Gonadectomy in Raccoons: Anesthetic and Cardiorespiratory Effects of Two Ketamine-Based Pre-Anesthetic Protocols before Sevoflurane-Sufentanil
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Procedures
2.2. Statistical Analysis
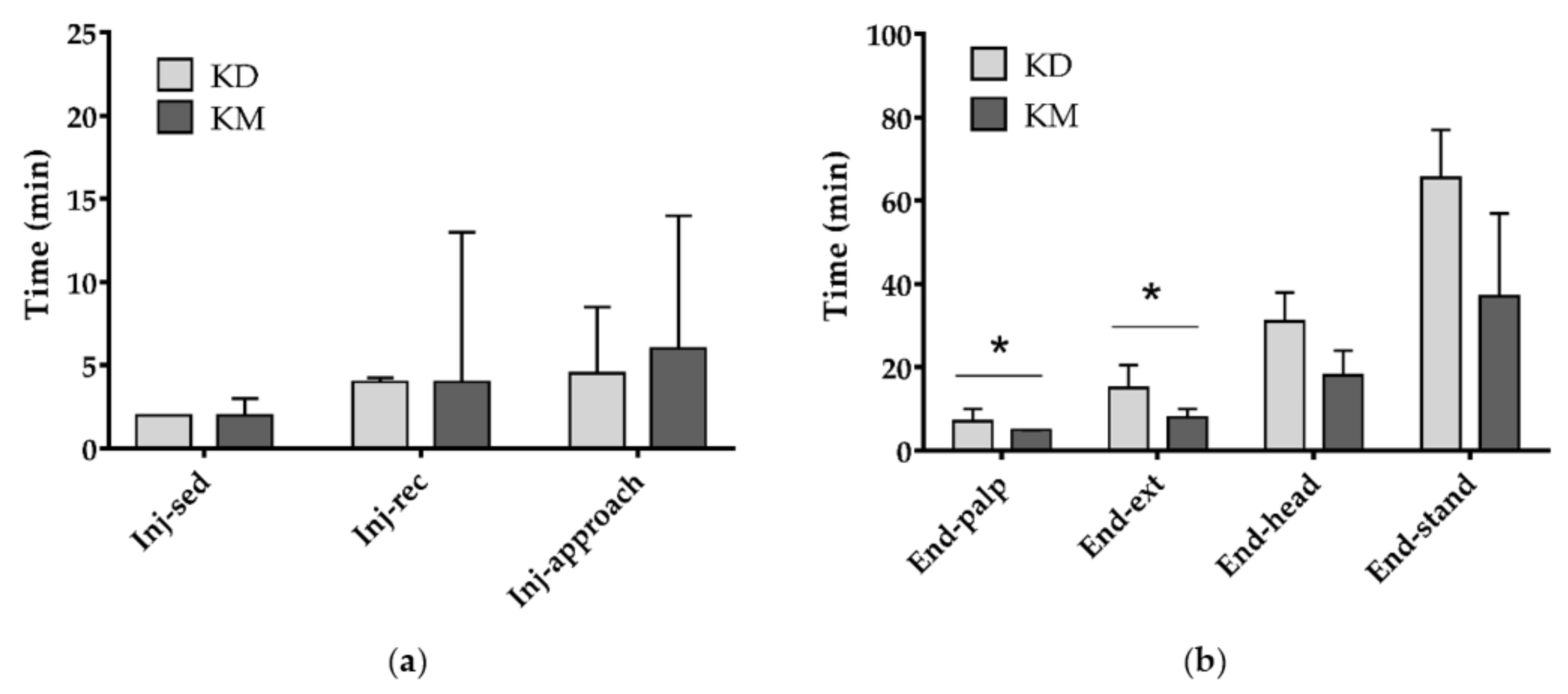
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Decreto del Ministero dell’Ambiente del 19 Aprile 1996. Elenco delle Specie Animali che Possono Costituire Pericolo per la Salute e L’incolumità Pubblica e di cui è Proibita la Detenzione. Available online: https://www.minambiente.it/sites/default/files/archivio/normativa/dim_19_04_1996_aggiornato_2001.pdf (accessed on 22 October 2020).
- Mazzamuto, M.V.; Wauters, L.A.; Bisi, F.; Martinoli, A. Procyon lotor. In Strategia di Azione e Degli Interventi per il Controllo e la Gestione delle Specie Alloctone in Regione Lombardia; Bisi, F., Montagnani, C., Cardarelli, E., Manenti, R., Trasforini, S., Gentili, R., Eds.; 2018; Available online: https://www.naturachevale.it/wp-content/uploads/2019/01/Strategia-per-il-controllo-e-la-gestione-delle-specie-aliene-invasive.pdf (accessed on 13 November 2020).
- Sorvillo, F.; Ash, L.; Berlin, O.G.V.; Morse, S.A. Baylisascaris procyonis: An emerging helminthic zoonosis. Emerg. Infect. Dis. 2002, 8, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duscher, T.; Hodžić, A.; Glawischnig, W.; Duscher, G.G. The raccoon dog (Nyctereutes procyonoides) and the raccoon (Procyon lotor)—Their role and impact of maintaining and transmitting zoonotic diseases in Austria, Central Europe. Parasitol. Res. 2017, 116, 1411–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathews, F.; Honess, P.; Wolfensohn, S. Use of inhalation anaesthesia for wild mammals in the field. Vet Rec. 2002, 150, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, S.K.; Strahl-Heldreth, D.; Fiorello, C.V.; Harms, C.A. Best-practice guidelines for field-based surgery and anesthesia of free-ranging wildlife. I. anesthesia and analgesia. J. Wildl. Dis. 2016, 52, S14–S27. [Google Scholar] [CrossRef] [PubMed]
- Seal, U.S.; Kreeger, T.J. Chemical immobilization of furbearers. In Wild Furbearer Management and Conservation in North America; Novak, M.J., Baker, A., Obbard, M.E., Malloch, B., Eds.; Ontario Trappers Association: North Bay, ON, Canada, 1987; pp. 191–215. [Google Scholar]
- Clutton, R.E.; Duggan, L.B. Saffan anesthesia in the Raccoon: A preliminary report. J. Zoo Anim. Med. 1986, 17, 91–99. [Google Scholar] [CrossRef]
- Hoilien, J.; Oates, D. Tranquilizer use in wildlife damage control. In Great Plains Wildlife Damage Control Workshop Proceedings; University of Nebraska: Lincoln, NE, USA, 1981; pp. 71–77. [Google Scholar]
- Belant, J.L. Field Immobilization of Raccoons (Procyon lotor) with Telazol and Xylazine. J. Wildl. Dis. 2004, 40, 787–790. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.; Larivière, S.; Messier, F. Efficacy of Zoletil® for field immobilization of raccoons. Wildl. Soc. Bull. 2006, 34, 1045–1048. [Google Scholar] [CrossRef]
- Bigler, W.J.; Hoff, G.L. Anesthesia of Raccoons with Ketamine Hydrochloride. J. Wildl. Manag. 1974, 38, 364–366. [Google Scholar] [CrossRef]
- Gregg, D.A.; Olson, L.D. The use of ketamine hydrochloride as an anesthetic for raccoons. J. Wildl. Dis. 1975, 11, 335–337. [Google Scholar] [CrossRef] [Green Version]
- Norment, J.L.; Elliott, C.L.; Costello, P.S. Another look at the chemical immobilization of raccoons (Procyon lotor) with ketamine hydrochloride. J. Wildl. Dis. 1994, 30, 541–544. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, J.R.; Winstead, J.B.; Hayden-Wing, L.D.; Kreeger, T.J.; Dzialak, M.R. Field sedation of coyotes, red foxes, and raccoons with medetomidine and atipamezole. J. Wildl. Manag. 2008, 72, 1267–1271. [Google Scholar] [CrossRef]
- Allan, M.R. The use of ketamine-xylazine and ketamine-medetomidine with and without their antagonists, yohimbine and atipamezole hydrochloride to immobilize Raccoons (Procyon lotor) in Ontario, Canada. Can. Field Nat. 2015, 129, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, P.S. When power calculations won’t do: Fermi approximation of animal numbers. Lab. Anim. 2019, 48, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, G.C. Techniques for Determining Age of Raccoons; Biol. Notes; Natural History Survey Division: Urbana, IL, USA, 1961; Volume 45, pp. 1–16. [Google Scholar]
- Nannarone, S.; Moretti, G.; Bellocchi, F.; Menchetti, L.; Bufalari, A. A comparative study of intramuscular alfaxalone- or ketamine-based anesthetic mixtures in gray squirrels undergoing gonadectomy: Clinical and physiologic findings. Animals 2020, 10, 1402. [Google Scholar] [CrossRef]
- Menchetti, L.; Guelfi, G.; Speranza, R.; Carotenuto, P.; Moscati, L.; Diverio, S. Benefits of dietary supplements on the physical fitness of German Shepherd dogs during a drug detection training course. PLoS ONE 2019, 14, e0218275. [Google Scholar] [CrossRef] [Green Version]
- Beck, C. Vetalar® (ketamine hydrochloride), a unique cataleptoid anesthetic agent for multispecies usage. J. Zoo Anim. Med. 1976, 7, 11–38. [Google Scholar] [CrossRef]
- Ramsden, R.O.; Coppin, P.F.; Johnston, D.H. Clinical observations on the use of ketamine hydrochloride in wild carnivores. J. Wildl. Dis. 1976, 2, 221–225. [Google Scholar] [CrossRef]
- Robert, K.; Garant, D.; Pelletier, F. Chemical immobilization of raccoons (Procyon lotor) with ketamine-medetomidine mixture and reversal with atipamezole. J. Wildl. Dis. 2012, 48, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Hime, J.M. Use of ketamine hydrochloride in non-domesticated cats. Vet. Rec. 1974, 31, 193–195. [Google Scholar] [CrossRef]
- Evans, R.H. Raccoons and Relatives (Carnivora, Procyonidae). In Zoological Restraint and Anesthesia; Heard, D., Ed.; International Veterinary Information Service: Ithaca/New York, NY, USA, 2002. [Google Scholar]
- Kollias, G.V.; Abou-Madi, N. Procyonids and Mustelids. In Zoo Animal & Wildlife Immobilization and Anesthesia—Zoo Animal and Wildlife Immobilization and Anesthesia, 1st ed.; West, G., Heard, D., Nigel Caulkett, N., Eds.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 417–427. [Google Scholar]
- Wellington, D.; Mikaelian, I.; Singer, L. Comparison of ketamine-xylazine and ketamine-dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 481–487. [Google Scholar] [CrossRef]
- Dupras, J.; Vachon, P.; Cuvelliez, S.; Blais, D. Anesthesie du lapin de Nouvelle-Zelande utilisant les combinaisons tiletamine-zolazepam et ketamine-midazolam avec ou sans xylazine. Can. Vet. J. 2001, 42, 455–460. [Google Scholar] [PubMed]
- Valverde, A.; Skelding, A.M. Alternatives to Opioid Analgesia in Small Animal Anesthesia: Alpha-2 Agonists. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Deresienski, D.T.; Rupprecht, C.E. Yohimbine reversal of ketamine-xylazine immobilization of raccoons (Procyon lotor). J. Wildl. Dis. 1989, 25, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogler, B.R.; Elias, K.; Steiner-Valentin, K.H.S. Anaesthesia in captive raccoons (Procyon lotor) during seasonal obesity. Proc. Int. Conf. Dis. Zoo Wild Anim. 2010, 6–9. [Google Scholar] [CrossRef]
- Cattet, M.R.L.; Obbard, M.E. Use of hyaluronidase to improve chemical immobilization of free-ranging polar bears (Ursus maritimus). J. Wildl. Dis. 2010, 46, 246–250. [Google Scholar] [CrossRef]
- Kropf, J.; Hughes, J.M.L. Effect of midazolam on the quality and duration of anaesthetic recovery in healthy dogs undergoing elective ovariohysterectomy or castration. Vet. Anaesth. Analg. 2019, 46, 587–596. [Google Scholar] [CrossRef]
- Abdul-Rasool, I.H.; Ward Denham, S. Ventilatory and Cardiovascular Responses to Sufentanil Infusion in Dogs Anesthetized with Isoflurane. Anesth. Analg. 1989, 69, 300–306. [Google Scholar] [CrossRef]
- Polis, I.; Moens, Y.; Hoebenà, D.; Tshamala, M.; Hoybergs, Y.; Gasthuys, F. Cardiopulmonary effects of sufentanil long acting on sevoflurane anaesthesia in dogs. Vet. Anaesth. Analg. 2006, 33, 111–121. [Google Scholar] [CrossRef]
- Reilly, S.; Seddighi, R.; Egger, C.M.; Rohrbach, B.W.; Doherty, T.J.; Qu, W.; Johnson, J.R. The effect of fentanyl on the end-tidal sevoflurane concentration needed to prevent motor movement in dogs. Vet. Anaesth. Analg. 2013, 40, 290–296. [Google Scholar] [CrossRef]
- Chinnadurai, S.K.; Williams, C. The minimum alveolar concentration of sevoflurane in ring-tailed lemurs (Lemur catta) and aye-ayes (Daubentonia madagascariensis). Vet. Anaesth. Analg. 2016, 43, 76–80. [Google Scholar] [CrossRef]


| Score | DEPTH OF ANESTHESIA |
| 1 | Inadequate: Responsive to approach, no safe handling. Additional ½ dose is required |
| 2 | Mild: Purposeful response to stimulation (toe pinch). Additional ½ dose is required |
| 3 | Profound: Relaxed and unresponsive to stimulation (toe pinch) |
| Score | MYORELAXATION |
| 1 | Absent: Muscle tone in the pelvic and thoracic limbs, pedal reflex, persistent jaw tone, no relaxed tongue |
| 2 | Mild: Some muscle tone in thoracic limbs and pedal reflex, no relaxed tongue |
| 3 | Moderate: Some muscle tone in the pelvic or thoracic limbs, no jaw tone |
| 4 | Excellent: No muscle tone in pelvic and thoracic limbs, no jaw tone, relaxed tongue |
| Score | CEPHALIC CATHETERIZATION |
| 1 | Possible, evident limb retraction |
| 2 | Possible, mild limb retraction |
| 3 | Possible, without limb retraction |
| Score | EASE OF INTUBATION |
| 1 | Trachea intubation possible after drug administration (tongue retraction, swallowing reflex) |
| 2 | Trachea intubation possible without drug (no jaw tone, relaxed tongue) |
| Parameter | Group | |
|---|---|---|
| KD | KM | |
| Body weight (kg, mean ± SD) | ||
| 7.00 ± 1.98 | 7.10 ± 0.81 | |
| Gender (n, %) | ||
| Female Male | 5 (50.0%) | 4 (57.1%) |
| 5 (50.0%) | 3 (42.9%) | |
| Pregnant animals (n, % of females) | ||
| No Yes | 2 (40.0%) | 2 (50.0%) |
| 3 (60.0%) | 2 (50.0%) | |
| Age * (n, %) | ||
| YoungAdult | 2 (20.0%) | 1 (14.3%) |
| 8 (80.0%) | 6 (85.7%) | |
| Parameter | Group | Total | p Value | ||
|---|---|---|---|---|---|
| KD | KM | ||||
| Depth of anesthesia | Inadequate | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.593 |
| Mild | 2 (20.0%) | 3 (42.9%) | 5 (29.4%) | ||
| Profound | 8 (80.0%) | 4 (57.1%) | 12 (70.6%) | ||
| Muscle relaxation | Absent | 2 a (20.0%) | 1 a (14.3%) | 3 (17.6%) | 0.003 |
| Mild | 0 a (0.0%) | 3 b (42.9%) | 3 (17.6%) | ||
| Moderate | 1 a (10.0%) | 3 a (42.9%) | 4 (23.5%) | ||
| Excellent | 7 a (70.0%) | 0 b (0.0%) | 7 (41.2%) | ||
| Cephalic catheterization | Evident limb retraction | 0 (0.0%) | 1 (14.3%) | 1 (5.9%) | 0.338 |
| Mild limb retraction | 1 (10.0%) | 2 (28.6%) | 3 (17.6%) | ||
| No limb retraction | 9 (90.0%) | 4 (57.1%) | 13 (76.5%) | ||
| Palpebral reflex | No | 1 (10.0%) | 1 (14.3%) | 2 (11.8%) | 1.000 |
| Yes | 9 (90.0%) | 6 (85.7%) | 15 (88.2%) | ||
| Response to tactile stimulus | No | 8 (80.0%) | 6 (85.7%) | 14 (82.4%) | 1.000 |
| Yes | 2 (20.0%) | 1 (14.3%) | 3 (17.6%) | ||
| Tongue relaxation | No | 3 (30.0%) | 5 (71.4%) | 8 (47.1%) | 0.153 |
| Yes | 7 (70.0%) | 2 (28.6%) | 9 (52.9%) | ||
| Ease of intubation | After propofol | 3 a (30.0%) | 7 b (100.0%) | 10 (58.8%) | 0.010 |
| Without other drugs | 7 a (70.0%) | 0 b (0.0%) | 7 (41.2%) | ||
| Parameter | Baseline Value * | Group | p Value | ||||
|---|---|---|---|---|---|---|---|
| KD | KM | Group | Time | Group × Time | Baseline | ||
| Temp. (°C) | 36.3 | 35.5 ± 0.1 | 35.6 ± 0.1 | 0.376 | 0.007 | 0.406 | <0.001 |
| SAP (mmHg) | 115.4 | 108.2 ± 1.7 | 116.2 ± 1.7 | 0.002 | 0.901 | 0.906 | <0.001 |
| MAP (mmHg) | 90.7 | 86.7 ± 1.5 | 92.4 ± 1.5 | 0.011 | 0.748 | 0.313 | <0.001 |
| DAP (mmHg) | 71.3 | 68.2 ± 1.8 | 75.7 ± 1.8 | 0.006 | 0.621 | 0.332 | <0.001 |
| SpO2 (%) | 97.8 | 98.6 ± 0.2 | 97.7 ± 0.2 | 0.009 | 0.703 | 0.286 | <0.001 |
| RR (breaths/min) | 13.3 | 10.2 ± 0.6 | 10.4 ± 0.6 | 0.788 | 0.098 | 0.106 | <0.001 |
| HR (beats/min) | 114.7 | 105.2 ± 2.5 | 109.3 ± 2.9 | 0.342 | 0.935 | 0.734 | <0.001 |
| etCO2 (mmHg) | 40.6 | 33.1 ± 1.3 | 34.1 ± 1.4 | 0.888 | 0.340 | 0.419 | 0.004 |
| Parameter | Group | p Value | |||
|---|---|---|---|---|---|
| KD | KM | ||||
| Median | IQR | Median | IQR | ||
| pH | 7.26 | (7.19–7.34) | 7.11 | (7.06–7.15) | 0.017 |
| PaCO2 (mmHg) | 49.10 | (40.50–56.50) | 63.10 | (60.40–70.10) | 0.033 |
| PaO2 (mmHg) | 535 | (473–562) | 442 | (423–562) | 0.517 |
| BE (mmol/L) | −4 | (−5–−3) | −7 | (−12–−7) | 0.067 |
| HCO3− (mmol/L) | 22.2 | (20.3–24.6) | 22.3 | (17.7–22.9) | 0.833 |
| TCO2 (mmol/L) | 24 | (22–24) | 24 | (20–25) | 1.000 |
| SaO2 (%) | 100 | (100–100) | 100 | (100–100) | 1.000 |
| Na+ (mmol/L) | 147 | (145–149) | 152 | (150–154) | 0.286 |
| K+ (mmol/L) | 3.8 | (3.6–4.0) | 3.4 | (3.2–3.5) | 0.143 |
| iCa2+ (mmol/L) | 1.26 | (1.22–1.29) | 1.13 | (1.12–1.14) | 0.286 |
| Hct (%) | 29 | (22–38) | 19 | (16–21) | 0.143 |
| Hb (g/dL) | 9.85 | (7.50–12.90) | 6.25 | (5.40–7.10) | 0.143 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nannarone, S.; De Monte, V.; Arcelli, R.; Menchetti, L.; Gialletti, R. Gonadectomy in Raccoons: Anesthetic and Cardiorespiratory Effects of Two Ketamine-Based Pre-Anesthetic Protocols before Sevoflurane-Sufentanil. Animals 2020, 10, 2110. https://doi.org/10.3390/ani10112110
Nannarone S, De Monte V, Arcelli R, Menchetti L, Gialletti R. Gonadectomy in Raccoons: Anesthetic and Cardiorespiratory Effects of Two Ketamine-Based Pre-Anesthetic Protocols before Sevoflurane-Sufentanil. Animals. 2020; 10(11):2110. https://doi.org/10.3390/ani10112110
Chicago/Turabian StyleNannarone, Sara, Valentina De Monte, Rolando Arcelli, Laura Menchetti, and Rodolfo Gialletti. 2020. "Gonadectomy in Raccoons: Anesthetic and Cardiorespiratory Effects of Two Ketamine-Based Pre-Anesthetic Protocols before Sevoflurane-Sufentanil" Animals 10, no. 11: 2110. https://doi.org/10.3390/ani10112110
APA StyleNannarone, S., De Monte, V., Arcelli, R., Menchetti, L., & Gialletti, R. (2020). Gonadectomy in Raccoons: Anesthetic and Cardiorespiratory Effects of Two Ketamine-Based Pre-Anesthetic Protocols before Sevoflurane-Sufentanil. Animals, 10(11), 2110. https://doi.org/10.3390/ani10112110







