacotherapy 2001, 21, 1061–1069. [Google Scholar] [CrossRef]
- Coetzee, J.F. A review of pain assessment techniques and pharmacological approaches to pain relief after bovine castration: Practical implications for cattle production within the United States. Appl. Anim. Behav. Sci. 2011, 135, 192–213. [Google Scholar] [CrossRef]
- Petersen, H.H.; Nielsen, J.P.; Heegaard, P.M.H. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 2004, 35, 163–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroedl, W.; Fuerll, B.; Reinhold, P.; Krueger, M.; Schuett, C. A novel acute phase marker in cattle: Lipopolysaccharide binding protein (LBP). J. Endotoxin Res. 2001, 7, 49–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Mazer, K.A.; Knickerbocker, P.L.; Kutina, K.L.; Huzzey, J.M. Changes in behavior and fecal cortisol metabolites when dairy cattle are regrouped in pairs versus individually after calving. J. Dairy Sci. 2020, 103, 4681–4690. [Google Scholar] [CrossRef] [PubMed]
- Heimbürge, S.; Kanitz, E.; Tuchscherer, A.; Otten, W. Within a hair’s breadth—Factors influencing hair cortisol levels in pigs and cattle. Gen. Comp. Endocrinol. 2020, 288, 113359. [Google Scholar] [CrossRef]
- Stroud, L.R.; Solomon, C.; Shenassa, E.; Papandonatos, G.; Niaura, R.; Lipsitt, L.P.; LeWinn, K.; Buka, S.L. Long-term stability of maternal prenatal steroid hormones from the National Collaborative Perinatal Project: Still valid after all these years. Psychoneuroendocrinology 2007, 32, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Kirschbaum, C.; Hellhammer, D.H. Salivary Cortisol in Psychobiological Research: An Overview. Neuropsychobiology 1989, 22, 150–169. [Google Scholar] [CrossRef]
- Garde, A.H.; Hansen, Å.M. Long-term stability of salivary cortisol. Scand. J. Clin. Lab. Investig. 2005, 65, 433–436. [Google Scholar] [CrossRef]
- Palmer, R.; Mostl, E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Proc. Z. Saugetierkd. 1997, 62, 192–197. [Google Scholar]
- Palme, R.; Robla, C.; Messmann, S.; Hofer, J.; Möstl, E. Measurement of faecal cortisol metabolites in ruminants: A non-invasive parameter of adrenocortical function. Wien. Tierarztl. Monatsschr. 1999, 86, 237–241. [Google Scholar]
- Yoshioka, M.; Watanabe, A.; Shimada, N.; Murata, H.; Yokomizo, Y.; Nakajima, Y. Regulation of haptoglobin secretion by recombinant bovine cytokines in primary cultured bovine hepatocytes. Domest. Anim. Endocrinol. 2002, 23, 425–433. [Google Scholar] [CrossRef]
- Morimatsu, M.; Sarikaputi, M.; Syuto, B.; Saito, M.; Yamamoto, S.; Naiki, M. Bovine haptoglobin: Single radial immunodiffusion assay of its polymeric forms and dramatic rise in acute-phase sera. Vet. Immunol. Immunopathol. 1992, 33, 365–372. [Google Scholar] [CrossRef]
- Coetzee, J.F.; Lubbers, B.V.; Toerber, S.E.; Gehring, R.; Thomson, D.U.; White, B.J.; Apley, M.D. Plasma concentrations of substance P and cortisol in beef calves after castration or simulated castration. Am. J. Vet. Res. 2008, 69, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Barragan, A.A.; Piñeiro, J.M.; Schuenemann, G.M.; Rajala-Schultz, P.J.; Sanders, D.E.; Lakritz, J.; Bas, S. Assessment of daily activity patterns and biomarkers of pain, inflammation, and stress in lactating dairy cows diagnosed with clinical metritis. J. Dairy Sci. 2018, 101, 8248–8258. [Google Scholar] [CrossRef]
- Lauder, J.K.; Marti, S.; Schwartzkopf-Genswein, K.S.; Jelinski, M.D.; Janzen, E.D. Measuring behavioral and physiological responses to pain mitigation for ovariectomy in Bos taurus yearling beef heifers. J. Anim. Sci. 2020, 98, skz386. [Google Scholar] [CrossRef] [PubMed]
- Tschoner, T.S.; Zablotski, Y.; Knubben-Schweizer, G.; Feist, M. Effect of xylazine administration before laparoscopic abomasopexy to correct left displaced abomasum on markers of stress in dairy cows. J. Dairy Sci. 2020, 103, 9318–9331. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, V.R.; Staker, C.; Williams, H.M.; Kastelic, J.P.; Kasimanickam, R.K. Aggressive attempted escape behavior during head-lock restraint reduced reproductive performances in Holstein heifers. Theriogenology 2018, 121, 147–152. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Schroeder, S.; Assay, M.; Kasimanickam, V.; Moore, D.; Gay, J.; Whittier, W. Influence of Temperament Score and Handling Facility on Stress, Reproductive Hormone Concentrations, and Fixed Time AI Pregnancy Rates in Beef Heifers. Reprod. Dom. Anim. 2014, 49, 775–782. [Google Scholar] [CrossRef]
- Ogino, M.; Matsuura, A.; Yamazaki, A.; Irimajiri, M.; Suzuki, Y.; Kushibiki, S.; Singu, H.; Kasuya, E.; Hasegawa, Y.; Hodate, K. Plasma cortisol and prolactin secretion rhythms in cattle under varying external environments and management techniques. Anim. Sci. J. 2014, 85, 58–68. [Google Scholar] [CrossRef]
- Lay, D.C.; Friend, T.H.; Randel, R.D.; Bowers, C.L.; Grissom, K.K.; Jenkins, O.C. Behavioral and physiological effects of freeze or hot-iron branding on crossbred cattle. J. Anim. Sci. 1992, 70, 330–336. [Google Scholar] [CrossRef]
- Grandin, T. Handling Methods and Facilities to Reduce Stress on Cattle. Vet. Clin. N. Am. Food Anim. Pract. 1998, 14, 325–341. [Google Scholar] [CrossRef]
- Krohn, C.C.; Jago, J.G.; Boivin, X. The effect of early handling on the socialisation of young calves to humans. Appl. Anim. Behav. Sci. 2001, 74, 121–133. [Google Scholar] [CrossRef]
- Grandin, T. Assessment of stress during handling and transport. J. Anim. Sci. 1997, 75, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmutz, S.M.; Stookey, J.M.; Winkelman-Sim, D.C.; Waltz, C.S.; Plante, Y.; Buchanan, F.C. A QTL Study of Cattle Behavioral Traits in Embryo Transfer Families. J. Hered. 2001, 92, 290–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasimanickam, V.; Abdel Aziz, R.; Williams, H.; Kasimanickam, R. Predictors of beef calf temperament at weaning and its impact on temperament at breeding and reproductive performance. Reprod. Dom. Anim. 2018, 53, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.F.; Bill, E. Kunkle Interdisciplinary Beef Symposium: Temperament and acclimation to human handling influence growth, health, and reproductive responses in Bos taurus and Bos indicus cattle. J. Anim. Sci. 2014, 92, 5325–5333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, R.F.; Schubach, K.M.; Marques, R.S.; Peres, R.F.G.; Silva, L.G.T.; Carvalho, R.S.; Cipriano, R.S.; Bohnert, D.W.; Pires, A.V.; Vasconcelos, J.L.M. Effects of temperament on physiological, productive, and reproductive responses in beef cows. J. Anim. Sci. 2017, 95, 1. [Google Scholar] [CrossRef] [Green Version]
- Cooke, R.F.; Arthington, J.D.; Araujo, D.B.; Lamb, G.C. Effects of acclimation to human interaction on performance, temperament, physiological responses, and pregnancy rates of Brahman-crossbred cows. J. Anim. Sci. 2009, 87, 4125–4132. [Google Scholar] [CrossRef] [Green Version]
- Cooke, R.F.; Arthington, J.D.; Austin, B.R.; Yelich, J.V. Effects of acclimation to handling on performance, reproductive, and physiological responses of Brahman-crossbred heifers. J. Anim. Sci. 2009, 87, 3403–3412. [Google Scholar] [CrossRef] [Green Version]
- Cooke, R.F.; Moriel, P.; Cappellozza, B.I.; Miranda, V.F.B.; Batista, L.F.D.; Colombo, E.A.; Ferreira, V.S.M.; Miranda, M.F.; Marques, R.S.; Vasconcelos, J.L.M. Effects of temperament on growth, plasma cortisol concentrations and puberty attainment in Nelore beef heifers. Animal 2019, 13, 1208–1213. [Google Scholar] [CrossRef]
- Cafe, L.M.; Robinson, D.L.; Ferguson, D.M.; McIntyre, B.L.; Geesink, G.H.; Greenwood, P.L. Cattle temperament: Persistence of assessments and associations with productivity, efficiency, carcass and meat quality traits. J. Anim. Sci. 2011, 89, 1452–1465. [Google Scholar] [CrossRef]
- Bouissou, M.F. Influence of body weight and presence of horns on social rank in domestic cattle. Anim. Behav. 1972, 20, 474–477. [Google Scholar] [CrossRef]
- Šárová, R.; Špinka, M.; Panamá, J.L.A.; Šimeček, P. Graded leadership by dominant animals in a herd of female beef cattle on pasture. Anim. Behav. 2010, 79, 1037–1045. [Google Scholar] [CrossRef]
- Šárová, R.; Špinka, M.; Stěhulová, I.; Ceacero, F.; Šimečková, M.; Kotrba, R. Pay respect to the elders: Age, more than body mass, determines dominance in female beef cattle. Anim. Behav. 2013, 86, 1315–1323. [Google Scholar] [CrossRef] [Green Version]
- Solano, J.; Galindo, F.; Orihuela, A.; Galina, C.S. The effect of social rank on the physiological response during repeated stressful handling in Zebu cattle (Bos indicus). Physiol. Behav. 2004, 82, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Šárová, R.; Špinka, M.; Ceacero, F. Higher dominance position does not result in higher reproductive success in female beef cattle. J. Anim. Sci. 2017, 95, 3301–3309. [Google Scholar] [CrossRef]
- Newberry, R.C.; Swanson, J.C. Implications of breaking mother–young social bonds. Appl. Anim. Behav. Sci. 2008, 110, 3–23. [Google Scholar] [CrossRef]
- Poindron, P. Mechanisms of activation of maternal behaviour in mammals. Reprod. Nutr. Dev. 2005, 45, 341–351. [Google Scholar] [CrossRef]
- Price, E.O.; Harris, J.E.; Borgward, R.E.; Sween, M.L.; Connor, J.M. Fenceline contact of beef calves with their dams at weaning reduces the negative effects of separation on behavior and growth rate. J. Anim. Sci. 2003, 81, 116–121. [Google Scholar] [CrossRef]
- Lynch, E.; Earley, B.; McGee, M.; Doyle, S. Effect of abrupt weaning at housing on leukocyte distribution, functional activity of neutrophils, and acute phase protein response of beef calves. BMC Vet. Res. 2010, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- McNeilly, A.S.; Glasier, A.F.; Howie, P.W.; Houston, M.J.; Cook, A.; Boyle, H. Fertility after childbirth: Pregnancy associated with breast feeding. Clin. Endocrinol. 1983, 19, 167–173. [Google Scholar] [CrossRef]
- Pérez, L.I.; Orihuela, A.; Galina, C.S.; Rubio, I.; Corro, M.; Cohen, A.; Hernández, A. Effect of different periods of maternal deprivation on behavioral and cortisol responses at weaning and subsequent growth rate in zebu (Bos indicus) type cattle. Livest. Sci. 2017, 197, 17–21. [Google Scholar] [CrossRef]
- Short, R.E.; Bellows, R.A.; Staigmiller, R.B.; Berardinelli, J.G.; Custer, E.E. Physiological mechanisms controlling anestrus and infertility in postpartum beef cattle. J. Anim. Sci. 1990, 68, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montiel, F.; Ahuja, C. Body condition and suckling as factors influencing the duration of postpartum anestrus in cattle: A review. Anim. Reprod. Sci. 2005, 85, 1–26. [Google Scholar] [CrossRef]
- Funston, R.N.; Summers, A.F. Effect of Prenatal Programming on Heifer Development. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Gasser, C.L. Joint Alpharma-beef Species Symposium: Considerations on puberty in replacement beef heifers. J. Anim. Sci. 2013, 91, 1336–1340. [Google Scholar] [CrossRef]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A Body Condition Scoring Chart for Holstein Dairy Cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Morrison, D.G.; Spitzer, J.C.; Perkins, J.L. Influence of prepartum body condition score change on reproduction in multiparous beef cows calving in moderate body condition. J. Anim. Sci. 1999, 77, 1048. [Google Scholar] [CrossRef] [Green Version]
- Ayres, H.; Ferreira, R.M.; Torres-Júnior, J.R.S.; Demétrio, C.G.B.; Sá Filho, M.F.; Gimenes, L.U.; Penteado, L.; D’Occhio, M.J.; Baruselli, P.S. Inferences of body energy reserves on conception rate of suckled Zebu beef cows subjected to timed artificial insemination followed by natural mating. Theriogenology 2014, 82, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Ginther, O.J. The theory of follicle selection in cattle. Domest. Anim. Endocrinol. 2016, 57, 85–99. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, J.A.; Sanz, A.; Ferrer, J.; Casasús, I. Influence of postweaning feeding management of beef heifers on performance and physiological profiles through rearing and first lactation. Domest. Anim. Endocrinol. 2018, 65, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Tena-Sempere, M. KiSS-1 and Reproduction: Focus on Its Role in the Metabolic Regulation of Fertility. Neuroendocrinology 2006, 83, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Odle, A.K.; Akhter, N.; Syed, M.M.; Allensworth-James, M.L.; Beneš, H.; Melgar Castillo, A.I.; MacNicol, M.C.; MacNicol, A.M.; Childs, G.V. Leptin Regulation of Gonadotrope Gonadotropin-Releasing Hormone Receptors As a Metabolic Checkpoint and Gateway to Reproductive Competence. Front. Endocrinol. 2018, 8, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wertz-Lutz, A.E.; Knight, T.J.; Pritchard, R.H.; Daniel, J.A.; Clapper, J.A.; Smart, A.J.; Trenkle, A.; Beitz, D.C. Circulating ghrelin concentrations fluctuate relative to nutritional status and influence feeding behavior in cattle. J. Anim. Sci. 2006, 84, 3285–3300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funston, R.N. Fat supplementation and reproduction in beef females. J. Anim. Sci. 2004, 82, E154–E161. [Google Scholar] [CrossRef]
- Warzych, E.; Pawlak, P.; Pszczola, M.; Cieslak, A.; Lechniak, D. Prepubertal heifers versus cows—The differences in the follicular environment. Theriogenology 2017, 87, 36–47. [Google Scholar] [CrossRef]
- Thatcher, W.; Santos, J.E.P.; Staples, C.R. Dietary manipulations to improve embryonic survival in cattle. Theriogenology 2011, 76, 1619–1631. [Google Scholar] [CrossRef]
- Lees, A.M.; Sejian, V.; Wallage, A.L.; Steel, C.C.; Mader, T.L.; Lees, J.C.; Gaughan, J.B. The Impact of Heat Load on Cattle. Animals 2019, 9, 322. [Google Scholar] [CrossRef] [Green Version]
- Sabés-Alsina, M.; Lundeheim, N.; Johannisson, A.; López-Béjar, M.; Morrell, J.M. Relationships between climate and sperm quality in dairy bull semen: A retrospective analysis. J. Dairy Sci. 2019, 102, 5623–5633. [Google Scholar] [CrossRef]
- St-Pierre, N.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries. J. Dairy Sci. 2003, 86, 52–77. [Google Scholar] [CrossRef] [Green Version]
- Polsky, L.; von Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.; Orellana, R.M.; Weng, X.; Marins, T.N.; Dahl, G.E.; Bernard, J.K. Symposium review: The influences of heat stress on bovine mammary gland function. J. Dairy Sci. 2018, 101, 5642–5654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Abilay, T.A.; Johnson, H.D.; Madan, M. Influence of Environmental Heat on Peripheral Plasma Progesterone and Cortisol During the Bovine Estrous Cycle. J. Dairy Sci. 1975, 58, 1836–1840. [Google Scholar] [CrossRef]
- Wise, M.E.; Armstrong, D.V.; Huber, J.T.; Hunter, R.; Wiersma, F. Hormonal Alterations in the Lactating Dairy Cow in Response to Thermal Stress. J. Dairy Sci. 1988, 71, 2480–2485. [Google Scholar] [CrossRef]
- Gwazdauskas, F.C.; Thatcher, W.W.; Kiddy, C.A.; Paape, M.J.; Wilcox, C.J. Hormonal patterns during heat stress following PGF2α-tham salt induced luteal regression in heifers. Theriogenology 1981, 16, 271–285. [Google Scholar] [CrossRef]
- Collier, R.J.; Doelger, S.G.; Head, H.H.; Thatcher, W.W.; Wilcox, C.J. Effects of Heat Stress during Pregnancy on Maternal Hormone Concentrations, Calf Birth Weight and Postpartum Milk Yield of Holstein Cows. J. Anim. Sci. 1982, 54, 309–319. [Google Scholar] [CrossRef]
- Badinga, L.; Thatcher, W.W.; Diaz, T.; Drost, M.; Wolfenson, D. Effect of environmental heat stress on follicular development and steroidogenesis in lactating Holstein cows. Theriogenology 1993, 39, 797–810. [Google Scholar] [CrossRef]
- Wilson, S.J.; Marion, R.S.; Spain, J.N.; Spiers, D.E.; Keisler, D.H.; Lucy, M.C. Effects of Controlled Heat Stress on Ovarian Function of Dairy Cattle. Lactating Cows. J. Dairy Sci. 1998, 81, 2124–2131. [Google Scholar] [CrossRef]
- Wilson, S.J.; Kirby, C.J.; Koenigsfeld, A.T.; Keisler, D.H.; Lucy, M.C. Effects of Controlled Heat Stress on Ovarian Function of Dairy Cattle. Heifers. J. Dairy Sci. 1998, 81, 2132–2138. [Google Scholar] [CrossRef]
- Biggers, B.G.; Geisert, R.D.; Wetteman, R.P.; Buchanan, D.S. Effect of Heat Stress on Early Embryonic Development in the Beef Cow. J. Anim. Sci. 1987, 64, 1512–1518. [Google Scholar] [CrossRef] [Green Version]
- Burke, J.M.; Spiers, D.E.; Kojima, F.N.; Perry, G.A.; Salfen, B.E.; Wood, S.L.; Patterson, D.J.; Smith, M.F.; Lucy, M.C.; Jackson, W.G.; et al. Interaction of Endophyte-Infected Fescue and Heat Stress on Ovarian Function in the Beef Heifer. Biol. Reprod. 2001, 65, 260–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadokawa, H.; Sakatani, M.; Hansen, P.J. Perspectives on improvement of reproduction in cattle during heat stress in a future Japan: New perspectives on heat stress in cattle. Anim. Sci. J. 2012, 83, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Landaeta-Hernández, A.J.; Yelich, J.V.; Lemaster, J.W.; Fields, M.J.; Tran, T.; Chase, C.C.; Rae, D.O.; Chenoweth, P.J. Environmental, genetic and social factors affecting the expression of estrus in beef cows. Theriogenology 2002, 57, 1357–1370. [Google Scholar] [CrossRef]
- White, F.J.; Wettemann, R.P.; Looper, M.L.; Prado, T.M.; Morgan, G.L. Seasonal effects on estrous behavior and time of ovulation in nonlactating beef cows. J. Anim. Sci. 2002, 80, 3053–3059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, E.C.; Boehmer, B.H.; Cooper-Prado, M.J.; Bailey, C.L.; Wettemann, R.P. Effect of elevated ambient temperature at parturition on duration of gestation, ruminal temperature, and endocrine function of fall-calving beef cows. J. Anim. Sci. 2014, 92, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Sakumoto, R.; Hayashi, K.-G.; Saito, S.; Kanahara, H.; Kizaki, K.; Iga, K. Comparison of the global gene expression profiles in the bovine endometrium between summer and autumn. J. Reprod. Dev. 2015, 61, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.E.P.; Thatcher, W.W.; Chebel, R.C.; Cerri, R.L.A.; Galvão, K.N. The effect of embryonic death rates in cattle on the efficacy of estrus synchronization programs. Anim. Reprod. Sci. 2004, 82–83, 513–535. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Baez, G.M.; Garcia-Guerra, A.; Toledo, M.Z.; Monteiro, P.L.J.; Melo, L.F.; Ochoa, J.C.; Santos, J.E.P.; Sartori, R. Pivotal periods for pregnancy loss during the first trimester of gestation in lactating dairy cows. Theriogenology 2016, 86, 239–253. [Google Scholar] [CrossRef]
- Fernandez-Novo, A.; Fargas, O.; Loste, J.M.; Sebastian, F.; Perez-Villalobos, N.; Pesantez-Pacheco, J.L.; Patron-Collantes, R.; Astiz, S. Pregnancy Loss (28–110 Days of Pregnancy) in Holstein Cows: A Retrospective Study. Animals 2020, 10, 925. [Google Scholar] [CrossRef]
- Eitam, H.; Brosh, A.; Orlov, A.; Izhaki, I.; Shabtay, A. Caloric stress alters fat characteristics and Hsp70 expression in milk somatic cells of lactating beef cows. Cell Stress Chaperones 2009, 14, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Dahlen, C.; Larson, J.; Lamb, G.C. Impacts of Reproductive Technologies on Beef Production in the United States. In Current and Future Reproductive Technologies and World Food Production, 1st ed.; Springer-Verlag Inc: New York, NY, USA, 2014; Volume 752, pp. 97–114. ISBN 978-1-4614-8886-6. [Google Scholar]
- Bó, G.A.; Baruselli, P.S. Synchronization of ovulation and fixed-time artificial insemination in beef cattle. Animal 2014, 8, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colazo, M.G.; Mapletoft, R.J. A review of current timed-AI (TAI) programs for beef and dairy cattle. Can. Vet. J. 2014, 55, 772–780. [Google Scholar] [PubMed]
- Looney, C.R.; Nelson, J.S.; Schneider, H.J.; Forrest, D.W. Improving fertility in beef cow recipients. Theriogenology 2006, 65, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, M.; Hasler, J.F.; Taponen, J. Factors affecting embryo production in superovulated Bos taurus cattle. Reprod. Fertil. Dev. 2020, 32, 104. [Google Scholar] [CrossRef]
- Edwards, L.M.; Rahe, C.H.; Griffin, J.L.; Wolfe, D.F.; Marple, D.N.; Cummins, K.A.; Pitchett, J.F. Effect of transportation stress on ovarian function in superovulated Hereford heifers. Theriogenology 1987, 28, 291–299. [Google Scholar] [CrossRef]
- Peixoto, M.G.C.D.; Fonseca, C.G.; Penna, V.M.; Alvim, M.T.T. Análise multivariada de resultados da ovulação múltipla seguida de transferência de embriões de doadoras zebuínas. Arq. Bras. Med. Vet. Zootec. 2002, 54, 492–500. [Google Scholar] [CrossRef]
- Biancucci, A.; Sbaragli, T.; Comin, A.; Sylla, L.; Monaci, M.; Peric, T.; Stradaioli, G. Reducing treatments in cattle superovulation protocols by combining a pituitary extract with a 5% hyaluronan solution: Is it able to diminish activation of the hypothalamic pituitary adrenal axis compared to the traditional protocol? Theriogenology 2016, 85, 914–921. [Google Scholar] [CrossRef]
- Imai, K.; Tagawa, M.; Yoshioka, H.; Matoba, S.; Narita, N.; Inaba, Y.; Aikawa, Y.; Ohtake, M.; Kobayashi, S. The efficiency of embryo production by ovum pick-up and in vitro fertilization in cattle. J. Reprod. Dev. 2006, 52, 19–29. [Google Scholar]
- Takuma, T.; Sakai, S.; Ezoe, D.; Ichimaru, H.; Jinnouchi, T.; Kaedei, Y.; Nagai, T.; Otoi, T. Effects of Season and Reproductive Phase on the Quality, Quantity and Developmental Competence of Oocytes Aspirated from Japanese Black Cows. J. Reprod. Dev. 2010, 56, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Binelli, M.; Thatcher, W.W.; Mattos, R.; Baruselli, P.S. Antiluteolytic strategies to improve fertility in cattle. Theriogenology 2001, 56, 1451–1463. [Google Scholar] [CrossRef]
- Purcell, S.H.; Beal, W.E.; Gray, K.R. Effect of a CIDR insert and flunixin meglumine, administered at the time of embryo transfer, on pregnancy rate and resynchronization of estrus in beef cattle. Theriogenology 2005, 64, 867–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geary, T.W.; Ansotegui, R.P.; MacNeil, M.D.; Roberts, A.J.; Waterman, R.C. Effects of flunixin meglumine on pregnancy establishment in beef cattle. J. Anim. Sci. 2010, 88, 943–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasimanickam, R.; Kasimanickam, V.; Gold, J.; Moore, D.; Kastelic, J.P.; Pyrdek, D.; Ratzburg, K. Injectable or transdermal flunixin meglumine improves pregnancy rates in embryo transfer recipient beef cows without altering returns to estrus. Theriogenology 2019, 140, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, R.K.; Hall, J.B.; Estill, C.T.; Kastelic, J.P.; Joseph, C.; Abdel Aziz, R.L.; Nak, D. Flunixin meglumine improves pregnancy rate in embryo recipient beef cows with an excitable temperament. Theriogenology 2018, 107, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Macedo, G.G.; Zúccari, C.E.S.N.; de Abreu, U.G.P.; Negrão, J.A.; da Costa e Silva, E.V. Human–animal interaction, stress, and embryo production in Bos indicus embryo donors under tropical conditions. Trop. Anim. Health. Prod. 2011, 43, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Marquezini, G.H.L.; Mercadante, V.R.G.; Olson, K.C.; Jaeger, J.R.; Perry, G.A.; Stevenson, J.S.; Lamb, G.C. Effects of equine chorionic gonadotropin on follicle development and pregnancy rates in suckled beef cows with or without calf removal. J. Anim. Sci. 2013, 91, 1216–1224. [Google Scholar] [CrossRef]
- De Medeiros Bastos, G.; Brenner, R.H.; Willke, F.W.; Neves, J.P.; de Oliveira, J.F.C.; Bragança, J.F.M.; Machado, S.A.; Porciúncula, P.M.; Gonçalves, P.B.D. Hormonal induction of ovulation and artificial insemination in suckled beef cows under nutritional stress. Theriogenology 2004, 62, 847–853. [Google Scholar] [CrossRef]
- Small, J.A.; Colazo, M.G.; Kastelic, J.P.; Mapletoft, R.J. Effects of progesterone presynchronization and eCG on pregnancy rates to GnRH-based, timed-AI in beef cattle. Theriogenology 2009, 71, 698–706. [Google Scholar] [CrossRef]
- Pincinato, D. Follicular Dynamics and Fertility in Beef Suckled Cows Synchronized with Progesterone Releasing Devices and GnRH; Faculty of Agriculture Sciences, National University of Cordoba: Cordoba, Argentina, 2012. [Google Scholar]
- Kawate, N.; Sakase, M.; Watanabe, K.; Fukushima, M.; Noda, M.; Takeda, K.; Ueno, S.; Inaba, T.; Kida, K.; Tamada, H.; et al. Ovsynch Plus CIDR Protocol for Timed Embryo Transfer in Suckled Postpartum Japanese Black Beef Cows. J. Reprod. Dev. 2007, 53, 811–817. [Google Scholar] [CrossRef] [Green Version]
- Baruselli, P.S.; Ferreira, R.M.; Vieira, L.M.; Souza, A.H.; Bó, G.A.; Rodrigues, C.A. Use of embryo transfer to alleviate infertility caused by heat stress. Theriogenology 2020, 155, 1–11. [Google Scholar] [CrossRef]
- Leite da Silva, W.A.; Poehland, R.; Carvalho de Oliveira, C.; Ribeiro Ferreira, M.G.C.; Garcia de Almeida, R.; Cáceres, M.B.S.; Macedo, G.G.; da Costa e Silva, E.V.; Alves, F.V.; Nogueira, E.; et al. Shading effect on physiological parameters and in vitro embryo production of tropical adapted Nellore heifers in integrated crop-livestock-forest systems. Trop. Anim. Health Prod. 2020, 52, 2273–2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fordyce, G.; Entwistle, K.; Norman, S.; Perry, V.; Gardiner, B.; Fordyce, P. Standardising bull breeding soundness evaluations and reporting in Australia. Theriogenology 2006, 66, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.D. Bull. Breeding Soundness, 3rd ed.; Western Canadian Association of Bovine Practitioners: Saskatoon, SK, Canada, 2013; p. 163. [Google Scholar]
- de Blockey, M.A.B. Observations on group mating of bulls at pasture. Appl. Anim. Ethol. 1979, 5, 15–34. [Google Scholar] [CrossRef]
- EU Beef Farms Report. 2012. Available online: https://ec.europa.eu/agriculture/rica/pdf/beef_report_2012.pdf (accessed on 8 October 2020).
- Miranda-de la Lama, G.C.; Pascual-Alonso, M.; Guerrero, A.; Alberti, P.; Alierta, S.; Sans, P.; Gajan, J.P.; Villarroel, M.; Dalmau, A.; Velarde, A.; et al. Influence of social dominance on production, welfare and the quality of meat from beef bulls. Meat Sci. 2013, 94, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, S.A.; Kattesh, H.G.; Rhinehart, J.D.; Strickland, L.G.; Krawczel, P.D.; Wilkerson, J.B.; Kirkpatrick, F.D.; Saxton, A.M. Relationships among temperament, acute and chronic cortisol and testosterone concentrations, and breeding soundness during performance testing of Angus bulls. Theriogenology 2017, 89, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rajak, S.; Kumar, P.; Kerketta, S.; Yogi, R. Nutrition and bull fertility: A review. J. Entomol. Zool. Stud. 2018, 6, 635–643. [Google Scholar]
- Barth, A.D.; Brito, L.F.C.; Kastelic, J.P. The effect of nutrition on sexual development of bulls. Theriogenology 2008, 70, 485–494. [Google Scholar] [CrossRef]
- Barth, A.D. Managing Bull Development to Optimize Fertility. In Proceedings of the Applied Reproductive Strategies in Beef Cattle Congress, Sioux Falls, SD, USA, 3–4 December 2012. [Google Scholar]
- Snoj, T.; Kobal, S.; Majdic, G. Effects of season, age, and breed on semen characteristics in different Bos taurus breeds in a 31-year retrospective study. Theriogenology 2013, 79, 847–852. [Google Scholar] [CrossRef]
- Brito, L.F.C.; Silva, A.E.D.F.; Rodrigues, L.H.; Vieira, F.V.; Deragon, L.A.G.; Kastelic, J.P. Effects of environmental factors, age and genotype on sperm production and semen quality in Bos indicus and Bos taurus AI bulls in Brazil. Anim. Reprod. Sci. 2002, 70, 181–190. [Google Scholar] [CrossRef]
- Morrell, J.M. Heat stress and bull fertility. Theriogenology 2020, 153, 62–67. [Google Scholar] [CrossRef]
- Llamas Luceño, N.; de Souza Ramos Angrimani, D.; de Cássia Bicudo, L.; Szymańska, K.J.; Van Poucke, M.; Demeyere, K.; Meyer, E.; Peelman, L.; Mullaart, E.; Broekhuijse, M.L.W.J.; et al. Exposing dairy bulls to high temperature-humidity index during spermatogenesis compromises subsequent embryo development in vitro. Theriogenology 2020, 141, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Al-Kanaan, A.; König, S.; Brügemann, K. Effects of heat stress on semen characteristics of Holstein bulls estimated on a continuous phenotypic and genetic scale. Livest. Sci. 2015, 177, 15–24. [Google Scholar] [CrossRef]
- Palmer, C.W. Welfare aspects of theriogenology: Investigating alternatives to electroejaculation of bulls. Theriogenology 2005, 64, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.D.; Arteaga, A.A.; Brito, L.F.C.; Palmer, C.W. Use of internal artificial vaginas for breeding soundness evaluation in range bulls: An alternative for electroejaculation allowing observation of sex drive and mating ability. Anim. Reprod. Sci. 2004, 84, 315–325. [Google Scholar] [CrossRef]
- Barth, A.D. Evaluation of Potential Breeding Soundness of the Bull. In Current Therapy in Large Animal Theriogenology, 2nd ed.; W.B. Saunders: Saint Louis, MO, USA, 2007; pp. 228–240. ISBN 978-0-7216-9323-1. [Google Scholar]
- Whitlock, B.K.; Coffman, E.A.; Coetzee, J.F.; Daniel, J.A. Electroejaculation increased vocalization and plasma concentrations of cortisol and progesterone, but not substance P, in beef bulls. Theriogenology 2012, 78, 737–746. [Google Scholar] [CrossRef]
- Falk, A.J.; Waldner, C.L.; Cotter, B.S.; Gudmundson, J.; Barth, A.D. Effects of epidural lidocaine anesthesia on bulls during electroejaculation. Can. Vet. J. 2001, 42, 116–120. [Google Scholar]
- Etson, C.J.; Waldner, C.L.; Barth, A.D. Evaluation of a segmented rectal probe and caudal epidural anesthesia for electroejaculation of bulls. Can. Vet. J. 2004, 45, 235–240. [Google Scholar]
- Pagliosa, R.C.; Derossi, R.; Costa, D.S.; Faria, F.J.C. Efficacy of caudal epidural injection of lidocaine, xylazine and xylazine plus hyaluronidase in reducing discomfort produced by electroejaculation in bulls. J. Vet. Med. Sci. 2015, 77, 1339–1345. [Google Scholar] [CrossRef] [Green Version]
- Kastelic, J.P.; Byrne Cook, R.; Coulter, G.H. Scrotal/Testicular Thermoregulation and the Effects of Increased Testicular Temperature in the Bull. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 271–282. [Google Scholar] [CrossRef]
- Hansen, P.J. Effects of heat stress on mammalian reproduction. Phil. Trans. R. Soc. B 2009, 364, 3341–3350. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Novo, A.; Pérez-Garnelo, S.S.; Villagrá, A.; Pérez-Villalobos, N.; Astiz, S. The Effect of Stress on Reproduction and Reproductive Technologies in Beef Cattle—A Review. Animals 2020, 10, 2096. https://doi.org/10.3390/ani10112096
Fernandez-Novo A, Pérez-Garnelo SS, Villagrá A, Pérez-Villalobos N, Astiz S. The Effect of Stress on Reproduction and Reproductive Technologies in Beef Cattle—A Review. Animals. 2020; 10(11):2096. https://doi.org/10.3390/ani10112096
Chicago/Turabian StyleFernandez-Novo, Aitor, Sonia S. Pérez-Garnelo, Arantxa Villagrá, Natividad Pérez-Villalobos, and Susana Astiz. 2020. "The Effect of Stress on Reproduction and Reproductive Technologies in Beef Cattle—A Review" Animals 10, no. 11: 2096. https://doi.org/10.3390/ani10112096
APA StyleFernandez-Novo, A., Pérez-Garnelo, S. S., Villagrá, A., Pérez-Villalobos, N., & Astiz, S. (2020). The Effect of Stress on Reproduction and Reproductive Technologies in Beef Cattle—A Review. Animals, 10(11), 2096. https://doi.org/10.3390/ani10112096





