Concentrations of Circulating Irisin and Myostatin in Race and Endurace Purebred Arabian Horses—Preliminary Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
2.2. Blood Sampling and Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laursen, P.B.; Jenkins, D.G. The scientific basis for high-intensity interval training: Optimising training programmes and maximising performance in highly trained endurance athletes. Sports Med. 2002, 32, 53–73. [Google Scholar] [CrossRef]
- Fazzio, F.; Assenza, A.; Tosto, F.; Casella, S.; Piccione, G.; Caola, G. Training and haematochemical profile in Thoroughbreds and Standardbreds: A longitudinal study. Livest. Sci. 2011, 141, 221–226. [Google Scholar] [CrossRef]
- Piccione, G.; Fazio, F.; Giudice, E. Oxidative stress in Standardbred horses during official races od 1600 and 2000 m. Med. Weter. 2007, 63, 1554–1557. [Google Scholar]
- Castejon-Riber, C.; Riber, C.; Rubio, M.D.; Agüera, E.; Muñoz, A. Objectives, principles, and methods of strength training for horses. J. Equine Vet. Sci. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Rajão, M.D.; Leite, C.S.; Nogueira, K.; Godoy, R.F.; Lima, E.M.M. The bone response in endurance long distance horse. Open Vet. J. 2019, 9, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Rogers, C.W.; Bolwell, C.F.; Tanner, J.C.; Van Weeren, P. Early exercise in the horse. J. Vet. Behav. 2012, 7, 375–379. [Google Scholar] [CrossRef]
- Westerterp, K.R. Exercise, energy balance and body composition. Eur. J. Clin. Nutr. 2018, 72, 1246–1250. [Google Scholar] [CrossRef]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef] [Green Version]
- Leal, L.G.; Lopes, M.A.; Batista, M.L., Jr. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Rodríguez, A.; Becerril, S.; Ezquerro, S.; Méndez-Giménez, L.; Frühbeck, G. Crosstalk between adipokines and myokines in fat browing. Acta Physiol. 2017, 219, 362–381. [Google Scholar] [CrossRef]
- Rodríguez, A.; Catalán, V.; Ramírez, B.; Unamuno, X.; Portincasa, P.; Gómez-Ambrosi, J.; Frühbeck, G.; Becerril, S. Impact of adipokines and myokines on fat browning. J. Physiol. Biochem. 2020, 76, 227–240. [Google Scholar] [CrossRef]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef]
- Ost, M.; Coleman, V.; Kasch, J.; Klaus, S. Regulation of myokine expression: Role of exercise and cellular stress. Free Radic. Biol. Med. 2016, 98, 78–89. [Google Scholar] [CrossRef]
- Buehring, B.; Binkley, N. Myostatin—The holy grail for muscle, bone, and fat? Curr. Osteoporos. Rep. 2013, 11, 407–414. [Google Scholar] [CrossRef]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharm. Res. 2018, 41, 14–29. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Wang, D.; AlQudsy, L.; Jiang, J.X.; Xu, H.; Shang, P. Muscle-bone crosstalk and potential therapies for sarco-osteoporosis. J. Cell. Biochem. 2019, 120, 14262–14273. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenia—The search for emerging biomarkers. Ageing Res. Rev. 2015, 22, 58–71. [Google Scholar] [CrossRef]
- Laurens, C.; Bergouignan, A.; Moro, C. Exercise-released myokines in the control of energy metabolism. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef]
- Raschke, S.; Eckel, J. Adipo-myokines: Two sides of the same coin-mediators of inflammation and mediators of exercise. Mediat. Inflamm. 2013, 320724. [Google Scholar] [CrossRef]
- Aydin, S. Three new players in energy regulation: Preptin, adropin and irisin. Peptides 2014, 56, 94–110. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine Response to High-Intensity Interval vs. Resistance Exercise: An Individual Approach. Front. Physiol. 2018, 9, 1735. [Google Scholar] [CrossRef] [PubMed]
- Planella-Farrugia, C.; Comas, F.; Sabater-Masdeu, M.; Moreno, M.; Moreno-Navarrete, J.M.; Rovira, O.; Ricart, W.; Fernández-Real, J.M. Circulating Irisin and Myostatin as Markers of Muscle Strength and Physical Condition in Elderly Subjects. Front. Physiol. 2019, 10, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Śliwicka, E.; Cisoń, T.; Kasprzak, Z.; Nowak, A.; Pilaszczyńska-Szcześniak, Ł. Serum irisin and myostatin levels after 2 weeks of high-altitude climbing. PLoS ONE 2017, 12, e0181259. [Google Scholar] [CrossRef] [Green Version]
- Pesce, M.; Ballerini, P.; Paolucci, T.; Puca, I.; Farzaei, M.H.; Patruno, A. Irisin in autophagy: First update. Int. J. Mol. Sci. 2020, 21, 7587. [Google Scholar] [CrossRef]
- Carnac, G.; Vernus, B.; Bonnieu, A. Myostatin in the pathophysiology of skeletal muscle. Curr. Genom. 2007, 8, 415–422. [Google Scholar]
- Guo, T.; Jou, W.; Chanturiya, T.; Portas, J.; Gavrilova, O.; McPerron, A.C. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS ONE 2009, 4, e4937. [Google Scholar] [CrossRef] [Green Version]
- Miyata, H.; Itoh, R.; Sato, F.; Takebe, N.; Hada, T.; Tozaki, T. Effect of myostatin SNP on muscle fiber properties in male Thoroughbred horses during training period. J. Physiol. Sci. 2018, 68, 639–646. [Google Scholar] [CrossRef]
- Sharma, M.; McFarlane, C.; Kambadur, R.; Kukreti, H.; Bonala, S.; Srinivasan, S. Myostatin: Expanding horizons. IUNMB Life 2015, 67, 589–600. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, X.; Chen, D. Myostatin: A novel insight into its role in metabolism, signal pathways, and expression regulation. Cell. Signal. 2011, 23, 1441–1446. [Google Scholar] [CrossRef]
- Bond, N.D.; Guo, J.; Hall, K.D.; McPherron, A.C. Modeling energy dynamics in mice with skeletal muscle hypertrophy fed high calorie diets. Int. J. Biol. Sci. 2016, 12, 617–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, X.; Sathiakumar, D.; Lua, B.J.G.; Kukreti, H.; Lee, M.; McFarlane, C. Myostatin signals through miR-34a to regulate Fndc5 expression and browning of white adipocytes. Int. J. Obes. 2017, 41, 137–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez Munoz, I.Y.; Camarillo Romero, E.D.S.; Garduno Garcia, J.J. Irisin a novel metabolic biomarker: Present knowledge and future directions. Int. J. Endocrinol. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostroem, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostroem, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Dong, J.; Dong, Y.; Dong, Y.; Chen, F.; Mitch, W.E.; Zhang, L.Z. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissue. Int. J. Obes. 2016, 40, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2013, 281, 739–749. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Zhang, X.-F.; Ma, Z.-M.; Pan, L.-L.; Chen, Z.; Han, H.-W.; Han, C.-K.; Zhuang, X.-J.; Lu, Y.; Li, X.-J.; et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J. Hepatol. 2013, 59, 557–562. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Landis-Piwowar, K.; Byrd, G.; Seimer, M.; Seigneurie, N.; Byrd, B.; Muzik, O. Plasma irisin in runners and nonrunners: No favorable metabolic associations in humans. Physiol. Rep. 2015, 3, 12262. [Google Scholar] [CrossRef] [Green Version]
- Brenmoehl, J.; Albrecht, E.; Komolka, K.; Schering, L.; Langhammer, M.; Hoeflich, A.; Maak, S. Irisin Is Elevated in Skeletal Muscle and Serum of Mice Immediately after Acute Exercise. Int. J. Biol. Sci. 2014, 10, 338–349. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, R.R.; Shockett, P.; Webb, N.D.; Shah, U.; Castracane, V.D. A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm. Metab. Res. 2014, 46, 150–154. [Google Scholar] [CrossRef]
- Albrecht, E.; Norheim, F.; Thiede, B.; Holen, T.; Ohashi, T.; Schering, L.; Lee, S.; Brenmoehl, J.; Thomas, S.; Drevon, C.A.; et al. Irisin—A myth rather than an exercise-inducible myokine. Sci. Rep. 2015, 5, 8889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fain, J.N.; Company, J.M.; Booth, F.W.; Laughlin, M.H.; Padilla, J.; Jenkins, N.T.; Bahouth, S.W.; Sacks, H.S. Exercise training does not increase muscle FNDC5 protein or mRNA expression in pigs. Metabolism 2013, 62, 1503–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biniaminov, N.; Bandt, S.; Roth, A.; Haertel, S.; Neumann, R.; Bub, A. Irisin, physical activity and fitness status in healthy humans: No association under resting conditions in a cross-sectional study. PLoS ONE 2018, 30, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Li, Y.; Duan, Y.; Hu, C.-A.A.; Tang, Y.; Yin, Y. Myokines and adipokines: Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev. 2017, 33, 73–82. [Google Scholar] [CrossRef]
- Desmecht, D.; Linden, A.; Amory, H.; Art, T.; Lekeux, P. Relationship of plasma lactate production to cortisol release following competition of different types of sporting events in horses. Vet. Res. Commun. 1996, 20, 371–379. [Google Scholar] [CrossRef]
- Trigo, P.; Castejon, F.; Riber, C.; Muñoz, A. Use of biochemical parameters to predict metabolic elimination in endurance rides. Equine Vet. J. 2011, 42 (Suppl. 38), 142–146. [Google Scholar] [CrossRef]
- Dundar, A.; Kocahan, S.; Sahin, L. Associations of apelin, leptin, irisin, ghrelin, insulin, glucose levels, and lipid parameters with physical activity during eight weeks of regular exercise training. Arch. Physiol. Biochem. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Ozbay, S.; Ulupınar, S.; Şebin, E.; Altınkaynak, K. Acute and chronic effects of aerobic exercise on serum irisin, adropin, and cholesterol levels in the winter season: Indoor training versus outdoor training. Chin. J. Physiol. 2020, 63, 21–26. [Google Scholar]
- Bagheri, R.; Moghadam, B.H.; Church, D.D.; Tinsley, G.M.; Eskandari, M.; Moghadam, B.H.; Motevalli, M.S.; Baker, J.S.; Robergs, R.A.; Wong, A. The effects of concurrent training order on body composition and serum concentrations of follistatin, myostatin and GDF11 in sarcopenic elderly men. Exp. Gerontol. 2020, 133, 110869. [Google Scholar] [CrossRef]
- Inoue, K.; Fujie, S.; Hasegawa, N.; Horii, N.; Uchida, M.; Iemitsu, K.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Aerobic exercise training-induced irisin secretion is associated with the reduction of arterial stiffness via nitric oxide production in adults with obesity. Appl. Physiol. Nutr. Metab. 2020, 45, 715–722. [Google Scholar] [CrossRef]
- Amri, J.; Parastesh, M.; Sadegh, M.; Latifi, S.A.; Alaee, M. High-intensity interval training improved fasting blood glucose and lipid profiles in type 2 diabetic rats more than endurance training; possible involvement of irisin and betatrophin. Physiol. Int. 2019, 106, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Belviranli, M.; Okudan, N. Exercise training increases cardiac, hepatic and circulating levels of brain-derived neurotrophic factor and irisin in young and aged rats. Horm. Mol. Biol. Clin. Investig. 2018, 36. [Google Scholar] [CrossRef] [PubMed]
- Kartinah, T.N.; Sianipar, R.I. The effects of exercise regimens on irisin levels in obese rats model: Comparing high-intensity intermittent with continuous moderate-intensity training. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirvani, H.; Arabzadeh, E. Metabolic cross-talk between skeletal muscle and adipose tissue in high-intensity interval training vs. moderate-intensity continuous training by regulation of PCG-1α. Eat. Weight Disord. 2020, 25, 17–24. [Google Scholar] [CrossRef]
- Tavassoli, H.; Heidarianpour, A.; Hedayati, M. The effects of resistance exercise training followed by de-training on irisin and some metabolic parameters in type 2 diabetic rat model. Arch. Physiol. Biochem. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine/Adipokine Response to “Aerobic” Exercise: Is It Just a Matter of Exercise Load? Front. Physiol. 2019, 10, 691. [Google Scholar] [CrossRef]
- Pekkala, S.; Wiklund, P.K.; Hulmi, J.J.; Ahtiainen, J.P.; Horttanainen, M.; Pöllänen, E.; Mäkelä, K.A.; Kainulainen, H.; Häkkinen, K.; Nyman, K.; et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013, 591, 5393–5400. [Google Scholar] [CrossRef]
- Wiecek, M.; Szymura, J.; Maciejczyk, M.; Kantorowicz, M.; Szygula, Z. Acute anaerobic exercise affects the secretion of asprosin, irisin, and other cytokines—A comparison between sexes. Front. Physiol. 2018, 10, 1782. [Google Scholar] [CrossRef] [Green Version]
- Liu, J. Irisin as an exercise-stimulated hormone binding crosstalk between organs. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 316–321. [Google Scholar]
- Qiu, S.; Bosnyák, E.; Treff, G.; Steinacker, J.M.; Nieß, A.M.; Krüger, K.; Mooren, F.-C.; Zügel, M.; Schumann, U. Acute exercise-induced irisin release in healthy adults: Associations with training status and exercise mode. Eur. J. Sport Sci. 2018, 18, 1226–1233. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ando, D.; Goto, K.; Kiuchi, M.; Yamakita, M.; Koyama, K. High-intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J. Exp. Med. 2014, 233, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kędzierski, W.; Cywińska, A.; Wawak, T.; Janczarek, I.; Wilk, I.; Kowalik, S. Plasma Apelin Concentration in Exercised Horses: Preliminary Study. J. Equine Vet. Sci. 2019, 80, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Kędzierski, W.; Janczarek, I.; Wilk, I.; Staniszewska, M.; Kowalik, S. Plasma visfatin response to the intensity of exercise and training in race-horses. Pferdeheilkunde 2018, 34, 525–530. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [Green Version]
- Kabak, B.; Belviranli, M.; Okudan, N. Irisin and myostatin responses to acute high-intensity interval exercise in humans. Horm. Mol. Biol. Clin. Investig. 2018, 35. [Google Scholar] [CrossRef]
- Wessner, B.; Ploder, M.; Tschan, H.; Ferunaj, P.; Erindi, A.; Strasser, E.-M.; Bachl, N. Effects of acute resistance exercise on proteolytic and myogenic markers in skeletal muscles of former weightlifters and age-matched sedentary controls. J. Sports Med. Phys. Fit. 2019, 59, 1915–1924. [Google Scholar] [CrossRef]
- Bagheri, R.; Rashidlamir, A.; Attarzadeh Hosseini, S.R. Effect of resistance training with blood flow restriction on follistatin to myostatin ratio, body composition and anaerobic power of trained-volleyball players. Med. Lab. J. 2018, 12, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, F. Myostatin alters with exercise training in diabetic rats; possible interaction with glycosylated hemoglobin and inflammatory cytokines. Cytokine 2019, 120, 99–106. [Google Scholar] [CrossRef]
- Shanazari, Z.; Faramarzi, M.; Banitalebi, E.; Hemmati, R. Effect of moderate and high-intensity endurance and resistance training on serum concentrations of MSTN and IGF-1 in old male Wistar rats. Horm. Mol. Biol. Clin. Investig. 2019, 38. [Google Scholar] [CrossRef]
- Arrieta, H.; Hervás, G.; Rezola-Pardo, C.; Ruiz-Litago, F.; Iturburu, M.; Yanguas, J.J.; Gil, S.M.; Rodriguez-Larrad, A.; Irazusta, J. Serum Myostatin Levels Are Higher in Fitter, More Active, and Non-Frail Long-Term Nursing Home Residents and Increase after a Physical Exercise Intervention. Gerontology 2019, 65, 229–239. [Google Scholar] [CrossRef]
- De Souza, E.O.; Tricoli, V.; Aoki, M.S.; Roschel, H.; Brum, P.C.; Bacurau, A.V.; Silva-Batista, C.; Wilson, J.M.; Neves, M.; Soares, A.G.; et al. Effects of Concurrent Strength and Endurance Training on Genes Related to Myostatin Signaling Pathway and Muscle Fiber Responses. J. Strength Cond. Res. 2014, 28, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- McKay, B.R.; Ogborn, D.I.; Bellamy, L.M.; Tarnopolsky, M.A.; Parise, G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J. 2012, 26, 2509–2521. [Google Scholar] [CrossRef] [PubMed]
- Poggioli, T.; Vujic, A.; Yang, P.; Macias-Trevino, C.; Uygur, A.; Loffredo, F.S.; Pancoast, J.R.; Cho, M.; Goldstein, J.M.; Tandias, R.M.; et al. Circulating Growth Differentiation Factor 11/8 Levels Decline with Age. Circ. Res. 2016, 118, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-Induced Irisin Secretion Is Independent of Age or Fitness Level and Increased Irisin May Directly Modulate Muscle Metabolism Through AMPK Activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef]
- Zhang, C.; McFarlane, C.; Lokireddy, S.; Bonala, S.; Ge, X.; Masuda, S.; Gluckman, P.D.; Sharma, M.; Kambadur, R. Myostatin-deficient mice exhibit reduced insulin resistance through activating the AMP-activated protein kinase signalling pathway. Diabetologia 2011, 54, 1491–1501. [Google Scholar] [CrossRef]
- Wang, M.; Yu, H.; Kim, Y.S.; Bidwell, C.A.; Kuang, S. Myostatin facilitates slow and inhibits fast myosin heavy chain expression during myogenic differentation. Biochem. Biophys. Res. Commun. 2012, 426, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Xuan, M.-F.; Luo, Z.-B.; Wang, J.-X.; Guo, Q.; Han, S.-Z.; Jin, S.-S.; Kang, J.-D.; Yin, X. Shift from slow- to fast-twitch muscle fibres in skeletal muscle of newborn heterozygous and homozygous myostatin-knockout piglets. Reprod. Fertil. Dev. 2019, 31, 1628. [Google Scholar] [CrossRef]
- Kaji, H. Effects of myokines on bone. BoneKEy Rep. 2016, 20, 826. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [Green Version]
- Battafarano, G.; Rossi, M.; Maramphon, F.; Minisola, S.; Del Fattore, A. Bone control of muscle function. Int. J. Mol. Sci. 2020, 21, 1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]



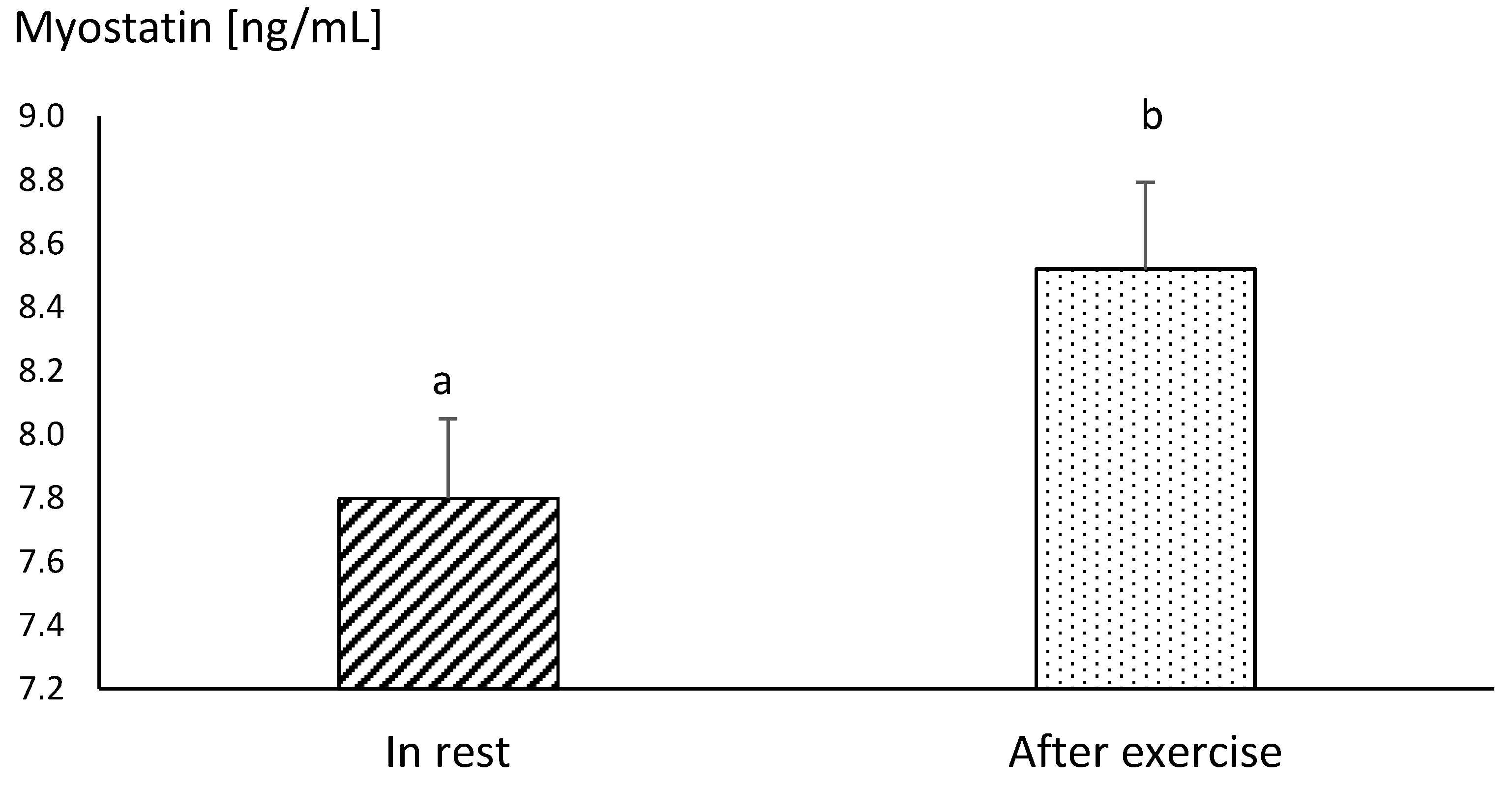
| Horses | n | Distance | Average Velocity | Average Duration | |
|---|---|---|---|---|---|
| Racehorses | MAY | 10 | 1200 m | 9.97 ± 0.25 m/s | 2.0 ± 0.05 min |
| SEP | 10 | 1200 m | 9.01 ± 0.22 m/s | 2.2 ± 0.06 min | |
| Endurance horses | 10 | 60 km | 3.33 ± 0.06 m/s | 300 ± 5.15 min | |
| Parameter | Racehorses | Endurance Horses | ||||
|---|---|---|---|---|---|---|
| Factor | F | p | Factor | F | p | |
| Irisin | Time | 0.03 | 0.9436 | Time | 1.33 | 0.2796 |
| Period | 6.62 | 0.0008 | Age | 1.83 | 0.1368 | |
| Interaction | 1.98 | 0.1687 | Interaction | 0.43 | 0.7821 | |
| Myostatin | Time | 3.81 | 0.0329 | Time | 7.91 | 0.0016 |
| Period | 0.81 | 0.4544 | Age | 0.89 | 0.4167 | |
| Interaction | 2.06 | 0.1376 | Interaction | 1.80 | 0.1403 | |
| Lactic acid | Time | 19.91 | 0.0001 | Time | 2.45 | 0.1024 |
| Period | 7.40 | 0.0023 | Age | 1.33 | 0.2791 | |
| Interaction | 3.59 | 0.0392 | Interaction | 0.76 | 0.3613 | |
| Cortisol | Time | 2.35 | 0.0931 | Time | 14.53 | 0.0006 |
| Period | 0.44 | 0.7821 | Age | 2.67 | 0.0843 | |
| Interaction | 1.21 | 0.2971 | Interaction | 1.28 | 0.3110 | |
| Proteins | Time | 1.53 | 0.1138 | Time | 5.18 | 0.0126 |
| Period | 0.47 | 0.4992 | Age | 1.33 | 0.2790 | |
| Interaction | 0.81 | 0.4543 | Interaction | 0.95 | 0.7803 | |
| AST | Time | 1.24 | 0.3011 | Time | 4.39 | 0.0034 |
| Period | 1.43 | 0.2503 | Age | 0.67 | 0.5167 | |
| Interaction | 0.85 | 0.3451 | Interaction | 0.13 | 0.8705 | |
| LDH | Time | 1.80 | 0.1400 | Time | 15.38 | 0.0002 |
| Period | 1.38 | 0.2119 | Age | 1.27 | 0.3083 | |
| Interaction | 0.08 | 0.8906 | Interaction | 1.68 | 0.1907 | |
| Time of the Test | At Rest | Immediately after the End of Exercise |
|---|---|---|
| MAY | 0.94 ± 0.02 a | 9.94 ± 1.27 b |
| SEP | 1.03 ± 0.01 a | 2.68 ± 0.91 c |
| Parameter | At Rest | After the End of Exercise |
|---|---|---|
| Lactic acid (mmol/L) | 0.82 ± 0.01 | 1.46 ± 0.12 |
| Cortisol (ng/mL) | 152 ± 5.08 | 237 ± 7.07 * |
| Total proteins (g/L) | 61.4 ± 3.31 | 65.3 ± 2.43 * |
| AST (U/L) | 235 ± 19.4 | 263 ± 16.2 * |
| LDH (U/L) | 458 ± 33.7 | 698 ± 60.4 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalik, S.; Wiśniewska, A.; Kędzierski, W.; Janczarek, I. Concentrations of Circulating Irisin and Myostatin in Race and Endurace Purebred Arabian Horses—Preliminary Study. Animals 2020, 10, 2268. https://doi.org/10.3390/ani10122268
Kowalik S, Wiśniewska A, Kędzierski W, Janczarek I. Concentrations of Circulating Irisin and Myostatin in Race and Endurace Purebred Arabian Horses—Preliminary Study. Animals. 2020; 10(12):2268. https://doi.org/10.3390/ani10122268
Chicago/Turabian StyleKowalik, Sylwester, Anna Wiśniewska, Witold Kędzierski, and Iwona Janczarek. 2020. "Concentrations of Circulating Irisin and Myostatin in Race and Endurace Purebred Arabian Horses—Preliminary Study" Animals 10, no. 12: 2268. https://doi.org/10.3390/ani10122268





