Influence of Feeding Quinoa (Chenopodium quinoa) Seeds and Prickly Pear Fruit (Opuntia ficus indica) Peel on the Immune Response and Resistance to Aeromonas sobria Infection in Nile Tilapia (Oreochromis niloticus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Rearing Conditions
2.2. Formulation of Tested Diets
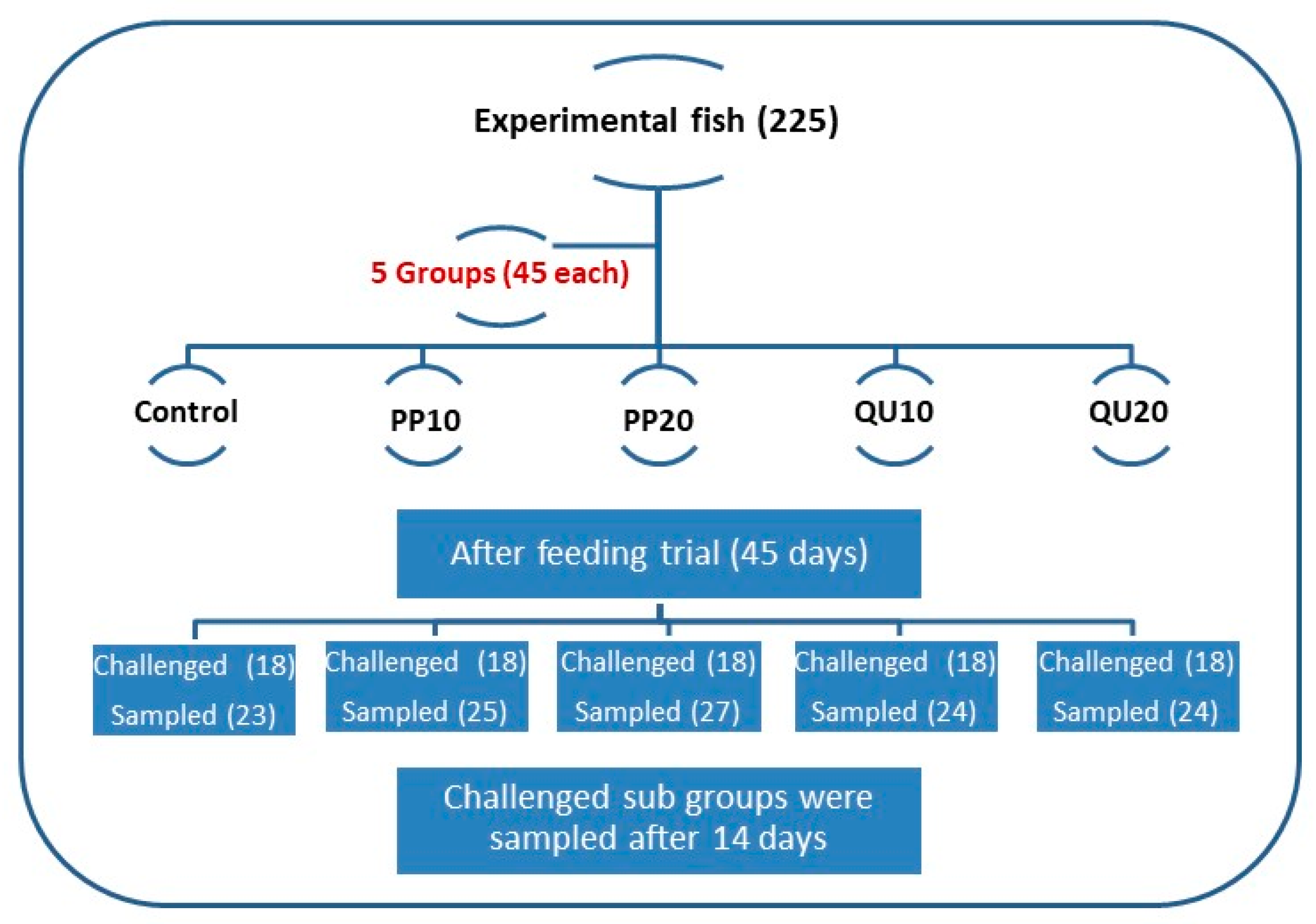
2.3. Experimental Design
2.4. Growth Performance
2.5. Blood and Tissue Sampling
2.6. Hematological Analysis
2.7. Serum Physiological Assays and Protein Profile
2.8. Antioxidant Status and Lipid Peroxidation Assays
2.9. Immune Response Assays
2.10. Transcriptional Analysis of Immune-Related Genes in the Spleen Tissue
2.11. Histological and Morphometric Methods
2.12. Aeromonas Sobria (A. sobria) Challenge Test
2.13. Statistical Analysis
3. Results
3.1. Survival Rate and Growth Performance in Nile Tilapia in Response to Dietary Supplementation with PP Peel or QU Seed
3.2. Hematological Variables of Nile Tilapia in Response to Dietary Supplementation with PP Peel or QU Seed
3.3. Physiological Biomarkers and Protein Profile in Nile Tilapia in Response to Dietary Supplementation with PP Peel or QU Seed
3.4. Antioxidant and Oxidative Stress indices in Nile Tilapia in Response to Dietary Supplementation with PP or QU
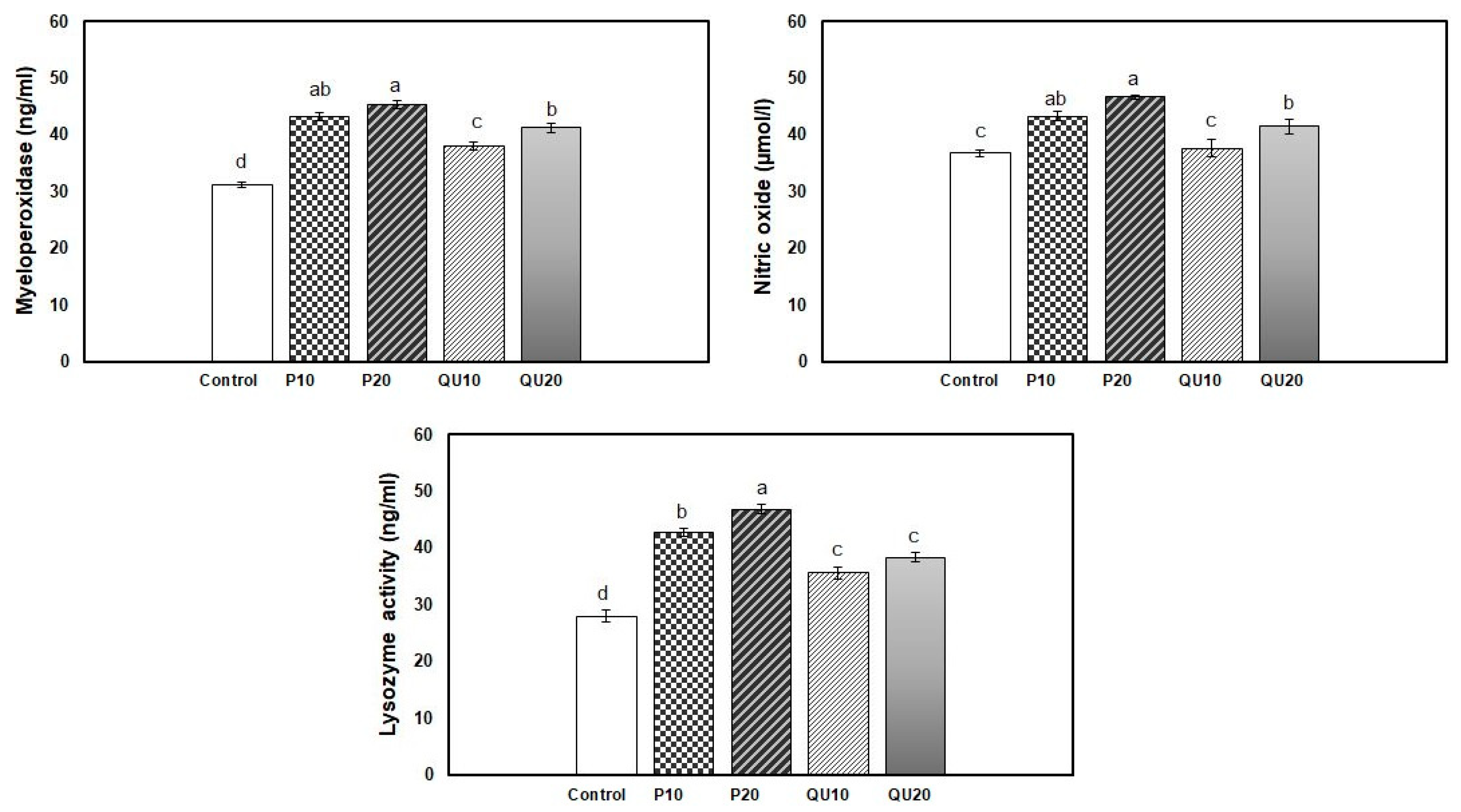
3.5. Indices of Immunological Response in Nile Tilapia Fed on Diets Supplemented with PP Peel or QU Seed
3.6. Gene expression Patterns of Immune-Encoding Genes in Nile Tilapia in Response to Dietary Supplementation with PP or QU
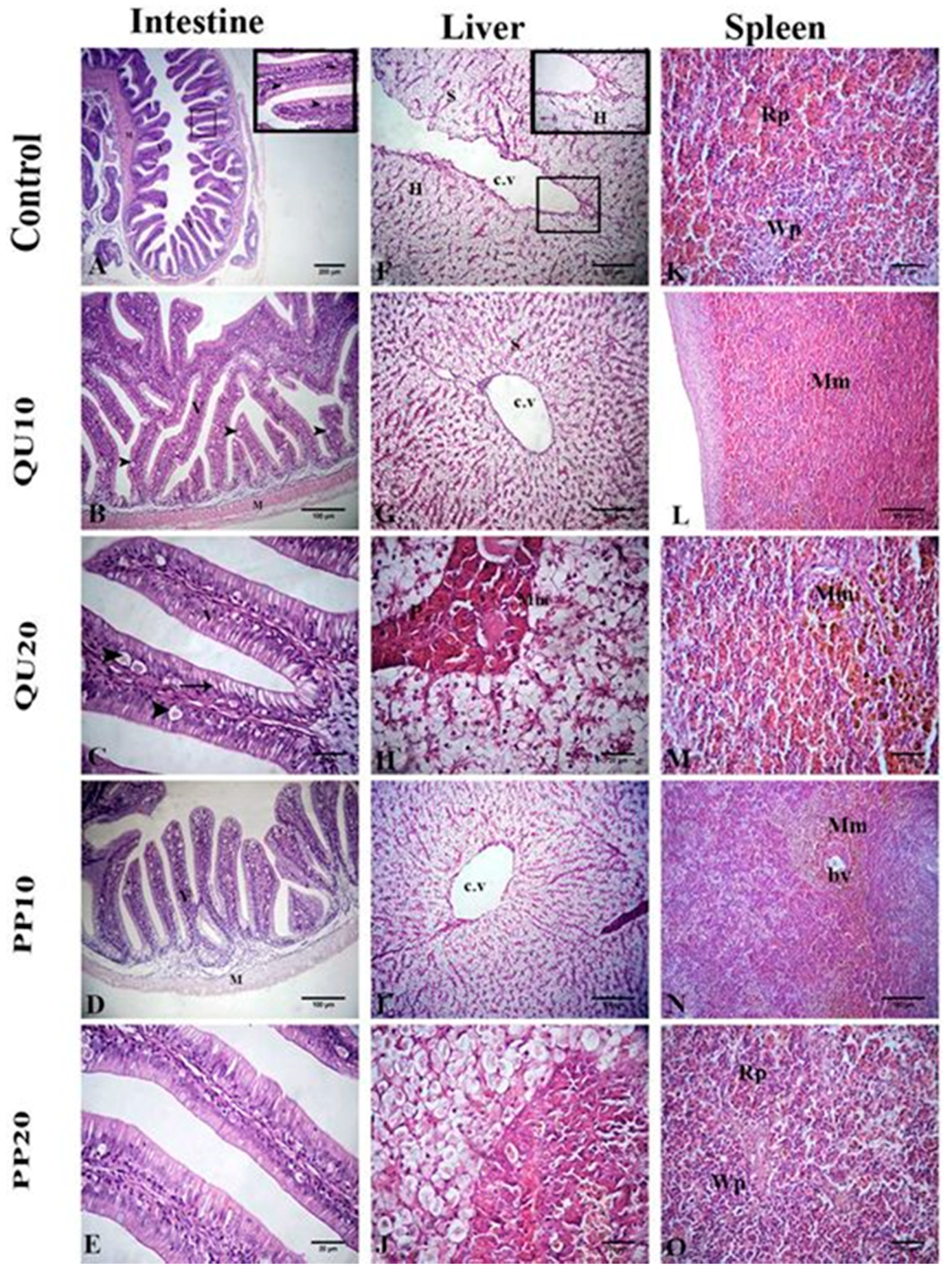
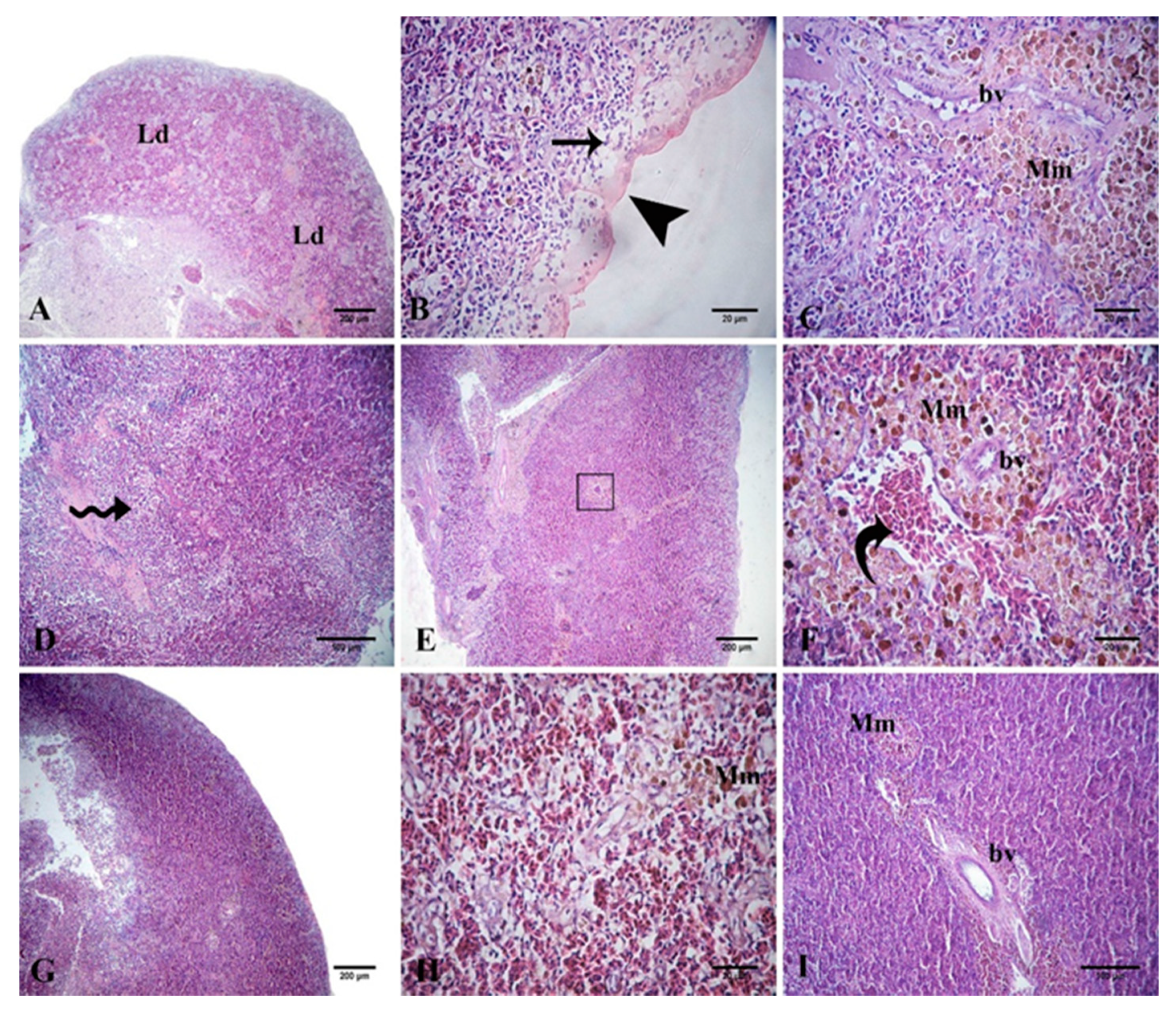
3.7. Histological Evidence in Nile Tilapia in Response to Dietary Supplementation with PP or QU
3.8. Host Resistance Against A. sobria
3.8.1. Mortality Rate, RPS, and Clinical Signs in the Surviving Nile Tilapia Supplemented with PP or QU and Challenged with A. sobria
3.8.2. Serum Physiological and Immunological Indices in Nile Tilapia Supplemented with PP or QU and Challenged with A. sobria
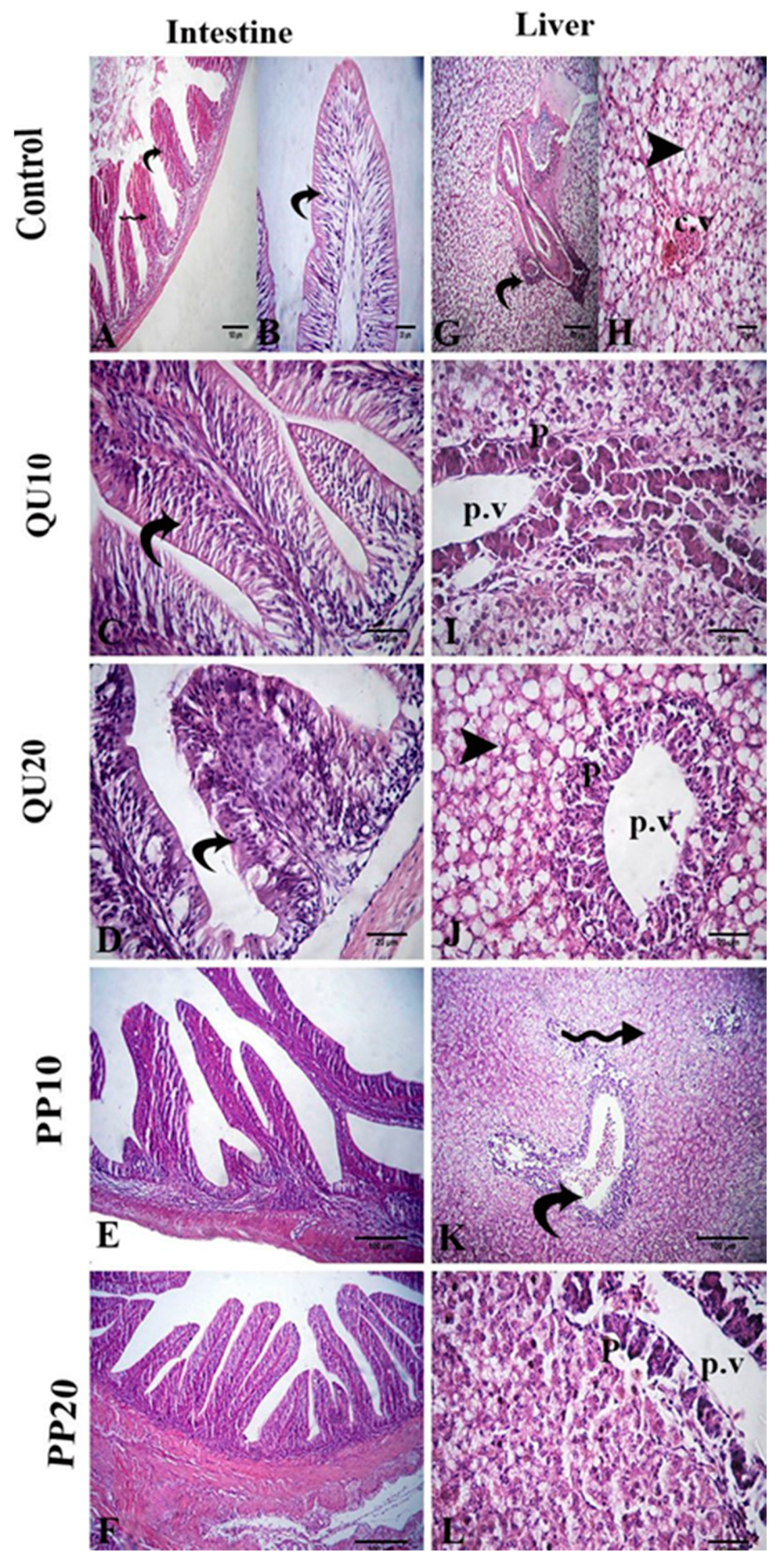
3.8.3. Histological Evidence in Nile Tilapia Supplemented with PP or QU and Challenged with A. sobria
3.8.4. Expression Pattern of the Immune-Encoding Genes in Nile Tilapia Supplemented with PP or QU and Challenged with A. sobria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Menanteau-Ledouble, S.; Krauss, I.; Santos, G.; Fibi, S.; Weber, B.; El-Matbouli, M. Effect of a phytogenic feed additive on the susceptibility of Onchorhynchus mykiss to Aeromonas salmonicida. Dis. Aquat. Org. 2015, 115, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Welker, T.L.; Lim, C. Use of Probiotics in Diets of Tilapia. J. Aquac. Res. Dev. 2011. [Google Scholar] [CrossRef] [Green Version]
- Haygood, A.M.; Jha, R. Strategies to modulate the intestinal microbiota of Tilapia (Oreochromis sp.) in aquaculture: A review. Rev. Aquac. 2016, 10, 320–333. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M. Tilapia Culture; CABI International: Oxfordshire, UK, 2006. [Google Scholar]
- Newaj-Fyzul, A.; Mutani, A.; Ramsubhag, A.; Adesiyun, A. Prevalence of bacterial pathogens and their antimicrobial resistance in tilapia and their pond water in Trinidad. Zoonoses Public Health 2008, 55, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Okaeme, A.N.; Ibiwoye, T.I.I. Hints on disease problems, prevention and control in the culture of tilapias and Clariid species in fresh water system in Nigeria. Tech. Rep. Ser. 2001, 18, 1–9. [Google Scholar]
- Subashinge, R.T. Physiological responses and depression of humoral components of the immune system in gilthead sea bream (Sparus aurata) following daily acute stress. Can. J. Fish. Aquat. Sci. 2005, 52, 2339–2346. [Google Scholar] [CrossRef]
- Hidalgo, R.B.; Figueras, M.J. Molecular detection and characterization of furunculosis and other Aeromonas fish infections. In Health and Environment in Aquaculture; IntechOpen Limited: London, UK, 2012; pp. 97–132. [Google Scholar]
- Yu, J.; Koo, B.H.; Kim, D.H.; Kim, D.W.; Park, S.W. Aeromonas sobria infection in farmed mud loach (Misgurnus mizolepis) in Korea, a bacteriological survey. Iran. J. Vet. Res. 2015, 16, 194. [Google Scholar]
- Coscelli, G.A.; Casabonne, C.; Morón-Alcain, E.; Arancegui, N.; Vigliano, F.A. Aeromonas sobria, an outbreak of natural infection in cultured silver catfish Rhamdia quelen (Quoy & Gaimard, 1824) in Argentina. J. Fish Dis. 2017, 40, 1929–1933. [Google Scholar]
- Noga, E.J. Fish Disease: Diagnosis and Treatment, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Korkoca, H.; Alan, Y.; Bozari, S.; Berktas, M.; Goz, Y. Detection € of putative virulence genes in Aeromonas isolates from humans and animals. J. Infect. Dev. Ctries. 2013, 8, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Maron, D.F.; Smith, T.J.S.; Nachman, K.E. Restrictions on antimicrobial use in food animal production: An international regulatory and economic survey. Glob. Health 2013, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Nobahar, Z.; Gholipour-Kanani, H.; Kakoolaki, S.; Jafaryan, H. Effects of garlic (Allium sativum) and nettle (Urtic adioica) on growth performance and on hematological parameters of beluga (Huso huso). Iran. J. Aquat. Anim. Health 2015, 1, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Hoseinifar, S.H.; Sun, Y.; Wang, A.; Zhou, Z. Probiotics as means of diseases control in aquaculture, A Review of current knowledge and future perspectives. Front Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Saad, A.; Dalel, B.; Rjeibi, I.; Smida, A.; Ncib, S.; Zouari, N.; Zourgui, L. Phytochemical, antioxidant and protective effect of cactus cladodes extract against lithium-induced liver injury in rats. Pharm. Biol. 2017, 55, 516–525. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; PérezÁlvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Iafelice, G.; Verardo, V.; Marconi, E.; Caboni, M.F. Influence of pearling process on phenolic and saponin content in quinoa (Chenopodium quinoa Willd). Food Chem. 2014, 157, 174–178. [Google Scholar] [CrossRef]
- Nowak, V.; Du, J.; Charrondière, U.R. Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Pasko, P.; Barton, H.; Zagrodzki, P.; Izewska, A.; Krosniak, M.; Gawlik, M.; Gorinstein, S. Effect of diet supplemented with quinoa seeds on oxidative status in plasma and selected tissues of high fructose-fed rats. Plant Foods Hum. Nutr. 2010, 65, 146–151. [Google Scholar] [CrossRef]
- Hirose, Y.; Fujita, T.; Ishii, T.; Ueno, N. Antioxidative properties and flavonoid composition of Chenopodium quinoa seeds cultivated in Japan. Food Chem. 2010, 119, 1300–1306. [Google Scholar] [CrossRef]
- Miranda, M.; Delatorre-Herrera, J.; Vega-Gálvez, A.; Jorquera, E.; Quispe-Fuentes, I.; Martínez, E.A. Antimicrobial potential and phytochemical content of six diverse sources of quinoa seeds (Chenopodium quinoa Willd.). Agric. Sci. 2014, 5, 1015. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Lee, Y.J.; Kim, Y.H.; Yoon, K.S. Antioxidant and antimicrobial activities of Quinoa (Chenopodium quinoa Willd.) seeds cultivated in Korea. Prev. Nutr. Food Sci. 2017, 22, 195–202. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Chenopodium quinoa-An Indian perspective. Ind. Crops. Prod. 2006, 23, 73–87. [Google Scholar] [CrossRef]
- Jacobsen, E.E.; Skadhauge, B.; Jacobsen, S.E. Effect of dietary inclusion of quinoa on broiler growth performance. Anim. Feed Sci. Technol. 1997, 65, 5–14. [Google Scholar] [CrossRef]
- Sataria, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of Sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [Green Version]
- Hegwood, D.A. Human Health Discoveries with Opuntia sp. (Prickly Pear). Hort Sci. 1990, 25, 1515–1516. [Google Scholar] [CrossRef] [Green Version]
- Barba, F.J.; Putnik, P.; Kovačević, D.B.; Poojary, M.M.; Roohinejad, S.; Lorenzo, J.M.; Koubaa, M. Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: From preservation of beverages to valorization of by-products. Trends Food Sci. Technol. 2017, 67, 260–270. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I. By-product recovery of Opuntia spp. peels: Betalainic and phenolic profiles and bioactive properties. Ind. Crops Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.M.; Sallam, E.M. Utilization of prickly pear peels to improve quality of pan bread. AJNSA 2016, 49, 151–163. [Google Scholar]
- Aragona, M.; Lauriano, E.R.; Pergolizzi, S.; Faggio, C. Opuntia ficus-indica (L.) Miller as a source of bioactivity compounds for health and nutrition. Nat. Prod. Res. 2018, 32, 2037–2049. [Google Scholar] [CrossRef]
- El Mostafa, K.; Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; Kebbaj, M.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [Green Version]
- Sepúlveda, L.; Romaní, A.; Aguilar, C.N.; Teixeira, J. Valorization of pineapple waste for the extraction of bioactive compounds and glycosides using autohydrolysis. Innov. Food Sci. Emerg Technol. 2018, 47, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Slimen, I.B.; Chabaane, H.; Chniter, M.; Mabrouk, M.; Ghram, A.; Miled, K.; Behi, I.; Abderrabba, M.; Najar, T. Thermoprotective properties of Opuntia ficus-indica f. inermis cladodes and mesocarps on sheep lymphocytes. J. Therm. Biol. 2019, 81, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Abdel Rahman, A.N.; ElHady, M.; Shalaby, S.I. Efficacy of the dehydrated lemon peels on the immunity, enzymatic antioxidant capacity and growth of Nile tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus). Aquaculture 2019, 505, 92–97. [Google Scholar] [CrossRef]
- Rosenzweig, S.D.; Holland, S.M. Defects in the interferon gamma and interleukin-12 pathways. Immunol. Rev. 2005, 203, 38–47. [Google Scholar] [CrossRef]
- Prabu, D.L.; Sahu, N.P.; Pal, A.K.; Dasgupta, S.; Narendra, A. Immunomodulation and interferon gamma gene expression in sutchi cat fish, Pangasianodon hypophthalmus: Effect of dietary fucoidan rich seaweed extract (FRSE) on pre and post challenge period. Aquac. Res. 2016, 47, 199–218.–218. [Google Scholar] [CrossRef] [Green Version]
- Maehr, T.; Costa, M.M.; Vecino, J.L.G.; Wadsworth, S.; Martin, S.A.; Wang, T.; Secombes, C.J. Transforming growth factor-β1b: A second TGF-β1 paralogue in the rainbow trout (Oncorhynchus mykiss) that has a lower constitutive expression but is more responsive to immune stimulation. Fish Shellfish. Immunol. 2013, 34, 420–432. [Google Scholar] [CrossRef]
- Awad, E.; Mitchell, W.J.; Austin, B. Effect of dietary supplements on cytokine gene expression in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2011, 34, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Forouhar Vajargah, M.; Mohamadi Yalsuyi, A.; Hedayati, A.; Faggio, C. Histopathological lesions and toxicity in common carp (Cyprinus carpio L. 1758) induced by copper nanoparticles. Microsc. Res. Tech. 2018, 81, 724–729. [Google Scholar] [CrossRef]
- Kollner, B.; Wasserrab, B.; Kotterba, G.; Fischer, U. Evaluation of immune functions of rainbow trout (Oncorhynchus mykiss)dhow can environmental influences be detected? Toxicol. Lett. 2002, 131, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Nordin, S.F.; Nordin, M.L.; Osman, A.Y.; Hamdan, R.H.; Shaari, R.; Arshad, M.M. The effect of Matricaria Chamomilla L. on the growth performance of Red Hybrid Tilapia. Biomed. Pharmacol. J. 2017, 10, 1905–1915. [Google Scholar] [CrossRef]
- APHA, A. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, Inc.: Washington, DC, USA, 1998. [Google Scholar]
- Adewolu, M.A.; Adamson, A.A. Amaranthus spinosus leaf meal as potential dietary protein source in the practical diets for Clarias gariepinus (Burchell, 1822) fingerlings. Int. J. Zool. Res. 2011, 7, 128–137. [Google Scholar] [CrossRef] [Green Version]
- El-Neney, B.A.; Zeedan, K.I.; El-Kotamy, E.M.; Gad, G.G.; Abdou, A. Effect of using prickly pear as a source of dietary feedstuffs on productive performance, physiological traits and immune response of rabbit. 2-prickly pear peels. EJNF 2019, 22, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Jobling, M. Fish bioenergetics. Oceanogr. Lit. Rev. 1995, 9, 785. [Google Scholar]
- Dacie, J.V.; Lewis, S.M. Practical Haematology; Churchill Livingstone: London, UK; New York, NY, USA, 1984. [Google Scholar]
- Murray, R. Aspartate Aminotransferase. In Clinical Chemistry: Theory Analysis and Correlation; Kaplan, L.A., Pesce, A.J., Eds.; C.V. Mosby publishing Co.: St. Louis, Mo, USA, 1984; pp. 1112–1116. [Google Scholar]
- Murray, R.L. Alanine aminotransferase. In Clinical Chemistry: Theory, Analysis, and Correlation, 2nd ed.; Kaplan, L.A., Pesce, A.J., Eds.; The C.V. Mosby publishing Co.: St. Louis, MO, USA, 1989; pp. 895–898. [Google Scholar]
- Wenger, C.; Kaplan, A.; Rubaltelli, F.F.; Hammerman, C. Alkaline phosphatase. In Clinical Chemistry: Theory Analysis and Correlation; Kaplan, L.A., Pesce, A.J., Eds.; The C.V. Mosby publishing Co.: St. Louis, MO, USA, 1984; pp. 1094–1098. [Google Scholar]
- Caraway, W.T. A stable starch substrate for the determination of amylase in serum and other body fluids. Am. J. Clin. Pathol. 1959, 32, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, I.G. Studies on the digestive lipases of Milkfish, Chanos chanos. Aquaculture 1990, 89, 315–325. [Google Scholar] [CrossRef]
- Doumas, B.T.; Bayse, D.D.; Carter, R.J.; Peters, T., Jr.; Schaffer, R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin. Chem. 1981, 27, 1642–1650. [Google Scholar] [CrossRef]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Ann. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ellis, A.E. Lysozyme assays. In Techniques in Fish Immunology; Stolen, J.S., Fletcher, T.C., Anderson, D.P., Roberson, B.S., Van Muiswinkel, W.B., Eds.; SOS Publications: Fair Haven, NJ, USA, 1990; pp. 101–103. [Google Scholar]
- Montgomery, H.A.C.D.J.; Dymock, J.F. Determination of nitrite in water. Analyst 1961, 86, 414. [Google Scholar]
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Suvarna, S.K.; Layton, C. Immunohistochemical and immunofluorescent techniques. In Bancroft’s Theory and Practice of Histological Techniques E-book, 8th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Khalil, S.R.; Reda, R.M.; Awad, A. Efficacy of Spirulina platensis diet supplements on disease resistance and immune-related gene expression in Cyprinus carpio L. exposed to herbicide atrazine. Fish Shellfish Immunol. 2017, 67, 119–128. [Google Scholar] [CrossRef]
- Amend, D.F. Potency testing of fish vaccines. In: Anderson, D.P., Hennessen, H. (Eds.), Fish Biologies: Serodiagnostics and Vaccines. Dev. Biol. Stand. 1981, 49, 447–454. [Google Scholar]
- Safari, R.; Hoseinifar, S.H.; Van Doan, H.; Dadar, M. The effects of dietary Myrtle (Myrtus communis) on skin mucus immune parameters and mRNA levels of growth, antioxidant and immune related genes in zebrafish (Danio rerio). Fish Shellfish Immunol. 2017, 66, 264–269. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.L.; Moate, R.; Davies, S.J.; Spring, P.; Sweetman, J.; Bradley, G. Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Anim. Sci. 2009, 87, 3226–3234. [Google Scholar] [CrossRef] [Green Version]
- Molina-Poveda, C.; Cárdenas, R.; Jover, M. Evaluation of amaranth (Amaranthus caudatus L.) and quinoa (Chenopodium quinoa) protein sources as partial substitutes for fish meal in Litopenaeus vannamei grow-out diets. Aquac. Res. 2017, 48, 822–835. [Google Scholar] [CrossRef]
- Tewary, A.; Patra, B.C. Oral administration of baker’s yeast (Saccharomyces cerevisiae) acts as a growth promoter and immunomodulator in Labeo rohita (Ham.). J. Aquac. Res. Dev. 2011, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gharaei, A.; Shafie, M.; Mirdar Harijani, J.; Hasanein, P.; Arshadi, A. Immune Responses and Haematological Parameters Changes of Rainbow Trout (Oncorhynchus mykiss) under Effects of Dietary Administration of Sumac (Rhus coriaria L.). J. Agric. Sci. Technol. 2020, 22, 173–186. [Google Scholar]
- Castro, L.M.; Alexandre, E.M.; Pintado, M.; Saraiva, J.A. Bioactive compounds, pigments, antioxidant activity and antimicrobial activity of yellow prickly pear peels. Int. J. Food Sci. Technol. 2019, 54, 1225–1231. [Google Scholar] [CrossRef] [Green Version]
- Dügenci, S.K.; Arda, N.; Candan, A. Some medicinal plants as immunostimulant for fish. J. Ethnopharmacol. 2003, 88, 99–106. [Google Scholar] [CrossRef]
- Cerezal, P.; Duarte, G. Use of skin in the elaboration of concentrated products of cactus pear (Opuntia ficus-indica (L.) Miller). J. Prof. Assoc. Cactus Dev. 2005, 7, 61–83. [Google Scholar]
- Jancurová, M.; Minarovičová, L.; Dandar, A. Quinoa—A review. Czech J. Food Sci. 2009, 27, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Talpur, A.D.; Ikhwanuddin, M.H.D. Dietary effects of garlic (Allium sativum) on haemato-immunological parameters, survival, growth, and disease resistance against Vibrio harveyi infection in Asian sea bass, Lates calcarifer (Bloch). Aquaculture 2012, 364, 6–12. [Google Scholar] [CrossRef]
- Fazlolahzadeh, F.; Keramati, K.; Nazifi, S.; Shirian, S.; Seifi, S. Effect of garlic (Allium sativum) on hematological parameters and plasma activities of ALT and AST of Rainbow trout in temperature stress. AJBAS 2011, 5, 84–90. [Google Scholar]
- Eggset, G.; Mikkelsen, H.; Killie, J.A. Immunocompetence and duration of immunity against Vibrio salmonicida and Aeromonas salmonicida after vaccination of Atlantic salmon (Salmo salar L.) at low and high temperatures. Fish Shellfish Immunol. 1997, 7, 247–260. [Google Scholar] [CrossRef]
- Udoh, J.P.; Emah, A.U.; George, I.E.; Philip, A.E. Growth performance and haematological response of Clarias gariepinus broodstock fed diets enriched with bitter leaf meal. Aquacult. Aquarium Conserv. Legis. 2017, 10, 1281–1296. [Google Scholar]
- Yao, Y.; Yao, J.; Du, Z.; Wang, P.; Ding, K. Structural elucidation and immune-enhancing activity of an arabinogalactan from flowers of Carthamus tinctorius L. Carbohydr. Polym. 2018, 202, 134–142. [Google Scholar] [CrossRef]
- Ennouri, M.; Ammar, I.; Khemakhem, B.; Attia, H. Chemical Composition and Antibacterial Activity of Opuntia Ficus-Indica F. Inermis (Cactus Pear) Flowers. J. Med. Food. 2014, 17, 908–914. [Google Scholar] [CrossRef]
- Ellis, A.E. Stress and the modulation of defense mechanisms in fish. Stress Fish 1981, 147–169. [Google Scholar]
- Řehulka, J. Aeromonas causes severe skin lesions in rainbow trout (Oncorhynchus mykiss): Clinical pathology, haematology, and biochemistry. Acta Vet. Brno 2002, 71, 351–360. [Google Scholar] [CrossRef]
- Řehulka, J.; Minařík, B. Blood parameters in brook trout Salvelinus fontinalis (Mitchill, 1815), affected by columnaris disease. Aquac. Res. 2007, 38, 1182–1197. [Google Scholar] [CrossRef]
- Kumar, V.; Makkar, H.; Becker, K. Nutritional, physiological and haematological responses in rainbow trout (Oncorhynchus mykiss) juveniles fed detoxified Jatropha curcas kernel meal. Aquac. Nutr. 2011, 17, 451–467. [Google Scholar] [CrossRef]
- Yu, W.; Wen, G.; Lin, H.; Yang, Y.; Huang, X.; Zhou, C.; Zhang, Z.; Duan, Y.; Huang, Z.; Li, T. Effects of dietary Spirulina platensis on growth performance, hematological and serum biochemical parameters, hepatic antioxidant status, immune responses and disease resistance of Coral trout Plectropomus leopardus (Lacepede, 1802). Fish Shellfish Immunol. 2018, 74, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Shahani, L.; Bhatnagar, P. Hepatoprotective effect of Chenopodium quinoa seed against CCL4-induced liver toxicity in Swiss albino male mice. Asian J. Pharm. Clin. Res. 2017, 10, 273–276. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; González-Rubio, M.G.Y.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787. [Google Scholar] [CrossRef]
- Paśko, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Saleem, M.; Ahmed, B.; Qadir, M.I.; Mahrukh, M.; Ahmad, M.; Ahmad, B. Hepatoprotective effect of Chenopodium murale in mice. Bangladesh J. Pharmacol. 2014, 9, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Nsimba, R.Y.; Kikuzaki, H.; Konishi, Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008, 106, 760–766. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elella, F.M.; Ali, R.F.M. Antioxidant and anti-cancer activities of different constituents extracted from Egyptian prickly pear Cactus (Opuntia Ficus-Indica) Peel. Biochem. Anal. Biochem. 2014, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Morán-Ramos, S.; Avila-Nava, A.; Tovar, A.R.; Pedraza-Chaverri, J.; López-Romero, P.; Torres, N. Opuntia ficus indica (nopal) attenuates hepatic steatosis and oxidative stress in obese Zucker (fa/fa) rats. J. Nutr. 2012, 142, 1956–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blando, F.; Russo, R.; Negro, C.; De Bellis, L.; Frassinetti, S. Antimicrobial and antibiofilm activity against Staphylococcus aureus of Opuntia ficus-indica (L.) Mill. cladode polyphenolic extracts. Antioxidants 2019, 8, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, S.W.; Liu, S.L. Effects of dietary quercetin on growth performance, serum lipids level and body composition of tilapia (Oreochromis niloticus). Ital. J. Anim. Sci. 2013, 12, e85. [Google Scholar] [CrossRef] [Green Version]
- Avila-Nava, A.; Calderón-Oliver, M.; Medina-Campos, O.N.; Zou, T.; Gu, L.; Torres, N.; Tovar, A.R.; Pedraza-Chaverri, J. Extract of cactus (Opuntia ficus indica) cladodes scavenges reactive oxygen species in vitro and enhances plasma antioxidant capacity in humans. J. Funct. Foods. 2014, 10, 13–24. [Google Scholar] [CrossRef]
- Awad, A.; Zaglool, A.W.; Ahmed, S.A.; Khalil, S.R. Transcriptomic profile change, immunologicalresponse and disease resistance of Oreochromis niloticus fed with conventional and Nano-Zinc oxide dietary supplements. Fish Shellfish Immunol. 2019, 93, 336–343. [Google Scholar] [CrossRef]
- Moustafa, E.M.; Dawood, M.A.O.; Assar, D.H.; Omar, A.A.; Elbialy, Z.I.; Farrag, F.A.; Shukry, M.; Zayed, M.M. Modulatory effects of fenugreek seeds powder on the histopathology, oxidative status, and immune related gene expression in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquaculture 2020, 515, 734589. [Google Scholar] [CrossRef]
- Paulsen, S.M.; Lunde, H.; Engstad, R.E.; Robertsen, B. In vivo effects of β-glucan and LPS on regulation of lysozyme activity and mRNA expression in Atlantic salmon (Salmo salar L.). Fish Shellfish Immunol. 2003, 14, 39–54. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Chi, C.; Kim, H.J.; Yun, S.; Park, S.C.; Sukumaran, V. Effect of guava leaves on the growth performance and cytokine gene expression of Labeo rohita and its susceptibility to Aeromonas hydrophila infection. Fish Shellfish Immunol. 2015, 46, 217–224. [Google Scholar] [CrossRef]
- Adel, M.; Yeganeh, S.; Dadar, M.; Sakai, M.; Dawood, M.A. Effects of dietary Spirulina platensis on growth performance, humoral and mucosal immune responses and disease resistance in juvenile great sturgeon (Huso huso Linnaeus, 1754). Fish Shellfish Immunol. 2016, 56, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.; Abd Elhakim, Y.; Abd El-fattah, A.H.; Farag, M.R.; Abd El-Hameed, N.E.; Abd Elhakeem, E.M. Dual immunological and oxidative responses in Oreochromis niloticus fish exposed to lambda cyhalothrin and concurrently fed with Thyme powder (Thymus vulgaris L.): Stress and immune encoding gene expression. Fish Shellfish Immunol. 2020, 100, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Engstad, R.E.; Robertsen, B.; Frivold, E. Yeast glucan induces increase in lysozyme and complement-mediated haemolytic activity in Atlantic salmon blood. Fish Shellfish Immunol. 1992, 2, 287–297. [Google Scholar] [CrossRef]
- Awad, E.; Cerezuela, R.; Esteban, M.Á. Effects of fenugreek (Trigonella foenum graecum) on gilthead seabream (Sparus aurata L.) immune status and growth performance. Fish Shellfish Immunol. 2015, 45, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, X.; Xue, P.; Zhang, Z.; Ren, G. Improved antibacterial effects of alkali-transformed saponin from quinoa husks against halitosis-related bacteria. BMC Complement. Altern. Med. 2019, 19, 46. [Google Scholar] [CrossRef]
- Dong, S.; Yang, X.; Zhao, L.; Zhang, F.; Hou, Z.; Xue, P. Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria. Ind. Crops Prod. 2020, 149, 112350. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Elmelegy, A.A.; Eldesoky, S.E.; Safwat, G. Phytochemical screening, antimicrobial, antioxidant, anti-cancer activities and nutritional values of cactus (Opuntia Ficus Indicia) pulp and peel. Fresenius. Environ. Bull. 2019, 28, 1545–1562. [Google Scholar]
- Aruwa, C.E.; Amoo, S.; Kudanga, T. Phenolic compound profile and biological activities of Southern African Opuntia ficus-indica fruit pulp and peels. LWT 2019, 111, 337–344. [Google Scholar] [CrossRef]
- Li, M.O.; Flavell, R.A. Contextual regulation of inflammation: A duet by transforming growth factor-β and interleukin-10. Immunity 2008, 28, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhang, J.; Zou, L.; Fu, C.; Li, P.; Zhao, G. Chemical characterization, antioxidant, immune-regulating and anti-cancer activities of a novel bioactive polysaccharide from Chenopodium quinoa seeds. Int. J. Biol. Macromol. 2017, 99, 622–629. [Google Scholar] [CrossRef]
- Yao, Y.; Shi, Z.; Ren, G. Antioxidant and immunoregulatory activity of polysaccharides from quinoa (Chenopodium quinoa Willd.). Int. J. Mol. Sci. 2014, 15, 19307–19318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, S.; Li, J.; Bai, B. Purification, structural elucidation and in vivo immunity-enhancing activity of polysaccharides from quinoa (Chenopodium quinoa Willd.) seeds. Biosci. Biotechnol. Biochem. 2019, 83, 2334–2344. [Google Scholar] [CrossRef] [PubMed]
- Zapata, A.; Diez, B.; Cejalvo, T.; Gutierrez-de Frias, C.; Cortes, A. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 2006, 20, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gupta, S.; Chandan, N.K.; Aklakur, M.; Pal, A.K.; Jadhao, S.B. Lipotropes protect against pathogen-aggravated stress and mortality in low dose pesticide-exposed fish. PLoS ONE 2014, 9, e93499. [Google Scholar] [CrossRef]
- Yardimci, B.; Aydin, Y. Pathological findings of experimental Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus). Ankara Üniv. Vet. Fak. Derg. 2011, 58, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Miwa, S.; Mano, N. Infection with Edwardsiella tarda causes hypertrophy of liver cells in the Japanese flounder Paralichthys olivaceus. Dis. Aquat. Org. 2000, 42, 227–231. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Dabrowski, K.; Hliwa, P.; Gomółka, P.; Kwasek, K. Nutritional regulation of intestine morphology in larval cyprinid fish, silver bream (Vimba vimba). Aquac. Res. 2008, 39, 1268–1278. [Google Scholar] [CrossRef]
- Owatari, M.S.; Jesus, G.F.A.; Brum, A.; Pereira, S.A.; Lehmann, N.B.; de Pádua Pereira, U.; Mouriño, J.L.P. Sylimarin as hepatic protector and immunomodulator in Nile tilapia during Streptococcus agalactiae infection. Fish Shellfish Immunol. 2018, 82, 565–572. [Google Scholar] [CrossRef]
- Tkachenko, H.; Kurhaluk, N.; Andriichuk, A.; Gasiuk, E.; Beschasniu, S. Oxidative stress biomarkers in liver of sea trout (Salmo trutta m. trutta L.) affected by ulcerative dermal necrosis syndrome. Turkish J. Fish. Aquat. Sci. 2014, 14, 391–402. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [Green Version]
- Steinel, N.C.; Bolnick, D.I. Melanomacrophage centers as a histological indicator of immune function in fish and other poikilotherms. Front. Immunol. 2017, 8, 827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Asely, A.M.; Amin, R.A.; El-Habashi, N.M. Effect of dietary administration of Echinacea purpurea on immune responses, histopathological alteration and microbial safety in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. In Proceedings of the 5th Global Fisheries and Aquaculture Research Conference, Faculty of Agriculture, Cairo University, Giza, Egypt, 1–3 October 2012; pp. 100–114. [Google Scholar]
- Magrone, T.; Fontana, S.; Laforgia, F.; Dragone, T.; Jirillo, E.; Passantino, L. Administration of a polyphenol-enriched feed to farmed sea bass (Dicentrarchus labrax L.) modulates intestinal and spleen immune responses. Oxid. Med. Cell. Longev. 2016, 2827567. [Google Scholar] [CrossRef] [Green Version]



| Gene | Forward | Amplicon Size (bp) | Accession No. |
|---|---|---|---|
| IFN-γ | F: 5′-AGC ACA ACG TAG CTT TCC CT-3′ R: 5′-TAA ACA GGG CAA ACA GGT CA-3′ | 132 | XM_003460533.2 |
| TGF-β | F: 5′-GTTTGAACTTCGGCGGTACTG-3′ R: 5′-TCCTGCTCATAGTCCCAGAGA-3′ | 80 | XM_003459454.2 |
| EF-1α | F: 5′-TGATCTACAAGTGCGGAGGAA-3′ R: 5′-GGAGCCCTTTCCCATCTCA-3′ | 80 | AB075952.1 |
| Parameters | Experimental Groups | ||||
|---|---|---|---|---|---|
| Control | PP10 | PP20 | QU10 | QU20 | |
| Survival rate | |||||
| Surviving fish/group (No./group) | 41/45 | 43/45 | 45/45 | 42/45 | 42/45 |
| Survival rate (%) | 91.11% | 95.50% | 100% | 93.33% | 93.33% |
| Bodyweight | |||||
| Initial body weight (g) | 23.12 ± 1.04 | 23.35 ± 0.48 | 23.59 ± 0.16 | 22.77 ± 0.83 | 23.61 ± 0.78 |
| Final body weight (g) | 32.40 ± 1.28 b | 35.33 ± 0.38 ab | 36.00 ± 0.36 a | 34.17 ± 0.54 ab | 34.00 ± 0.46 ab |
| Body weight gain (g) | 9.28 ± 0.32 b | 11.98 ± 0.86 ab | 12.41 ± 0.42 a | 11.40 ± 0.67 ab | 10.38 ± 0.67 ab |
| Specific growth rate (%/day) | 0.76 ± 0.04 | 0.92 ± 0.07 | 0.94 ± 0.03 | 0.88 ± 0.06 | 0.81 ± 0.06 |
| Parameters | Experimental Groups | ||||
|---|---|---|---|---|---|
| Control | PP10 | PP20 | QU10 | QU20 | |
| Erythrogram | |||||
| RBCs (106/µL) | 2.82 ± 0.11 c | 3.47 ± 0.10 ab | 3.63 ± 0.11 a | 2.91 ± 0.13 c | 3.08 ± 0.11 bc |
| Hb (g/dL) | 8.27 ± 0.21 c | 9.73 ± 0.12 a | 10.17 ± 0.20 a | 8.55 ± 0.13 bc | 9.02 ± 0.12 b |
| PCV (%) | 29.16 ± 1.29 c | 36.39 ± 1.53 ab | 38.72 ± 1.08 a | 30.1 ± 1.26 c | 32.71 ± 1.20 bc |
| MCV (fL) | 103.4 ± 0.81 | 104.87 ± 1.27 | 106.67 ± 0.96 | 103.43 ± 0.63 | 106.2 ± 0.86 |
| MCH (pg) | 29.33 ± 0.65 | 28.05 ± 0.66 | 27.97 ± 0.45 | 29.38 ± 0.79 | 29.29 ± 0.64 |
| MCHC (%) | 28.37 ± 0.65 | 26.74 ± 1.07 | 26.25 ± 0.35 | 28.4 ± 0.78 | 27.59 ± 0.62 |
| Leukogram | |||||
| WBCs (103/µL) | 6.51 ± 0.16 c | 8.54 ± 0.25 ab | 9.01 ± 0.02 a | 7.06 ± 0.16 c | 7.87 ± 0.19 b |
| Lymphocytes (103/µL) | 3.89 ± 0.10 c | 5.28 ± 0.11 a | 5.55 ± 0.16 a | 4.23 ± 0.12 c | 4.8 ± 0.13 b |
| Heterophils (103/µL) | 1.89 ± 0.076 c | 2.40 ± 0.14 ab | 2.59 ± 0.10 a | 2.07 ± 0.10 bc | 2.26 ± 0.10 abc |
| Monocytes (103/µL) | 0.46 ± 0.01 d | 0.58 ± 0.02 ab | 0.62 ± 0.02 a | 0.51 ± 0.01 cd | 0.54 ± 0.01 bc |
| Eosinophils (103/µL) | 0.20 ± 0.01 | 0.21 ± 0.009 | 0.19 ± 0.01 | 0.18 ± 0.02 | 0.22 ± 0.01 |
| Basophils(103/µL) | 0.065 ± 0.001 | 0.061 ± 0.001 | 0.064 ± 0.001 | 0.066 ± 0.002 | 0.067 ± 0.002 |
| Liver function tests | |||||
| ALT (U/L) | 24.02 ± 0.26 | 23.21 ± 0.19 | 22.85 ± 0.60 | 23.77 ± 0.44 | 22.73 ± 0.41 |
| AST(U/L) | 34.20 ± 0.32 a | 34.15 ± 0.28 a | 32.77 ± 0.19 b | 33.90 ± 0.30 ab | 33.20 ± 1.00 ab |
| ALP(U/L) | 49.00 ± 0.26 a | 48.02 ± 0.22 a | 46.93 ± 0.47 b | 48.98 ± 0.62 ab | 47.73 ± 0.18 ab |
| Protein profile | |||||
| Total protein (g/dL) | 6.76 ± 0.10 | 6.86 ± 0.13 | 6.73 ± 0.19 | 6.68 ± 0.10 | 6.93 ± 0.14 |
| Albumin (A) | 3.62 ± 0.12 | 3.82 ± 0.13 | 3.62 ± 0.15 | 3.57 ± 0.11 | 3.84 ± 0.15 |
| Globulin (G) | 3.15 ± 0.03 | 3.04 ± 0.02 | 3.11 ± 0.07 | 3.11 ± 0.02 | 3.10 ± 0.03 |
| Digestive enzymes | |||||
| Amylase (U/L) | 40.67 ± 0.61 d | 55.33 ± 0.71 b | 63.33 ± 1.33 a | 48.50 ± 0.99 c | 58.33 ± 0.92 b |
| Lipase (U/L) | 25.50 ± 1.64 e | 46.83 ± 1.49 b | 52.00 ± 1.37 a | 30.50 ± 1.23 d | 41.16 ± 1.05 c |
| Parameters | Experimental Groups | ||||
|---|---|---|---|---|---|
| Control | PP10 | PP20 | QU10 | QU20 | |
| Pre challenge | |||||
| Intestinal villi length (μm) | 300.06 ± 7.77 | 310.36 ± 17.68 | 342.51 ± 29.00 | 298.87 ± 13.75 | 314.42 ± 9.56 |
| Intestinal villi width (μm) | 37.37 ± 1.94 c | 46.34 ± 1.39 b | 52.96 ± 1.39 a | 40.31 ± 1.71 c | 46.92 ± 1.84 b |
| Goblet cell count (cell/ mm2) | 20.83 ± 1.25 b | 26.00 ± 1.06 b | 32.17 ± 2.18 a | 21.67 ± 1.20 b | 24.50 ± 1.18 b |
| Control (Challenged) | PP10 (Challenged) | PP20 (Challenged) | QU10 (Challenged) | QU20 (Challenged) | |
| Post challenge | |||||
| Intestinal villi length (μm) | 208.20 ± 12.44 c | 289.83 ± 9.46 a | 291.83 ± 14.15 a | 223.34 ± 18.28 bc | 277.88 ± 24.37 ab |
| Intestinal villi width (μm) | 34.35 ± 0.97 c | 38.33 ± 1.59 bc | 47.45 ± 2.61 a | 38.38 ± 1.51 bc | 43.00 ± 2.15 ab |
| Goblet cell count (cell/ mm2) | 16.33 ± 1.28 b | 22.00 ± 1.93 a | 26.00 ± 1.46 a | 16.83 ± 0.70 b | 22.83 ± 1.01 a |
| Parameters | Experimental Groups | |||||
|---|---|---|---|---|---|---|
| Control (Non-Challenged) | Control (Challenged) | PP10 (Challenged) | PP20 (Challenged) | QU10 (Challenged) | QU20 (Challenged) | |
| Mortality (number and rate) | - | 9/18 (50%) | 3/18 (16.66%) | 2/18 (11.11%) | 5/18 (27.77%) | 3/18 (16.66%) |
| RPS | - | 0.00% | 66.66% | 77.77% | 44.44% | 66.66% |
| Liver function tests | ||||||
| ALT (U/L) | 24.02 ± 0.26 f | 56.13 ± 0.60 a | 31.63 ± 0.49 d | 28.9 ± 0.36 e | 36.17 ± 0.90 b | 33.77 ± 0.37 c |
| AST(U/L) | 34.20 ± 0.32 d | 57.02 ± 0.82 a | 39.50 ± 1.36 c | 35.77 ± 0.77 d | 44.8 ± 0.54 b | 42.28 ± 0.70 bc |
| ALP(U/L) | 49.00 ± 0.26 f | 113.33 ± 1.63 a | 73.17 ± 1.47 c | 57.83 ± 1.47 e | 81.92 ± 1.80 b | 67.37 ± 1.54 d |
| Protein profile | ||||||
| Total protein (g/dL) | 6.76 ± 0.10 a | 4.34 ± 0.12 d | 5.07 ± 0.10 c | 5.85 ± 0.10 b | 4.58 ± 0.08 d | 5.09 ± 0.10 c |
| Albumin (A) | 3.62 ± 0.12 a | 2.40 ± 0.10 c | 2.81 ± 0.14 bc | 3.04 ± 0.19 b | 2.61 ± 0.08 bc | 2.97 ± 0.14 b |
| Globulin (G) | 3.15 ± 0.03 a | 1.93 ± 0.12 b | 2.25 ± 0.08 b | 2.81 ± 0.20 a | 1.97 ± 0.07 b | 1.95 ± 0.11 b |
| Immune status | ||||||
| Lysozyme activity (ng/mL) | 28.00 ± 1.06 d | 36.00 ± 0. 82 c | 38.67 ± 1.05 c | 50.33 ± 0.76 a | 37.16 ± 0.95 c | 42.33 ± 1.05 b |
| Nitric oxide (µmol/L) | 36.83 ± 0.60 d | 43.30 ± 0.88 c | 49.73 ± 1.06 b | 55.06 ± 1.47 a | 45.73 ± 0.89 bc | 48.82 ± 1.56 b |
| Myeloperoxidase (ng/mL) | 31.18 ± 0.48 d | 40.17 ± 0.77 c | 41.77 ± 0.92 c | 53.42 ± 1.13 a | 42.10 ± 2.08 c | 48.67 ± 0.88 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.A.A.; Abd El-Rahman, G.I.; Behairy, A.; Beheiry, R.R.; Hendam, B.M.; Alsubaie, F.M.; Khalil, S.R. Influence of Feeding Quinoa (Chenopodium quinoa) Seeds and Prickly Pear Fruit (Opuntia ficus indica) Peel on the Immune Response and Resistance to Aeromonas sobria Infection in Nile Tilapia (Oreochromis niloticus). Animals 2020, 10, 2266. https://doi.org/10.3390/ani10122266
Ahmed SAA, Abd El-Rahman GI, Behairy A, Beheiry RR, Hendam BM, Alsubaie FM, Khalil SR. Influence of Feeding Quinoa (Chenopodium quinoa) Seeds and Prickly Pear Fruit (Opuntia ficus indica) Peel on the Immune Response and Resistance to Aeromonas sobria Infection in Nile Tilapia (Oreochromis niloticus). Animals. 2020; 10(12):2266. https://doi.org/10.3390/ani10122266
Chicago/Turabian StyleAhmed, Shaimaa A. A., Ghada I. Abd El-Rahman, Amany Behairy, Rasha R. Beheiry, Basma M. Hendam, Faisal M. Alsubaie, and Samah R. Khalil. 2020. "Influence of Feeding Quinoa (Chenopodium quinoa) Seeds and Prickly Pear Fruit (Opuntia ficus indica) Peel on the Immune Response and Resistance to Aeromonas sobria Infection in Nile Tilapia (Oreochromis niloticus)" Animals 10, no. 12: 2266. https://doi.org/10.3390/ani10122266
APA StyleAhmed, S. A. A., Abd El-Rahman, G. I., Behairy, A., Beheiry, R. R., Hendam, B. M., Alsubaie, F. M., & Khalil, S. R. (2020). Influence of Feeding Quinoa (Chenopodium quinoa) Seeds and Prickly Pear Fruit (Opuntia ficus indica) Peel on the Immune Response and Resistance to Aeromonas sobria Infection in Nile Tilapia (Oreochromis niloticus). Animals, 10(12), 2266. https://doi.org/10.3390/ani10122266





