Maternal Folic Acid Supplementation Differently Affects the Small Intestinal Phenotype and Gene Expression of Newborn Lambs from Differing Litter Sizes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Data and Sample Collection
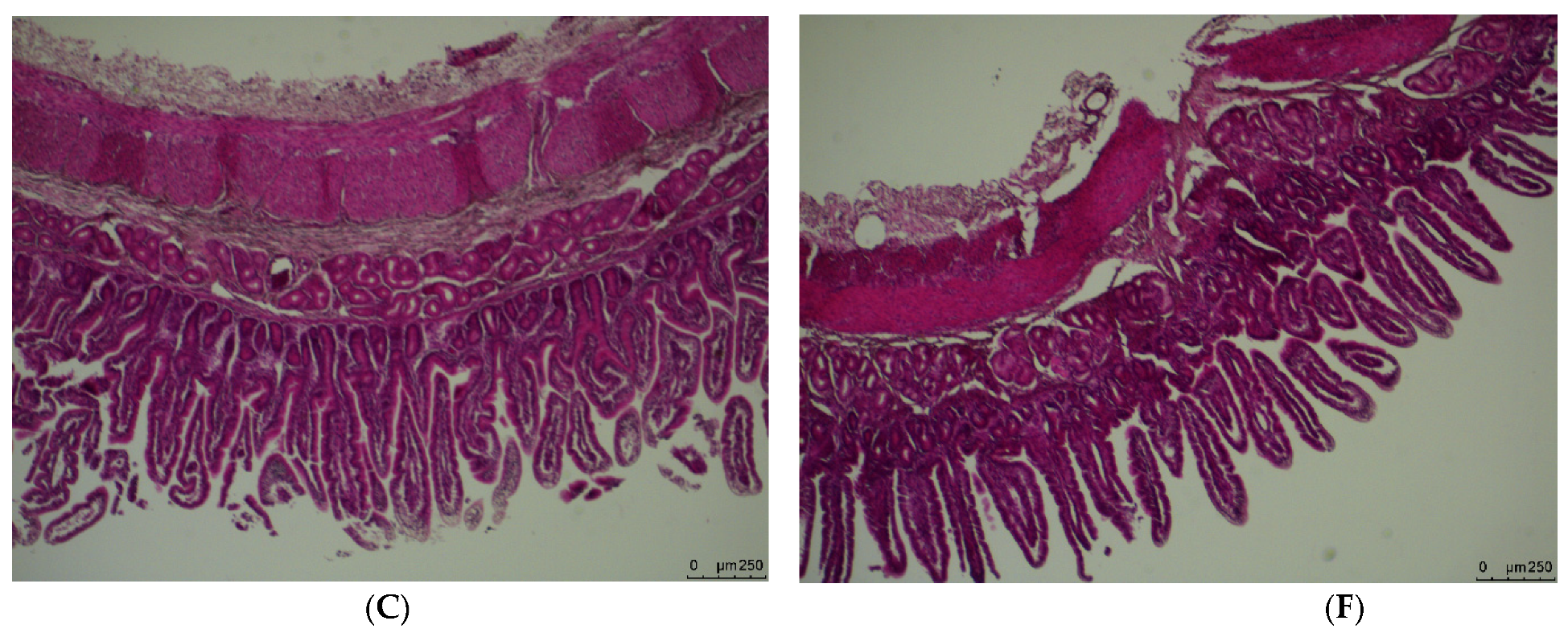
2.3. Small Intestinal Morphology
2.4. qRT-PCR
2.5. Statistical Analysis
3. Results
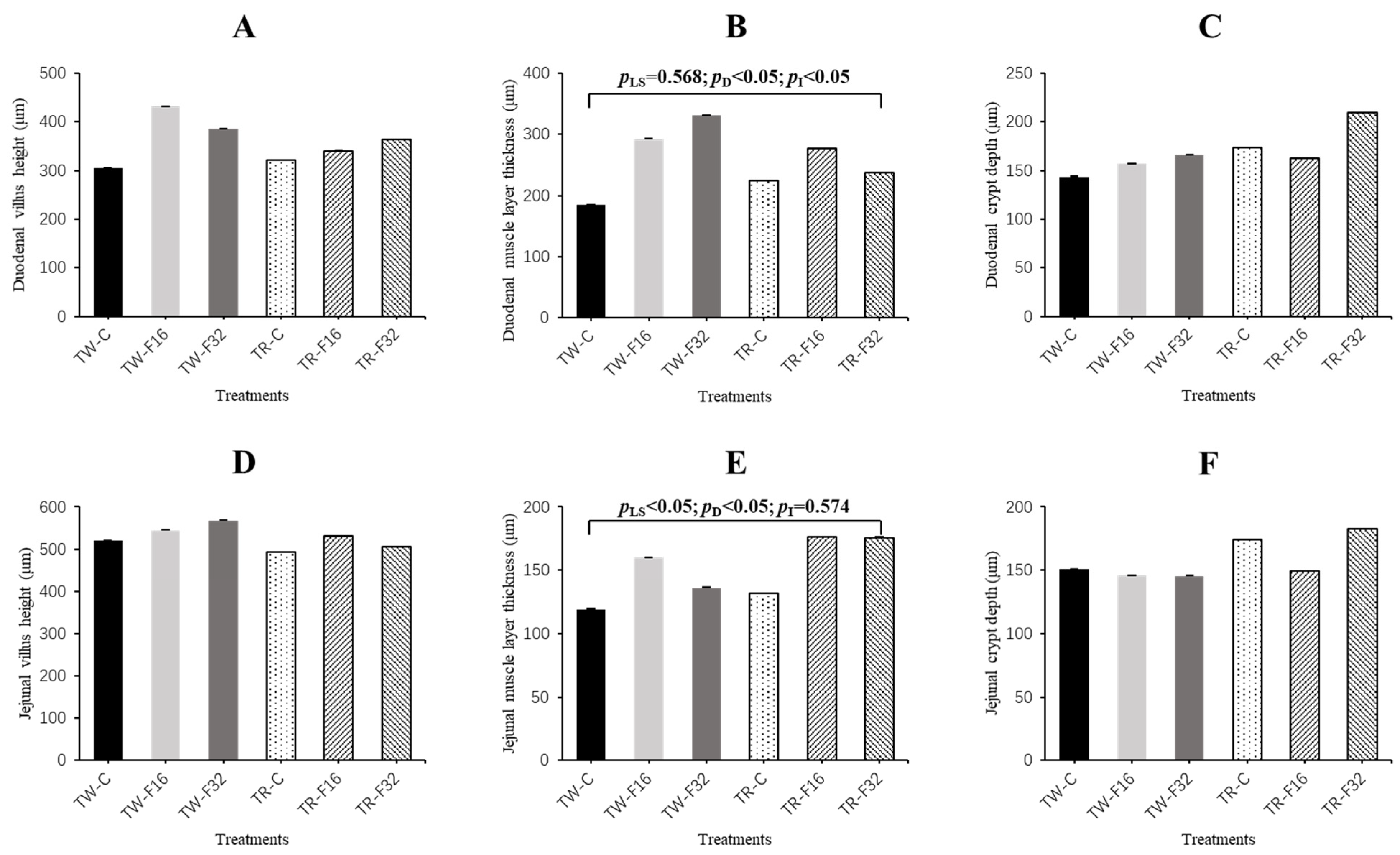
3.1. Weight and Morphology of the Small Intestine
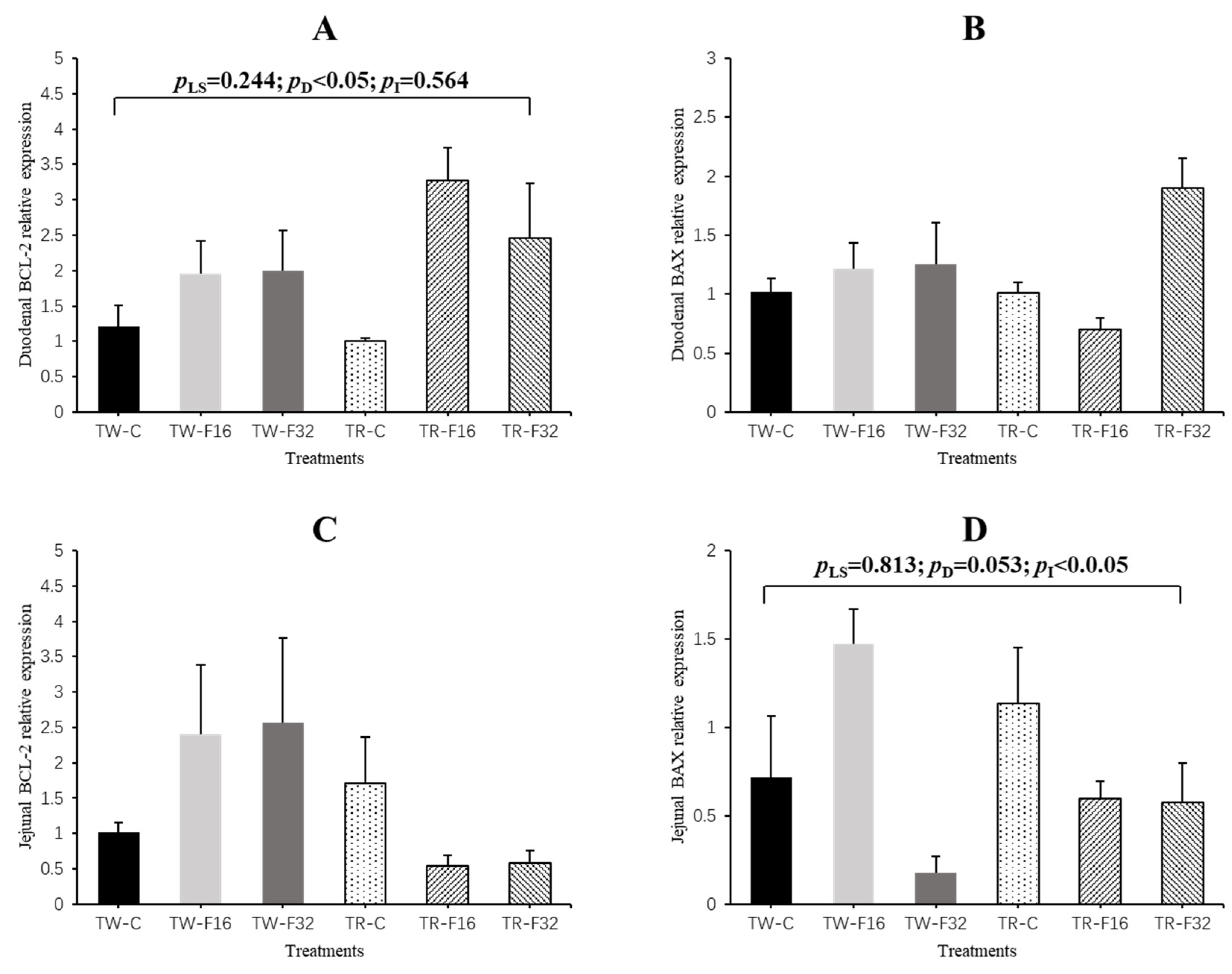
3.2. Gene Expression
3.3. Pearson Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bauer, M.K.; Breier, B.H.; Harding, J.E.; Veldhuis, J.D.; Gluckman, P.D. The fetal somatotropic axis during long term maternal undernutrition in sheep: Evidence for nutritional regulation in utero. Endocrinology 1995, 136, 1250–1257. [Google Scholar]
- Wilson, M.E. Role of placental function in mediating conceptus growth and survival. J. Anim. Sci. 2002, 80 (Suppl. 2), E195–E201. [Google Scholar]
- Liu, Y.; Li, H.; Sha, Q.; Hai, R.; Wang, Y.; Song, Y.; Gao, F. Effects of maternal undernutrition on the growth, development and antioxidant status of ovine placentome subtypes during late pregnancy. Theriogenology 2018, 110, 96–102. [Google Scholar]
- Bailey, L.B.; Gregory, J.F. Folate metabolism and requirements. J. Nutr. 1999, 129, 779–782. [Google Scholar] [PubMed] [Green Version]
- Frédéric, G.; Matte, J.J.; Girard, C.L.; Palin, M.F.; Alain, G.; Laforest, J.P. Effect of folic acid plus glycine supplement on uterine prostaglandin and endometrial granulocyte-macrophage colony-stimulating factor expression during early pregnancy in pigs. Theriogenology 2004, 61, 485–498. [Google Scholar]
- Cho, C.E.; Sánchezhernández, D.; Rezalópez, S.A.; Huot, P.S.P.; Kim, Y.I.; Anderson, G.H. High folate gestational and post-weaning diets alter hypothalamic feeding pathways by DNA methylation in Wistar rat offspring. Epigenetics 2013, 8, 710–719. [Google Scholar] [PubMed] [Green Version]
- Zhao, Y.; Jin, C.; Xuan, Y.D.; Zhou, P.; Fang, Z.F.; Che, L.Q.; Xu, S.Y.; Feng, B.; Li, J.; Jiang, X.M.; et al. Effect of maternal or post-weaning methyl donor supplementation on growth performance, carcass traits and meat quality of pig offspring. J. Sci. Food. Agr. 2018, 99, 2096–2107. [Google Scholar]
- Meyer, A.M.; Hess, B.W.; Paisley, S.I.; Du, M.; Caton, J.S. Small intestinal growth measures are correlated with feed efficiency in market weight cattle, despite minimal effects of maternal nutrition during early to midgestation. J. Anim. Sci. 2014, 92, 3855–3867. [Google Scholar] [PubMed]
- Mckay, J.A.; Williams, E.A.; Mathers, J.C. Effect of maternal and post-weaning folate supply on gene-specific DNA methylation in the small intestine of weaning and adult apc+/min and wild type mice. Front. Genet. 2011, 2, 23. [Google Scholar]
- Bressenot, A.; Pooya, S.; Bossenmeyer, P.C.; Gauchotte, G.; Germain, A.; Chevau, J.B. Methyl donor deficiency affects small-intestinal differentiation and barrier function in rats. Brit. J. Nutr. 2013, 109, 667–677. [Google Scholar]
- Kahraman, O.; Citil, O.B.; Alatas, M.S.; Ozbilgin, A. Folic acid in ruminant nutrition. Anim. Sci. 2015, 58, 140–143. [Google Scholar]
- Ragaller, V.; Hüther, L.; Lebzien, P. Folic acid in ruminant nutrition: A review. Brit. J. Nutr. 2009, 101, 153–164. [Google Scholar] [PubMed] [Green Version]
- Girard, C.L.; Castonguay, F.; Fahmy, M.H.; Matte, J.J. Serum and milk folates during the first two gestations and lactations in Romanov, Finnsheep, and Suffolk ewes. J. Anim. Sci. 1996, 74, 1711–1715. [Google Scholar] [PubMed]
- Yue, G.H. Reproductive characteristics of Chinese Hu sheep. Anim. Reprod. Sci. 1996, 44, 223–230. [Google Scholar]
- Wang, B.; Li, H.Q.; Li, Z.; Jian, L.Y.; Gao, Y.F.; Qu, Y.H.; Liu, C.; Xu, C.C.; Li, Y.X.; Diao, Z.C.; et al. Maternal folic acid supplementation modulates the growth performance, muscle development and immunity of Hu sheep offspring of different litter size. J. Nutr. Biochem. 2019, 70, 194–201. [Google Scholar]
- Li, H.Q.; Wang, B.; Li, Z.; Luo, H.L.; Wang, Y.J.; Zhang, C.; Jian, L.Y.; Gao, Y.F.; Lu, W.; Liu, M.; et al. Effects of rumen-protected folic acid addition in maternal and post-weaning diets on growth performance, total tract digestibility, ruminal fermentation and blood metabolites in lambs. Anim. Feed. Sci. Tech. 2020, 260, 114364. [Google Scholar]
- NRC. Nutrient Requirements of Small Ruminants; The National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Li, X.T.; Yin, J.D.; Li, D.F.; Chen, X.J. Dietary supplementation with zinc oxide increases Igf-I and Igf-I receptor gene expression in the small intestine of weanling piglets. J. Nutr. 2006, 136, 1786–1791. [Google Scholar]
- Vaughan, T.J.; James, P.S.; Pascall, J.C.; Kenneth, D.B. Expression of the genes for TGF alpha, EGF and the EGF receptor during early pig development. Development 1992, 116, 663–669. [Google Scholar]
- Lindsten, T.; Ross, A.J.; King, A.; Zong, W.X.; Jeffrey, C.R.; Helena, A.S.; Eugen, U.; Katrina, G.W.; Patryce, M.; Kenneth, F.; et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 2000, 6, 1389–1399. [Google Scholar]
- Amiri, M.; Naim, H. Characterization of mucosal disaccharidases from human intestine. Nutrients 2017, 9, 1106–1113. [Google Scholar]
- Yukio, K.; Tomoko, S.; Yasuhito, N.; Mikihiro, S.; Kubo, Y.; Sato, T.; Harada, A.; Tsuji, A. Investigation of the role of oligopeptide transporter pept1 and sodium/glucose cotransporter sglt1 in intestinal absorption of their substrates using small gtp-binding protein rab8-null mice. Drug. Metabol. Dispos. 2009, 37, 602–607. [Google Scholar]
- Sinéad, M.W.; Keogh, K.; Buckley, F.; David, A.K. Effect of genotype on duodenal expression of nutrient transporter genes in dairy cows. J. Anim. Sci. Biotechnol. 2013, 4, 49–57. [Google Scholar]
- Day, A.J.; Gee, J.M.; Dupont, M.S.; Johnson, I.T.; Williamson, G. Absorption of quercetin-3-glucoside and quercetin-4’-glucoside in the rat small intestine: The role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharmacol. 2003, 65, 1199–1206. [Google Scholar] [PubMed]
- Antony, A.C. In utero physiology: Role of folic acid in nutrient delivery and fetal development. Am. J. Clin. Nutr. 2007, 85, 598S–603S. [Google Scholar] [PubMed] [Green Version]
- Van Wettere, W.H.E.J.; Smits, R.J.; Hughes, P.E. Methyl donor supplementation of gestating sow diets improves pregnancy outcomes and litter size. Anim. Reprod. Sci. 2013, 53, 1–7. [Google Scholar]
- Bechdel, S.I.; Honeywell, H.E.; Dutcher, R.A.; Knutsen, M.H. Synthesis of vitamin B in the rumen of the cow. J. Biol. Chem. 1928, 80, 231–238. [Google Scholar]
- Girard, C.L.; Matte, J.J. Serum clearance and urinary excretion of pteroylmonoglutamic acid in gestating and lactating dairy cows. Brit. J. Nutr. 1995, 74, 857–865. [Google Scholar]
- Avila, C.G.; Harding, R.; Rees, S.; Robinson, P. Small intestinal development in growth-retarded fetal sheep. J. Pediatr. Gastr. Nutr. 1989, 8, 507–515. [Google Scholar]
- Li, D.B.; Liu, X.G.; Zhang, C.Z.; Kao, G.L.; Hou, X.Z. Effects of nutrient restriction followed by realimentation on growth, visceral organ mass, cellularity, and jejunal morphology in lambs. Livest. Sci. 2015, 173, 24–31. [Google Scholar]
- Meyer, A.M.; Caton, J.S. Role of the small intestine in developmental programming: Impact of maternal nutrition on the dam and offspring. Adv. Nutr. 2016, 7, 169–178. [Google Scholar]
- Chang, Y.L.; Cai, H.Y.; Liu, G.H.; Chang, W.H.; Zheng, A.J.; Zhang, S.; Liao, R.B.; Liu, W.; Li, Y.; Tian, J. Effects of dietary leucine supplementation on the gene expression of mammalian target of rapamycin signaling pathway and intestinal development of broilers. Anim. Nutr. 2015, 1, 313–319. [Google Scholar] [PubMed]
- Davidson, G.P.; Townley, R.R.W. Structural and functional abnormalities of the small intestine due to nutritional folic acid deficiency in infancy. J. Pediatr. US. 1977, 90, 590–594. [Google Scholar]
- Yuan, Z.H.; Zhang, J.X.; Li, W.H.; Wang, W.M.; Li, F.D.; Yue, X.P. Association of polymorphisms in candidate genes with the litter size in two sheep breeds. Animals 2019, 9, 958–971. [Google Scholar]
- Li, Y.; Zhang, H.; Su, W.P.; Ying, Z.X.; Chen, Y.P.; Zhang, L.L.; Lu, Z.X.; Wang, T. Effects of dietary Bacillus amyloliquefaciens supplementation on growth performance, intestinal morphology, inflammatory response, and microbiota of intra-uterine growth retarded weanling piglets. J. Anim. Sci. Biotechno. 2018, 9, 480–495. [Google Scholar]
- Hamed, K.V.; Giulia, R.; Azita, H.; Somaye, F.; Cheng, T.S.; Mahdi, S.; Vahid, K.; Mohammad, M.S.; Meysam, Z.; Shekoufeh, S.; et al. The influence of vitamin D supplementation on IGF-1 levels in humans: A systematic review and meta-analysis. Ageing. Res. Rev. 2020, 57, 100996. [Google Scholar]
- Burrin, D.G.; Stoll, B.; Fan, M.Z.; Dudley, M.A.; Donovan, S.M.; Reeds, P.J. Oral IGF-I alters the posttranslational processing but not the activity of lactase-phlorizin hydrolase in formula-fed neonatal pigs. J. Nutr. 2001, 131, 2235–2241. [Google Scholar] [PubMed]
- Trahair, J.F.; Wing, S.J.; Quinn, K.J.; Owens, P.C. Regulation of gastrointestinal growth in fetal sheep by luminally administered insulin-like growth factor-I. J. Endocrinol. 1997, 152, 29–38. [Google Scholar]
- Grether, G.F. Environmental change, phenotypic plasticity, and genetic compensation. Am. Nat. 2005, 166, E115–E123. [Google Scholar]
- Pinheiro, D.F.; Pinheiro, P.F.; José, B.J.; Castilho, A.C.S.; Vicentini, P.M.L.M. Maternal protein restriction during pregnancy affects gene expression and immunolocalization of intestinal nutrient transporters. Clin. Sci. 2013, 125, 281–289. [Google Scholar]
- Liu, J.B.; Chen, D.W.; Mao, X.B.; Yu, B. Effects of maternal folic acid supplementation on morphology and apoptosis-related gene expression in jejunum of newborn intrauterine growth retarded piglets. Arch. Anim. Nutr. 2011, 65, 376–385. [Google Scholar]
- Bagnyukova, T.V.; Powell, C.L.; Pavliv, O.; Tryndyak, V.P.; Pogribny, I.P. Induction of oxidative stress and DNA damage in rat brain by a folate/methyl-deficient diet. Brain. Res. 2008, 1237, 44–51. [Google Scholar] [PubMed]
- Anup, R.; Muniswamy, M.; Kunissery, A.B. Apoptosis in the intestinal epithelium: Its relevance in normal and pathophysiological conditions. J. Gastroen. Hepatol. 2000, 15, 109–120. [Google Scholar]
- McCoard, S.A.; Cristobal, C.O.; Knol, F.W.; Heiser, A.; Khan, M.A.; Hennes, N.; Johnstone, P.; Lewis, S.; Stevens, D.R. Impact of early weaning on small intestine, metabolic, immune and endocrine system development, growth and body composition in artificially reared lambs. J. Anim. Sci. 2020, 98, skz356. [Google Scholar] [PubMed]
- Ronald, J.T.; Manuel, A.V.H.; Kimberly, A.V.; Kendall, C.S. Effects of nutrient restriction during midgestation to late gestation on maternal and fetal postruminal carbohydrase activities in sheep. J. Anim. Sci. 2020, 98, 1–12. [Google Scholar]
- Yin, D.F.; Selle, P.H.; Moss, A.F.; Wang, Y.L.; Dong, X.Y.; Xiao, Z.B.; Guo, Y.M.; Yuan, J.M. Influence of starch sources and dietary protein levels on intestinal functionality and intestinal mucosal amino acids catabolism in broiler chickens. J. Anim. Sci. Biotechno. 2019, 10, 658–672. [Google Scholar]
- Wang, Z.; Maravelias, C.; Sibley, E. Lactase gene promoter fragments mediate temporal expression patterns in transgenic mice. DNA. Cell. Biol. 2006, 25, 215–222. [Google Scholar]
- Barone, S.; Fussell, S.J.; Singh, A.K.; Xu, J.; Amlal, H.; Seidler, U.E.; Zuo, J.; Soleimani, M. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J. Biol. Chem. 2009, 284, 5056–5066. [Google Scholar]
- Teirlynck, E.; Bjerrum, L.; Eeckhaut, V.; Huygebaert, G.; Pasmans, F.; Haesebrouck, F. The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens. Brit. J. Nutr. 2009, 102, 1453–1461. [Google Scholar]



| Constitution of TMR | Formula of Concentrate | Levels of Nutrients in TMR | |||||
|---|---|---|---|---|---|---|---|
| EG | Ingredients | EG | LG | Nutrients | EG | LG | |
| Peanut vines | 50.00 | Corn | 53.00 | 50.00 | DM | 92.35 | 90.96 |
| Whole corn silage | 45.00 | Soybean meal | 9.70 | 22.50 | CP | 9.60 | 10.00 |
| Concentrate | 5.00 | Rapeseed meal | 12.00 | 7.00 | EE | 3.51 | 4.52 |
| Total | 100.00 | Wheat bran | 15.70 | 11.50 | Ash | 11.19 | 8.85 |
| LG | Limestone | 1.00 | 1.00 | NDF | 49.19 | 34.15 | |
| Peanut vines | 27.00 | CaHPO4 | 0.60 | 0.60 | ADF | 29.98 | 24.86 |
| Whole corn silage | 28.00 | NaHCO3 | 1.30 | 1.30 | ME 2) | 1.93 | 2.15 |
| Concentrate | 45.00 | NaCl | 0.80 | 0.40 | Ca | 0.54 | 0.56 |
| Total | 100.00 | Vitamin E | 0.40 | 0.10 | P | 0.37 | 0.26 |
| Soybean oil | 0.30 | 0.40 | |||||
| Premix 1) | 5.00 | 5.00 | |||||
| De-mold agent | 0.20 | 0.20 | |||||
| Total | 100.00 | 100.00 | |||||
| Genes Name | Primers Sequence | Recommended Melting Temperature (℃) | Product Size (bp) | |
|---|---|---|---|---|
| SGLT1 | Forward | AGAGGTCACAGTTGGAATGGC | 60 | 110 |
| Reverse | TGATTATGCTCCTGGGGTCTT | |||
| PEPT1 | Forward | GTAGACGATGGACAGCGACAC | 60 | 211 |
| Reverse | CAATGAGTTCTGCGAAAGGTT | |||
| IGF-I | Forward | CCAGCCTGCTGTTATTTCTTT | 60 | 80 |
| Reverse | CATTGCGGTTCTGTTGATAGT | |||
| BAX | Forward | CTTCCAGATGGTGAGTGAGGC | 60 | 242 |
| Reverse | GGGTTGTCGCCCTTTTCTACT | |||
| BCL-2 | Forward | ACTGCTTTCACGAACCTTTTG | 60 | 142 |
| Reverse | TTGATTTCTCCTGGCTGTCTC | |||
| SLC2A5 | Forward | ACAAGGATTCCGATGGTGATA | 60 | 113 |
| Reverse | GCAGGTCTGTCTTCCAATGTC | |||
| GLP2R | Forward | CCCCAGCACCCTGTATTCTCC | 60 | 175 |
| Reverse | ATTACTCGTGGCTGCTCGTCG | |||
| EGF | Forward | CAGACAAGTCGCCAGCAAACG | 60 | 195 |
| Reverse | GCCCTCCCACCTCCTCCAAGT | |||
| SLC2A2 | Forward | GGCACAAACAAACATTCCACT | 60 | 166 |
| Reverse | CTCAACCAGCATTTTCCAGAC | |||
| SI | Forward | TCAAACCACCGAGCATTAGGG | 60 | 125 |
| Reverse | AATGAAAAGCCAACCTGGGATA | |||
| LCT | Forward | TCTCCAGTGGCGTTGTCTTTC | 60 | 118 |
| Reverse | TGTCCTCGTCAGCCTATCAGA | |||
| MGAM | Forward | GGCCCTAAAACCACATAAAAG | 60 | 162 |
| Reverse | CTACGGTGTCCACCCCTACTA | |||
| GAPDH | Forward | GGCGTGAACCACGAGAAGTA | 60 | 141 |
| Reverse | GGCGTGGACAGTGGTCATAA |
| Items | Twins | Triplets | Litter size | Diet | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | F16 | F32 | CON | F16 | F32 | Twins | Triplets | CON | F16 | F32 | Litter Size | Diet | I× | |
| Small intestine weight (g) | 118.75 ± 3.09 | 128.93 ± 5.76 | 142.00 ± 7.46 | 106.96 ± 8.33 | 114.98 ± 3.62 | 113.01 ± 5.44 | 127.51 ± 3.22 | 112.54 ± 3.14 | 115.39 ± 3.34 | 121.96 ± 3.65 | 123.55 ± 5.26 | 0.001 | 0.356 | 0.244 |
| Small intestine/live body weight (%) (1) | 3.15 ± 0.07 | 3.31 ± 0.09 | 3.48 ± 0.14 | 3.17 ± 0.17 | 3.45 ± 0.09 | 3.49 ± 0.09 | 3.28 ± 0.06 | 3.41 ± 0.06 | 3.15 ± 0.07 | 3.38 ± 0.07 | 3.48 ± 0.08 | 0.122 | 0.005 | 0.769 |
| Items | Genes | Twins | Triplets | Litter Size | Diet | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | F16 | F32 | CON | F16 | F32 | Twins | Triplets | CON | F16 | F32 | Litter Size | Diet | I× | ||
| Duodenum | EGF | 1.24 ± 0.36 | 3.69 ± 0.94 | 1.58 ± 0.32 | 1.09 ± 0.23 | 0.90 ± 0.25 | 1.08 ± 0.32 | 2.01 ± 0.46 | 1.02 ± 0.14 | 1.08 ± 0.21 | 3.41 ± 0.65 | 0.89 ± 0.21 | 0.055 | 0.099 | 0.285 |
| GLP2R | 1.08 ± 0.19 | 1.00 ± 0.16 | 0.62 ± 0.13 | 1.04 ± 0.12 | 1.00 ± 0.13 | 1.93 ± 0.47 | 0.89 ± 0.11 | 1.28 ± 0.18 | 0.94 ± 0.11 | 0.83 ± 0.10 | 0.91 ± 0.33 | 0.064 | 0.675 | 0.399 | |
| IGF-I (1) | 1.07 ± 0.16 | 0.78 ± 0.12 | 0.78 ± 0.15 | 1.08 ± 0.19 | 2.43 ± 0.66 | 2.55 ± 0.23 | 0.88 ± 0.09 | 1.95 ± 0.30 | 1.14 ± 0.12 | 1.46 ± 0.40 | 1.52 ± 0.31 | 0.003 | 0.422 | 0.005 | |
| Jejunum | EGF | 0.98 ± 0.35 | 0.98 ± 0.50 | 0.80 ± 0.46 | 1.14 ± 0.35 | 0.49 ± 0.19 | 0.45 ± 0.22 | 1.00 ± 0.23 | 0.74 ± 0.15 | 1.07 ± 0.23 | 0.74 ± 0.21 | 0.65 ± 0.24 | 0.352 | 0.255 | 0.691 |
| GLP2R | 0.80 ± 0.80 | 0.60 ± 0.32 | 1.40 ± 0.81 | 1.05 ± 0.19 | 1.24 ± 0.44 | 1.13 ± 0.11 | 1.10 ± 0.20 | 1.12 ± 0.17 | 0.95 ± 0.21 | 0.92 ± 0.25 | 1.29 ± 0.20 | 0.947 | 0.729 | 0.481 | |
| IGF-I | 0.69 ± 0.63 | 0.95 ± 0.20 | 0.14 ± 0.09 | 2.02 ± 0.70 | 0.88 ± 0.17 | 0.89 ± 0.41 | 0.60 ± 0.16 | 1.36 ± 0.29 | 1.45 ± 0.42 | 0.92 ± 0.10 | 0.46 ± 0.28 | 0.041 | 0.390 | 0.360 | |
| Terms | Villus Height | Thickness of Muscle Layer | Crypt Depth |
|---|---|---|---|
| R (p) | R (p) | R (p) | |
| BCL-2 | 0.328 (0.127) | −0.092 (0.660) | 0.039 (0.851) |
| EGF | 0.279 (0.197) | 0.279 (0.177) | −0.220 (0.280) |
| MGAM | 0.120 (0.577) | −0.226 (0.266) | 0.510 (0.007) |
| PEPT1 | 0.287 (0.195) | 0.161 (0.453) | 0.060 (0.785) |
| SLC2A2 | 0.012 (0.959) | −0.228 (0.262) | 0.147 (0.484) |
| BAX | 0.294 (0.173) | 0.124 (0.554) | −0.015 (0.943) |
| GLP2R | 0.337 (0.093) | −0.286 (0.132) | −0.147 (0.448) |
| IGF1 | 0.189 (0.367) | −0.145 (0.461) | 0.208 (0.288) |
| LCT | −0.103 (0.639) | −0.039 (0.849) | 0.586 (0.002) |
| SGLT1 | −0.032 (0.890) | −0.181 (0.397) | 0.646 (0.001) |
| SI | 0.104 (0.630) | −0.130 (0.519) | 0.541 (0.004) |
| SLC2A5 | −0.090 (0.683) | −0.153 (0.454) | 0.418 (0.034) |
| Terms | Villus Height | Thickness of Muscle Layer | Crypt Depth |
|---|---|---|---|
| R (p) | R (p) | R (p) | |
| BCL | −0.115 (0.593) | −0.304 (0.169) | 0.173 (0.441) |
| EGF | −0.129 (0.556) | −0.193 (0.377) | 0.008 (0.969) |
| MGAM | 0.429 (0.029) | −0.181 (0.357) | 0.053 (0.787) |
| PEPT1 | −0.058 (0.779) | 0.499 (0.008) | 0.155 (0.441) |
| SLC2A2 | 0.196 (0.338) | 0.011 (0.957) | −0.093 (0.645) |
| BAX | 0.085 (0.700) | −0.172 (0.422) | −0.229 (0.283) |
| GLP2R | −0.189 (0.377) | −0.113 (0.600) | −0.047 (0.827) |
| IGF1 | −0.351 (0.093) | 0.112 (0.621) | 0.472 (0.027) |
| LCT | 0.083 (0.705) | −0.015 (0.946) | −0.042 (0.852) |
| SGLT1 | −0.360 (0.091) | 0.275 (0.227) | 0.189 (0.412) |
| SI | −0.190 (0.408) | 0.059 (0.801) | 0.178 (0.440) |
| SLC2A5 | −0.430 (0.036) | 0.024 (0.915) | 0.248 (0.266) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, B.; Li, H.; Jian, L.; Luo, H.; Wang, B.; Zhang, C.; Zhao, X.; Xue, Y.; Peng, S.; et al. Maternal Folic Acid Supplementation Differently Affects the Small Intestinal Phenotype and Gene Expression of Newborn Lambs from Differing Litter Sizes. Animals 2020, 10, 2183. https://doi.org/10.3390/ani10112183
Li Z, Wang B, Li H, Jian L, Luo H, Wang B, Zhang C, Zhao X, Xue Y, Peng S, et al. Maternal Folic Acid Supplementation Differently Affects the Small Intestinal Phenotype and Gene Expression of Newborn Lambs from Differing Litter Sizes. Animals. 2020; 10(11):2183. https://doi.org/10.3390/ani10112183
Chicago/Turabian StyleLi, Zhen, Bo Wang, Heqiong Li, Luyang Jian, Hailing Luo, Bing Wang, Can Zhang, Xingang Zhao, Ying Xue, Sijia Peng, and et al. 2020. "Maternal Folic Acid Supplementation Differently Affects the Small Intestinal Phenotype and Gene Expression of Newborn Lambs from Differing Litter Sizes" Animals 10, no. 11: 2183. https://doi.org/10.3390/ani10112183
APA StyleLi, Z., Wang, B., Li, H., Jian, L., Luo, H., Wang, B., Zhang, C., Zhao, X., Xue, Y., Peng, S., & Zuo, S. (2020). Maternal Folic Acid Supplementation Differently Affects the Small Intestinal Phenotype and Gene Expression of Newborn Lambs from Differing Litter Sizes. Animals, 10(11), 2183. https://doi.org/10.3390/ani10112183






