Stocking Rate Has No Confounding Effect on the Use of Internal and Inert Markers to Predict Botanical Composition, Diet Quality, Degradability and Passage Rate Kinetics in Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Management of Animals
2.2. In Sacco Degradability Estimation of Diets
2.3. Determination of Passage Rate of Digesta Using Inert Markers
2.4. Chemical Analysis of Feed and Faecal Samples
2.5. Prediction ofDiet Composition, Digestibility and IntakeUsing Internal Markers
2.6. Statistical Analysis
3. Results
3.1. Diet Chemical Composition and In Sacco Degradability
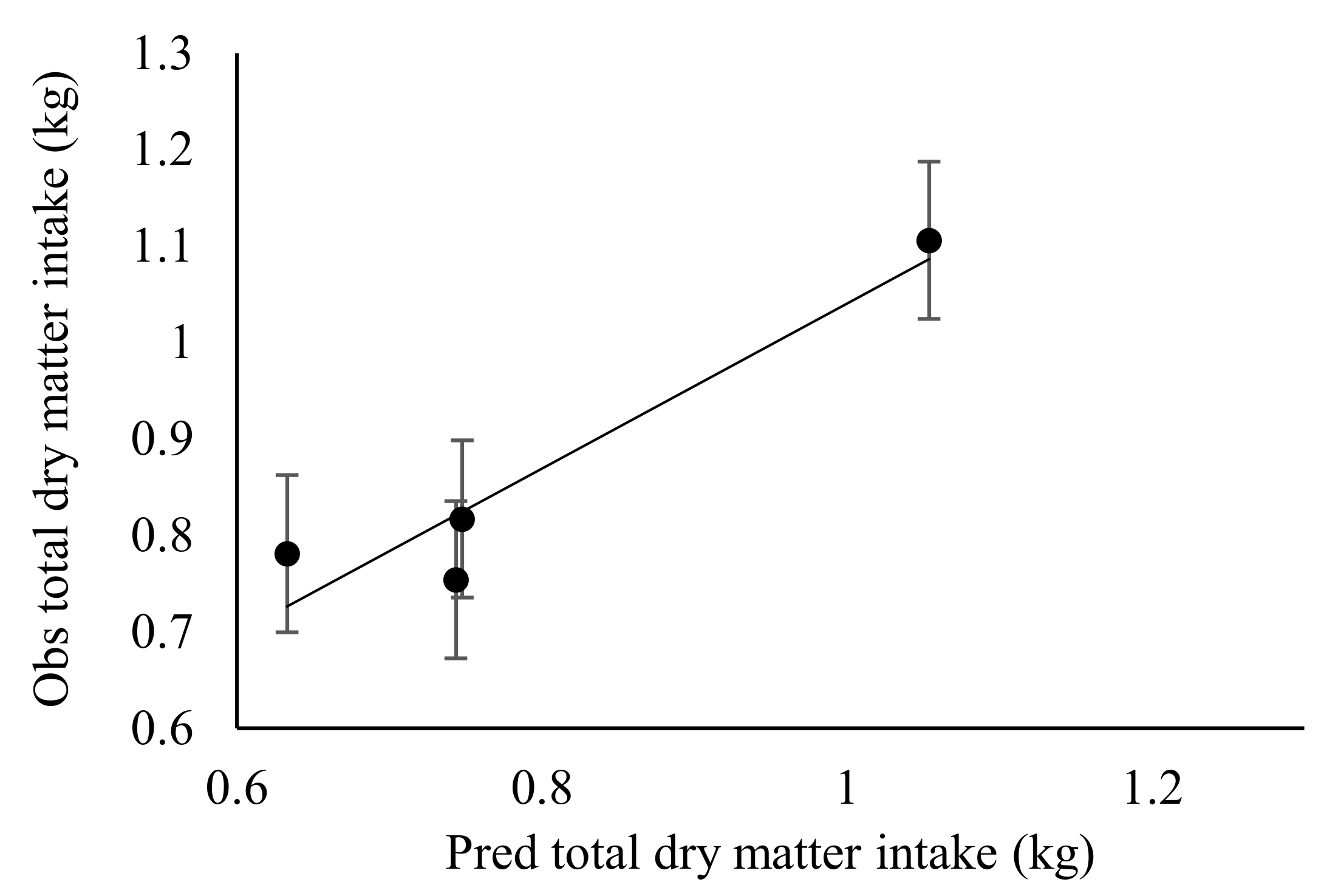
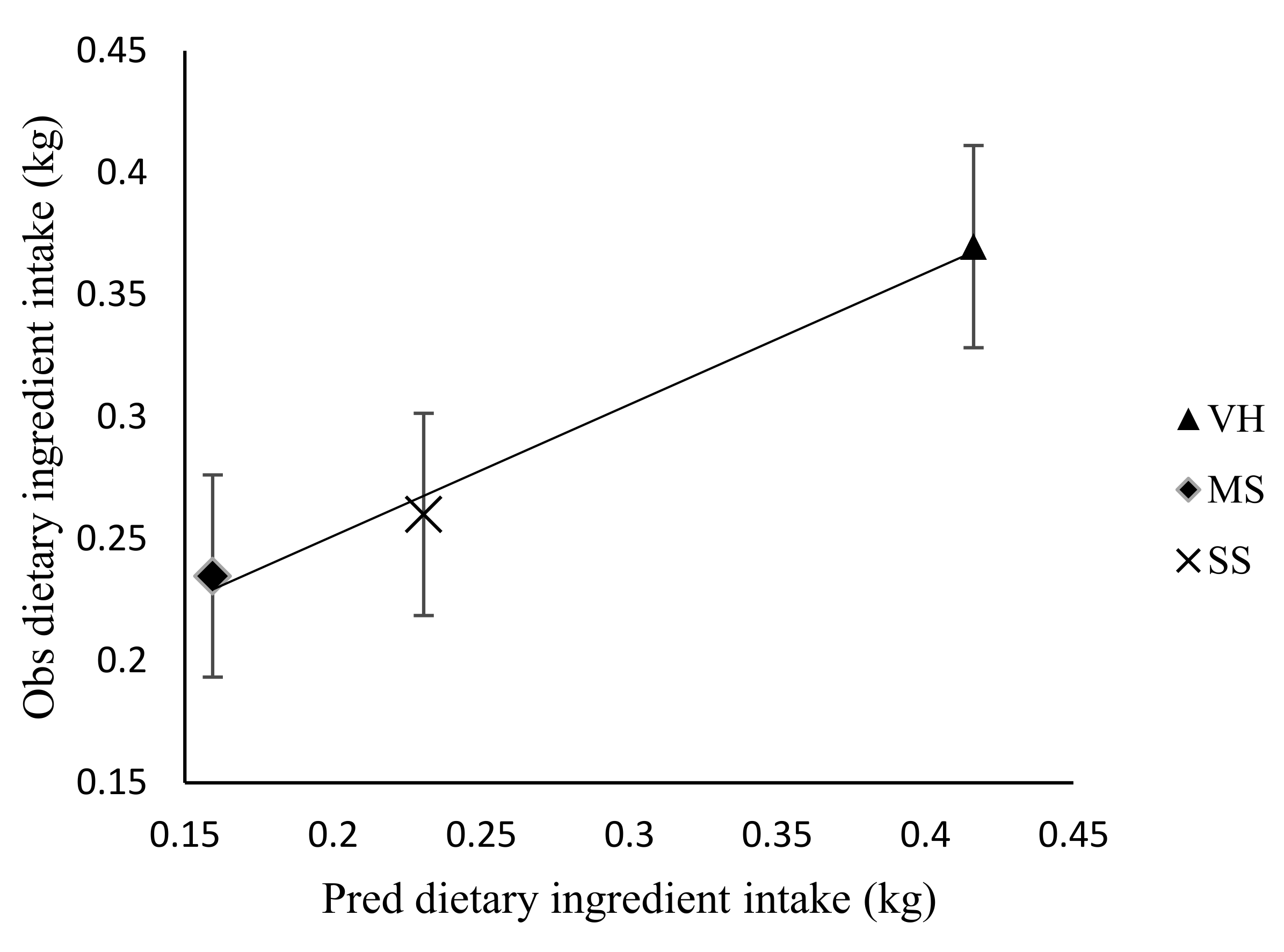
3.2. Effect of Stocking Rate on Diet and Nutrient Intake, Diet Selection, Digestibility and Passage Rate in Sheep
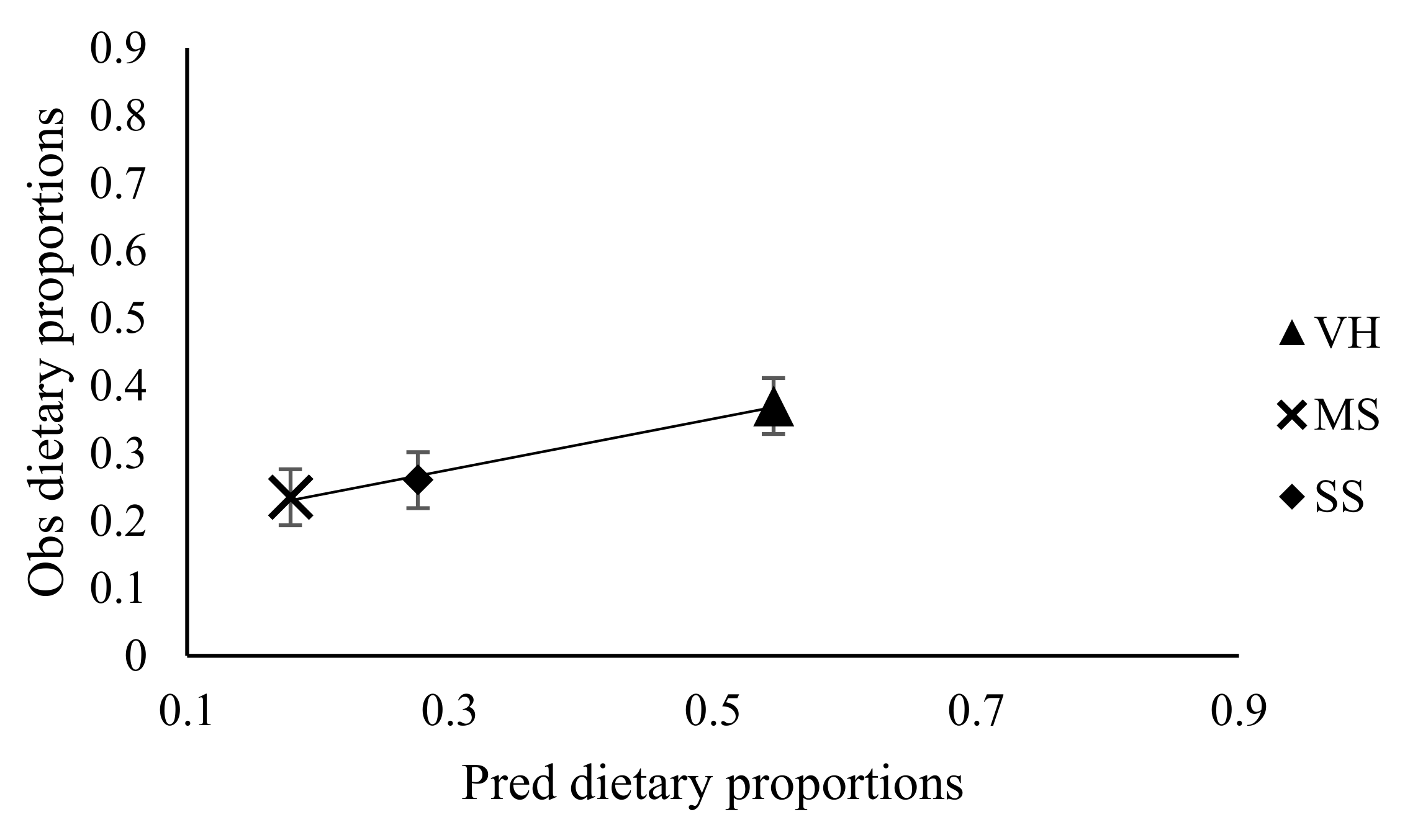
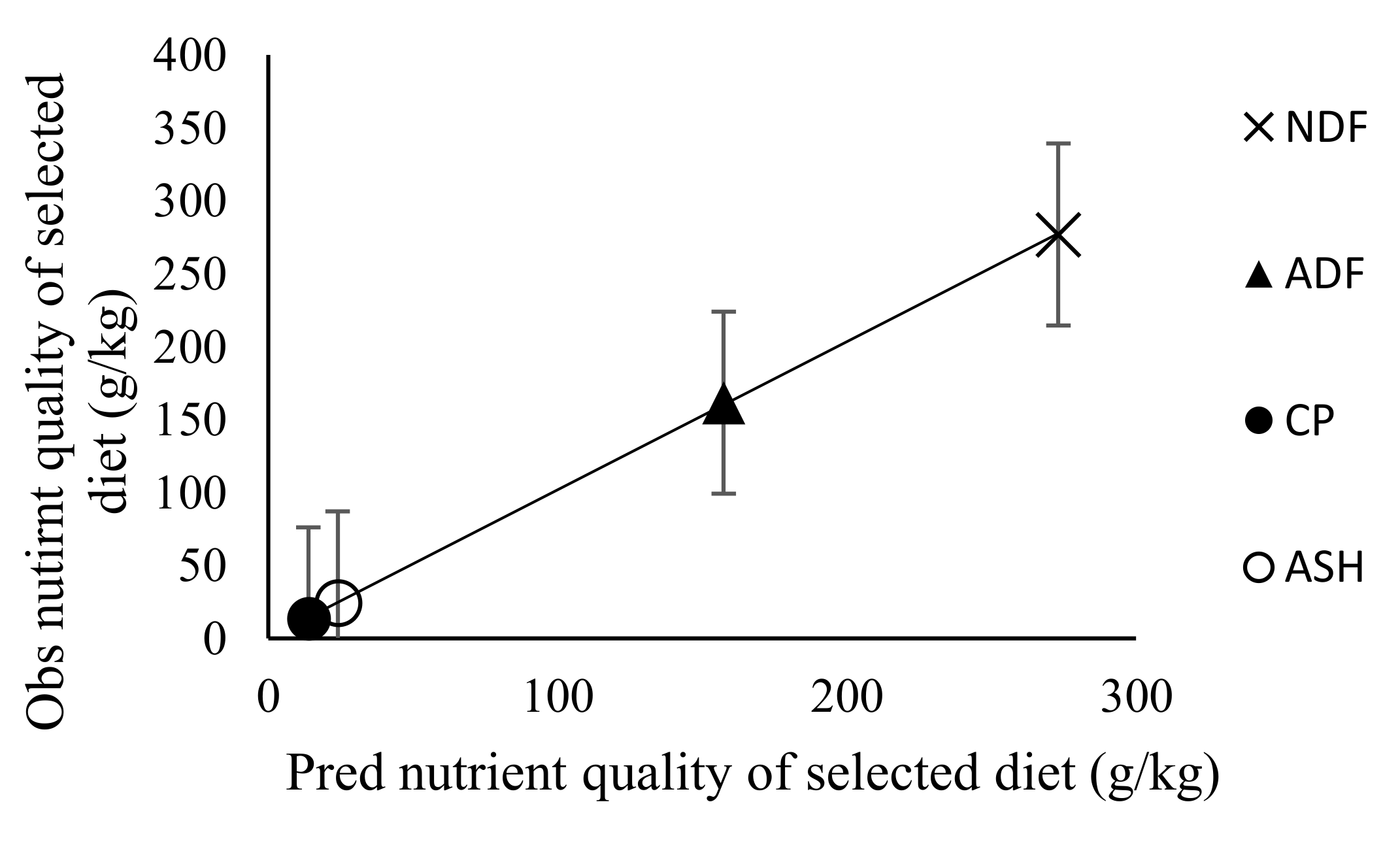
3.3. Effect of Stocking Rate on Faecal Recovery of Markers and Prediction of Diet Components Selected by Sheep
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Egea, A.V.; Allegretti, L.; Lama, S.P.; Grilli, D.; Sartor, C.; Fucili, M.; Guevara, J.C.; Passera, C. Selective behavior of Creole goats in response to the functional heterogeneity of native forage species in the central Monte desert, Argentina. Small Rumin. Res. 2014, 120, 90–99. [Google Scholar] [CrossRef]
- Salem, H.B.; Smith, T. Feeding strategies to increase small ruminant production in dry environments. Small Rumin.Res. 2008, 77, 174–194. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A. In vitro methane production of Eragrostis hay treated with graded levels of urea or nitrate. J. Anim. Plant Sci. 2018, 28, 679–685. [Google Scholar]
- Ferreira, L.M.M.; Celaya, R.; Benavides, R.; Jáuregui, B.M.; García, U.; Santos, A.S.; Osoro, K. Foraging behaviour of domestic herbivore species grazing on heathlands associated with improved pasture areas. Livest. Sci. 2013, 155, 373–383. [Google Scholar] [CrossRef]
- Magadlela, A.M. An evaluation of n-alkanes as markers to estimate dry matter intake, diet selection and solid digesta passage rates in ruminants. PhD Thesis, University of Edinburgh, Edinburgh, UK, 2001. [Google Scholar]
- Moyo, M.; Adebayo, R.A.; Nsahlai, I.V. Effects of roughage quality, period of day and time lapse after meal termination on rumen digesta load in goats and sheep. Asian Australas. J. Anim. Sci. 2018, 31, 1183–1196. [Google Scholar] [CrossRef]
- Ramirez, H.R.; Harvatine, K.J.; Kononoff, P.J. Forage particle size and fat intake affect rumen passage, the fatty acid profile of milk, and milk fat production in dairy cows consuming dried distillers grains with solubles. J. Dairy Sci. 2016, 99, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Mkhize, N.R.; Scogings, P.F.; Nsahlai, I.V.; Dziba, L.E. Diet selection of goats depends on season: Roles of plant physical and chemical traits. Afr. J. Range Forage Sci. 2014, 31, 209–214. [Google Scholar] [CrossRef]
- Chen, X.J.; Hou, F.J.; Matthew, C.; He, X.Z. Stocking rate effects on metabolizable energy intake and grazing behaviour of Tan sheep in steppe grassland on the Loess Plateau of Northwest China. J. Agric. Sci. 2010, 148, 709–721. [Google Scholar] [CrossRef]
- Pepeta, B.N. Use of inert markers to predict diet composition, forage intake, digestibility and passage rate in sheep. Master’s Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2019. [Google Scholar]
- Rankins, D.L., Jr.; Bransby, D.I. Fermentation of steers grazing Sorghum halepense at three stocking rates. Trop. Grasslands. 1995, 29, 102–110. [Google Scholar]
- Martínez, M.E.; De la Barra, R. Diet composition of two sheep breeds grazing in Chiloe Archipelago. Chile J. Anim. Vet. Adv. 2014, 13, 871–876. [Google Scholar]
- Ferreira, L.M.M.; Celaya, R.; Santos, A.S.; Mayes, R.W.; Rodrigues, M.A.M.; Osoro, K. Application of long-chain alcohols as diet-composition markers in sheep fed on grass–white clover and heather-gorse plant species. Grass Forage Sci. 2015, 70, 30–43. [Google Scholar] [CrossRef]
- Alonso-Díaz, M.A.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Hoste, H.; Aguilar-Caballero, A.J.; Capetillo-Leal, C.M. Sheep preference for different tanniniferous tree fodders and its relationship with in vitro gas production and digestibility. Anim. Feed Sci. Technol. 2009, 151, 75–85. [Google Scholar] [CrossRef]
- Orskov, E.R.; DeBHovell, F.D.; Mould, F. The use of the nylon bag technique for the evaluation of feedstuffs. Trop. Anim. Prod. 1980, 5, 195–213. [Google Scholar]
- McDonald, I. A revised model for the estimation of protein degradability in the rumen. J. Agric. Sci. 1981, 96, 251–252. [Google Scholar] [CrossRef]
- Udén, P.; Colucci, P.E.; Van Soest, P.J. Investigation of chromium, cerium and cobalt as markers in digesta. Rate of passage studies. J. Sci. Food Agric. 1980, 31, 625–632. [Google Scholar] [CrossRef]
- Hatfield, P.G.; Clanton, D.C.; Sanson, D.W.; Eskridge, K.M. Methodsof administering ytterbium for estimation of faecal output. J. Range Manag. 1990, 316–320. [Google Scholar] [CrossRef]
- Grovum, W.L.; Williams, V.J. Rate of passage of digesta in sheep: 4. Passage of marker through the alimentary tract and the biological relevance of rate-constants derived from the changes in concentration of marker in faeces. Br. J. Nutr. 1973, 30, 313–329. [Google Scholar] [CrossRef] [Green Version]
- Grovum, W.L.; Phillips, G.D. Rate of passage of digesta in sheep: 5. Theoretical considerations based on a physical model and computer simulation. Br. J. Nutr. 1973, 30, 377–390. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 17th ed.Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 2000. [Google Scholar]
- ANKOM Technology. Method for Determining Acid Detergent Fiber. 2011. Available online: https://www.ankom.com/procedures.aspx (accessed on 15 October 2018).
- Collier, R.E.; Foulds, S.M. A measure of the ruggedness of the modified acid-detergent fibre method. J. Sci. Food Agric. 1975, 26, 949–952. [Google Scholar] [CrossRef]
- Van Keulen, J.Y.B.A.; Young, B.A. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J. Animal Sci. 1977, 44, 282–287. [Google Scholar] [CrossRef]
- Krebs, C.J. Measurement of dietary preferences. In Ecological methodology; Harper Collins: New York, NY, USA, 1989; pp. 392–407. [Google Scholar]
- Ferreira, L.M.M.; Garcia, U.; Rodrigues, M.A.M.; Celaya, R.; Dias-da-Silva, A.; Osoro, K. Estimation of feed intake and apparent digestibility of equines and cattle grazing on heathland vegetation communities using the n-alkane markers. Livest. Sci. 2007, 110, 46–56. [Google Scholar] [CrossRef]
- Langlands, J.P. Assessing the nutrient status of herbivores. In The nutrition of herbivores; Hacker, J.B., Ternouth, J.H., Eds.; Academic Press: London, UK, 1987; pp. 363–390. [Google Scholar]
- Gerber, N.D. The effect of feed type and diet quality on kinetics of digestion: The degradation properties of certain protein supplements, and the effect of concentrate supplementation and basal roughage quality on eating behaviour and particle passage from the rumen of sheep. Master’s Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2014. [Google Scholar]
- Moyo, M.; Bhiya, S.T.; Masande, K.; Nsahlai, I.V. Evaluation and prediction of the nutritive value of underutilised forage crops as potential feeds for ruminants. In Forage Groups; Edvan, R.L., Santos, E.M., Eds.; IntechOpen: London, UK, 2019; pp. 87–106. [Google Scholar]
- Nsahlai, I.V.; Siaw, D.E.K.A.; Umunna, N.N. Inter-relationships between chemical constituents, rumen dry matter and nitrogen degradability in fresh leaves of multipurpose trees. J. Sci Food Agric. 1995, 69, 235–246. [Google Scholar] [CrossRef]
- Arnold, G.W. Regulation of forage intake. In Bioenergetics of wild herbivores; Hudson, R.J., White, R.G., Eds.; CRC Press: New York, NY, USA, 2017; pp. 81–102. [Google Scholar]
- Chua, B.; Coenen, E.; Van Delen, J.; Weary, D. Effects of pair versus individual housing on the behaviour and performance of dairy calves. J. Dairy Sci. 2002, 85, 360–364. [Google Scholar] [CrossRef]
- Vallentine, J.F. Grazing Management, 2nd ed.; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Odoi, F.N.; Owen, E. Encouraging store lambs to eat barley straw at housing: Influence upon intake of pen to pen visibility and number of lambs per pen. Proc. Br. Soc. Anim. Prod. 1993, 56, 455. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional ecology of the ruminant., 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Catanese, F.; Distel, R.A.; Provenza, F.D.; Villalba, J.J. Early experience with diverse foods increases intake of nonfamiliar flavors and feeds in sheep. J. Anim. Sci. 2012, 90, 2763–2773. [Google Scholar] [CrossRef]
- Ruckstuhl, K.E.; Festa-Bianchet, M.; Jorgenson, J.T. Bite rates in Rocky Mountain bighorn sheep (Ovis canadensis): Effects of season, age, sex and reproductive status. Behav. Ecol. Sociobiol. 2003, 54, 167–173. [Google Scholar] [CrossRef]
- Owen-Smith, N. Credible models for herbivore-vegetation systems: Towards an ecology of equations. S. Afr. J. Sci. 2002, 98, 445–449. [Google Scholar]
- Illius, A.W.; Gordon, I.J. Constraints on diet selection and foraging behaviour in mammalian herbivores. In Behavioural mechanisms of food selection; Hughes, R.N., Ed.; Springer: Berlin, Germany, 1990; pp. 369–393. [Google Scholar]
- Huhtanen, P.; Kaustell, K.; Jaakkola, S. The use of internal markers to predict total digestibility and duodenal flow of nutrients in cattle given six different diets. Anim. Feed Sci. Tech. 1994, 48, 211–227. [Google Scholar] [CrossRef]


| Diet | 1 Chemical Composition (g/kg DM) | 2 Degradability Parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | NDF | ADF | MADF | AIA | ADL | CP | Ash | HEM | a | b | c | PDDM | EDDM | |
| Maize stover | 910 | 893 | 542 | 771 | 17.1 | 21.1 | 49.9 | 41.4 | 351 | 101 | 534 | 0.02 | 634 | 322 |
| Sorghum stover | 882 | 877 | 512 | 695 | 26.4 | 39.0 | 43.4 | 110.8 | 365 | 118 | 573 | 0.02 | 691 | 347 |
| Veld hay | 911 | 767 | 430 | 497 | 20.1 | 73.3 | 30.4 | 74.6 | 337 | 188 | 528 | 0.02 | 716 | 414 |
| Maize stover stem | 937 | 858 | 545 | 531 | 25.7 | 26.1 | 52.9 | 34.4 | 313 | 130 | 583 | 0.01 | 713 | 300 |
| Maize stover leaves | 921 | 837 | 450 | 578 | 13.3 | 13.6 | 52.2 | 39.5 | 387 | 101 | 536 | 0.04 | 636 | 393 |
| Sorghum stover stem | 917 | 844 | 464 | 538 | 45.4 | 28.7 | 43.8 | 56.0 | 381 | 175 | 580 | 0.03 | 754 | 390 |
| Sorghum stover leaves | 898 | 850 | 472 | 400 | 16.3 | 19.3 | 40.1 | 66.4 | 378 | 108 | 513 | 0.02 | 621 | 337 |
| Veld hay stem | 907 | 699 | 419 | 449 | 17.4 | 54.5 | 31.5 | 48.2 | 310 | n.d | n.d | n.d | n.d | n.d |
| Veld hay leaves | 899 | 756 | 460 | 429 | 14.7 | 59.9 | 32.4 | 38.5 | 296 | n.d | n.d | n.d | n.d | n.d |
| SEM | 4.94 | 12.4 | 13.5 | 38.6 | 3.13 | 6.53 | 2.79 | 7.67 | 10.7 | 12.5 | 9.93 | 0.003 | 17.8 | 14.7 |
| Parameter | NDF | ADF | ADL | CP | Ash | HEM | a | b | c | PDDM | EDDM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NDF | 0.990 (0.093) | −0.509 (0.660) | 0.978 (0.135) | −0.091 (0.942) | 0.803 (0.407) | −0.997 (0.048) | 0.495 (0.670) | −0.756 (0.455) | −0.809 (0.400) | −0.988 (0.100) | |
| ADF | - | −0.628 (0.568) | 0.997 (0.043) | −0.234 (0.850) | 0.708 (0.500) | −0.998 (0.045) | 0.364 (0.763) | −0.653 (0.547) | −0.886 (0.307) | −0.999 (0.008) | |
| ADL | - | - | −0.679 (0.525) | 0.904 (0.282) | 0.106 (0.933) | 0.572 (0.613) | 0.497 (0.669) | −0.178 (0.885) | 0.917 (0.261) | 0.637 (0.560) | |
| CP | - | - | - | −0.299 (0.807) | 0.658 (0.542) | −0.991 (0.088) | 0.301 (0.806) | −0.600 (0.590) | −0.915 (0.264) | −0.999 (0.035) | |
| Ash | - | - | - | - | 0.521 (0.651) | 0.165 (0.895) | 0.820 (0.388) | −0.584 (0.603) | 0.658 (0.543) | 0.246 (0.842) | |
| HEM | - | - | - | - | - | −0.756 (0.455) | 0.916 (0.263) | −0.997 (0.048) | −0.299 (0.807) | −0.699 (0.507) | |
| a | - | - | - | - | - | - | −0.429 (0.718) | 0.704 (0.502) | 0.851 (0.352) | 0.997 (0.053) | |
| b | - | - | - | - | - | - | - | −0.943 (0.216) | 0.110 (0.930) | −0.353 (0.771) | |
| c | - | - | - | - | - | - | - | - | 0.227 (0.855) | 0.644 (0.555) | |
| PDDM | - | - | - | - | - | - | - | - | - | 0.891 (0.300) | |
| EDDM | - | - | - | - | - | - | - | - | - | - |
| 1 Y | 2 X | N | Models | AIC | p-Value | RMSE | R2 |
|---|---|---|---|---|---|---|---|
| a | NDF | 7 | 678 (±232.6) − 0.65 (±0.275) NDF | 48.8 | 0.07 | 27.0 | 0.525 |
| CP | 7 | 264 (±68.62) − 2.98 (±1.517) CP | 49.0 | 0.11 | 29.5 | 0.435 | |
| NDF, CP | 7 | 580 (±290.7) − 0.46 (±0.412) NDF − 1.34 (±2.085) CP | 49.1 | 0.19 | 28.8 | 0.569 | |
| NDF, ADF | 7 | 740 (±285.3) − 0.84 (±0.512) NDF + 0.21 (±0.452) ADF | 49.4 | 0.20 | 29.4 | 0.550 | |
| NDF, Ash | 7 | 660 (±260.5) − 0.64 (±0.303) NDF + 0.17 (±0.457) Ash | 49.6 | 0.21 | 29.7 | 0.541 | |
| b | ADF | 7 | 423 (±124.0) + 0.26 (±0.253) ADF | 48.4 | 0.35 | 28.3 | 0.174 |
| CP | 7 | 489 (±67.0) + 1.35 (±1.481) CP | 48.0 | 0.41 | 28.8 | 0.142 | |
| CP, Ash | 7 | 420 (±110.1) + 2.27 (±1.913) CP + 0.46 (±0.570) Ash | 49.6 | 0.54 | 29.9 | 0.263 | |
| ADF, CP | 7 | 0.07 (±0.026) − 0.0002 (±0.0001) ADF + 0.001(±0.0004) CP | 70.0 | 0.18 | 0.006 | 0.572 | |
| c | NDF, ADF | 7 | 0.002 (±0.060) + 0.0001 (±0.0001) NDF − 0.0002(±0.0001) ADF | 69.0 | 0.24 | 0.006 | 0.513 |
| NDF, CP | 7 | 0.03 (±0.067) + 0.0001 (±0.0001) NDF + 0.0005(±0.0005) CP | 69.0 | 0.33 | 0.006 | 0.633 | |
| EDDM | ADF | 7 | 771 (±79.10) − 0.85 (±0.162) ADF | 42.1 | <0.01 | 18.0 | 0.846 |
| ADF, Ash | 7 | 761 (±94.33) − 0.84 (±0.182) ADF + 0.08 (±0.311) Ash | 44.0 | 0.02 | 20.0 | 0.849 | |
| ADF, CP | 7 | 772 (±89.01) − 0.86 (±0.231) ADF + 0.14 (±1.325) CP | 44.1 | 0.02 | 20.1 | 0.847 | |
| NDF, ADF | 7 | 780 (±195.3) − 0.02 (±0.350) NDF − 0.83 (±0.309) ADF | 44.1 | 0.02 | 20.1 | 0.846 | |
| PDDM | NDF | 7 | 1053 (±449.8) − 0.44 (±0.531) NDF | 57.0 | 0.45 | 52.3 | 0.121 |
| 1 Diet Intake | Stocking Rate (Sheep per Pen) | 2 Mixed Model | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | SEM | Significance | |
| Veld hay | 301 | 306 | 446 | 434 | 70 | NS |
| Maize stover | 146b | 416a | 143b | 209b | 62 | * |
| Sorghum stover | 262 | 384 | 163 | 175 | 73 | NS |
| Total DM intake | 709 | 1111 | 766 | 817 | 171 | NS |
| FR-total DM intake | 651.4 | 1000 | 713.0 | 737.4 | 110 | NS |
| KSI = 85.5% | - | - | - | - | - | - |
| Nutrient intake | ||||||
| CP | 28.3 | 45.6 | 31.5 | 34.5 | 4.70 | NS |
| NDF | 591 | 943 | 613 | 672 | 94.50 | NS |
| ADF | 343 | 553 | 353 | 389 | 55.6 | NS |
| Ash | 49.5 | 84.8 | 56.0 | 62.7 | 9.13 | NS |
| 1 Parameter | Stocking Rate | 2 Mixed Model | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | SEM | Significance | |
| Diet selection(g/g) | Observed | |||||
| Veld hay | 0.409 | 0.286 | 0.632 | 0.557 | 0.09 | NS |
| Maize stover | 0.236 | 0.369 | 0.162 | 0.239 | 0.07 | NS |
| Sorghum stover | 0.356 | 0.346 | 0.205 | 0.205 | 0.09 | NS |
| KSI = 90.9% | - | - | - | - | - | - |
| Diet selection (g/g) | Predicted | |||||
| Veld hay | 0.655 | 0.393 | 0.632 | 0.557 | 0.07 | NS |
| Maize stover | 0.114 | 0.262 | 0.147 | 0.170 | 0.04 | NS |
| Sorghum stover | 0.231 | 0.346 | 0.222 | 0.273 | 0.04 | NS |
| Diet quality (g/kg DM) | ||||||
| CP | 40.27 | 41.26 | 41.77 | 42.26 | 1.91 | NS |
| NDF | 770.91 | 779.11 | 761.70 | 766.53 | 4.27 | NS |
| ADF | 450.99 | 460.44 | 439.14 | 444.75 | 5.18 | NS |
| Ash | 71.30 | 76.50 | 73.68 | 76.44 | 4.408 | NS |
| DMDigestibility | 369b | 590a | 428b | 451b | 53.00 | * |
| FR-digestibility | 290 | 529 | 430 | 409 | 67.78 | NS |
| KSI = 94.74% | - | - | - | - | - | - |
| 1 Parameter | Stocking Rate (Sheep per Pen) | 2 Mixed Model | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | SEM | Significance | |
| Fractional passage rate (h−1) | ||||||
| Rumen solids (kS) | 0.027 | 0.031 | 0.030 | 0.029 | 0.005 | NS |
| Hindgut solids (kS) | 0.040 | 0.042 | 0.046 | 0.039 | 0.009 | NS |
| Rumen liquids (kL) | 0.078 | 0.054 | 0.07 | 0.08 | 0.367 | NS |
| Hindgut liquids (kL) | 0.115 | 0.076 | 1.04 | 0.101 | 0.409 | NS |
| Mean retention time (h) | ||||||
| Rumensolids | 37.49 | 41.03 | 30.88 | 35.72 | 7.49 | NS |
| Hindgutsolids | 30.00 | 18.80 | 28.18 | 27.33 | 5.93 | NS |
| Rumenliquids | 16.3 | 19.7 | 13.9 | 13.0 | 3.37 | NS |
| Hindgutliquids | 10.4 | 12.6 | 13.2 | 10.1 | 3.93 | NS |
| Total tract mean retention time (h) | ||||||
| Solid fraction MRT | 70.1 | 62.9 | 60.7 | 65.42 | 11.61 | NS |
| Liquid fraction MRT | 30.3 | 34.2 | 23.8 | 25.4 | 6.82 | NS |
| 1 Marker | Stocking Rate (Sheep per Pen) | 2 General Linear Model | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | RMSE | Significance | |
| MADF | 35.5 | 31.3 | 32.9 | 34.9 | 0.07 | NS |
| ADL | 49.1 | 49.3 | 53.1 | 47.4 | 0.15 | NS |
| AIA | 81.0 | 118 | 76.1 | 76.9 | 0.41 | NS |
| Diet | Proportion of Plant Fraction in Refusals (g/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Offered | 1 | 2 | 4 | 8 | ||||||
| Stem | Leaf | Stem | Leaf | Stem | Leaf | Stem | Leaf | Stem | Leaf | |
| MS | 0.713 | 0.287 | 0.770 | 0.230 | 0.865 | 0.135 | 0.773 | 0.227 | 0.882 | 0.118 |
| SS | 0.701 | 0.299 | 0.836 | 0.164 | 0.809 | 0.191 | 0.766 | 0.234 | 0.923 | 0.077 |
| VH | 0.606 | 0.394 | 0.364 | 0.636 | 0.654 | 0.346 | 0.285 | 0.715 | 0.412 | 0.588 |
| SEM | 0.028 | 0.120 | 0.052 | 0.132 | 0.134 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepeta, B.N.; Moyo, M.; Hassen, A.; Nsahlai, I.V. Stocking Rate Has No Confounding Effect on the Use of Internal and Inert Markers to Predict Botanical Composition, Diet Quality, Degradability and Passage Rate Kinetics in Sheep. Animals 2020, 10, 2232. https://doi.org/10.3390/ani10122232
Pepeta BN, Moyo M, Hassen A, Nsahlai IV. Stocking Rate Has No Confounding Effect on the Use of Internal and Inert Markers to Predict Botanical Composition, Diet Quality, Degradability and Passage Rate Kinetics in Sheep. Animals. 2020; 10(12):2232. https://doi.org/10.3390/ani10122232
Chicago/Turabian StylePepeta, Bulelani Nangamso, Mehluli Moyo, Abubeker Hassen, and Ignatius Verla Nsahlai. 2020. "Stocking Rate Has No Confounding Effect on the Use of Internal and Inert Markers to Predict Botanical Composition, Diet Quality, Degradability and Passage Rate Kinetics in Sheep" Animals 10, no. 12: 2232. https://doi.org/10.3390/ani10122232







